

Deconstructing Chronic Low Back Pain in the Older Adult -
Step by Step Evidence and Expert-Based Recommendations
for Evaluation and Treatment.
Part VII: InsomniaThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Med. 2016 (May); 17 (5): 851–863 ~ FULL TEXT
OPEN ACCESS Adam D. Bramoweth PhD, Jenna G. Renqvist PhD, Anne Germain PhD,
Daniel J. Buysse MD, Angela Gentili MD, Gary Kochersberger MD,
Eric Rodriguez MD, Michelle I. Rossi MD, Debra K. Weiner MD
Mental Illness Research, Education & Clinical Center (MIRECC),
VA Pittsburgh Healthcare System,
Pittsburgh, Pennsylvania Department of Psychiatry,
University of Pittsburgh School of Medicine,
Pittsburgh, Pennsylvania.OBJECTIVE: To present the seventh in a series of articles designed to deconstruct chronic low back pain (CLBP) in older adults. This article focuses on insomnia and presents a treatment algorithm for managing insomnia in older adults, along with a representative clinical case.
METHODS: A modified Delphi process was used to develop the algorithm and supportive materials. A multidisciplinary expert panel representing expertise in health psychology and sleep medicine developed the algorithm and supporting documents that were subsequently refined through an iterative process of input from a primary care provider panel.
RESULTS: We present an illustrative clinical case and an algorithm to help guide the care of older adults with insomnia, an important contributor to CLBP and disability. Multicomponent cognitive behavioral therapy for insomnia (CBTI) and similar treatments (e.g., brief behavioral treatment for insomnia [BBTI]) are the recommended first-line treatment. Medications should be considered only if BBTI/CBTI is suboptimal or not effective and should be prescribed at the lowest effective dose for short periods of time (< 90 days).
CONCLUSIONS: Insomnia is commonly comorbid with CLBP in older adults and should be routinely evaluated and treated because it is an important contributor to pain and disability. The algorithm presented was structured to assist primary care providers in planning treatment for older adults with CLBP and insomnia.
KEYWORDS: Chronic Low Back Pain; Chronic Pain; Elderly; Insomnia; Low Back Pain; Older Adults; Sleep Disorders
From the FULL TEXT Article:
Introduction
Sleep problems are a highly prevalent comorbidity and consequence of chronic low back pain (CLBP), impacting an estimated 50–80% of individuals with CLBP. [1–3] Insomnia — dissatisfaction with sleep quantity or quality related to difficulty initiating, maintaining, and/or early morning awakenings [4] — is the most common sleep disorder in the general population and among those with CLBP. [5] Insomnia also significantly increases the risk of developing CLBP, even after controlling for socioeconomic, self-reported health, lifestyle behaviors, and anthropometric variables. [6] Prolonged sleep onset latency and poor sleep quality, key symptoms of insomnia, are associated with poor physical functioning and longer pain duration. [7] Also, self-reported insomnia severity is associated with pain intensity and vice versa. [2] The bidirectional relationship of pain and sleep is supported by multiple shared neurobiological underpinnings between the two disorders. Several studies have implicated dopaminergic signaling and opioidergic signaling, as well as negative and positive affect. [8] Structural and functional changes to similar brain structures, such as the activation to the limbic area, have been implicated in both pain and insomnia. [9] Also, dysregulation of the hypothalamus-pituitary-adrenal axis and decreased brain-derived neurotrophic factor have been linked to chronic pain and insomnia. [9]
Comorbid chronic pain and insomnia is an important public health issue, as the combined impact magnifies the clinical and economic consequences and correlates related to each disorder. [10] Both insomnia and CLBP are independently related to significant reductions in quality of life [11, 12], medical morbidity, and disability. [13, 14] Furthermore, both are linked with a significant economic impact, each exceeding $100 billion annually. [15–17] Fortunately, effective pharmacological [18, 19] and nonpharmacological treatments [20, 21] are available that reduce pain, improve physical functioning, and decrease sleep disturbance. The most commonly prescribed medications for chronic pain and insomnia are opioid analgesics and sedative hypnotics [18, 19], respectively. Unfortunately, these medications are linked with side effects, such as risk of falls and tolerance and withdrawal [22–24], all issues particularly important in older adults. Opioid analgesics may actually disturb sleep, change sleep architecture, and induce sleep disordered breathing. [25, 26] Effective nonpharmacological treatments for insomnia with fewer associated risks are available but are often underutilized by clinicians. Assessment tools and treatment guidelines are often too general and global to meet the needs of a diverse patient population. Furthermore, the most commonly recommended nonpharmacological approach for managing insomnia — sleep hygiene [27] — lacks evidence [28] as a stand-alone treatment, especially in the context of chronic pain. Accordingly, there is a great deal of variation in how older adults with chronic pain and insomnia are treated, and there is limited evidence on effective strategies for the management of comorbid pain and insomnia in real-world clinical settings.
A substantial proportion of older adults with CLBP report insomnia. We present a pragmatic, evidence-based approach to the management of insomnia in older adults and a case illustrating the application and efficacy of nonpharmacological strategies.
Methods
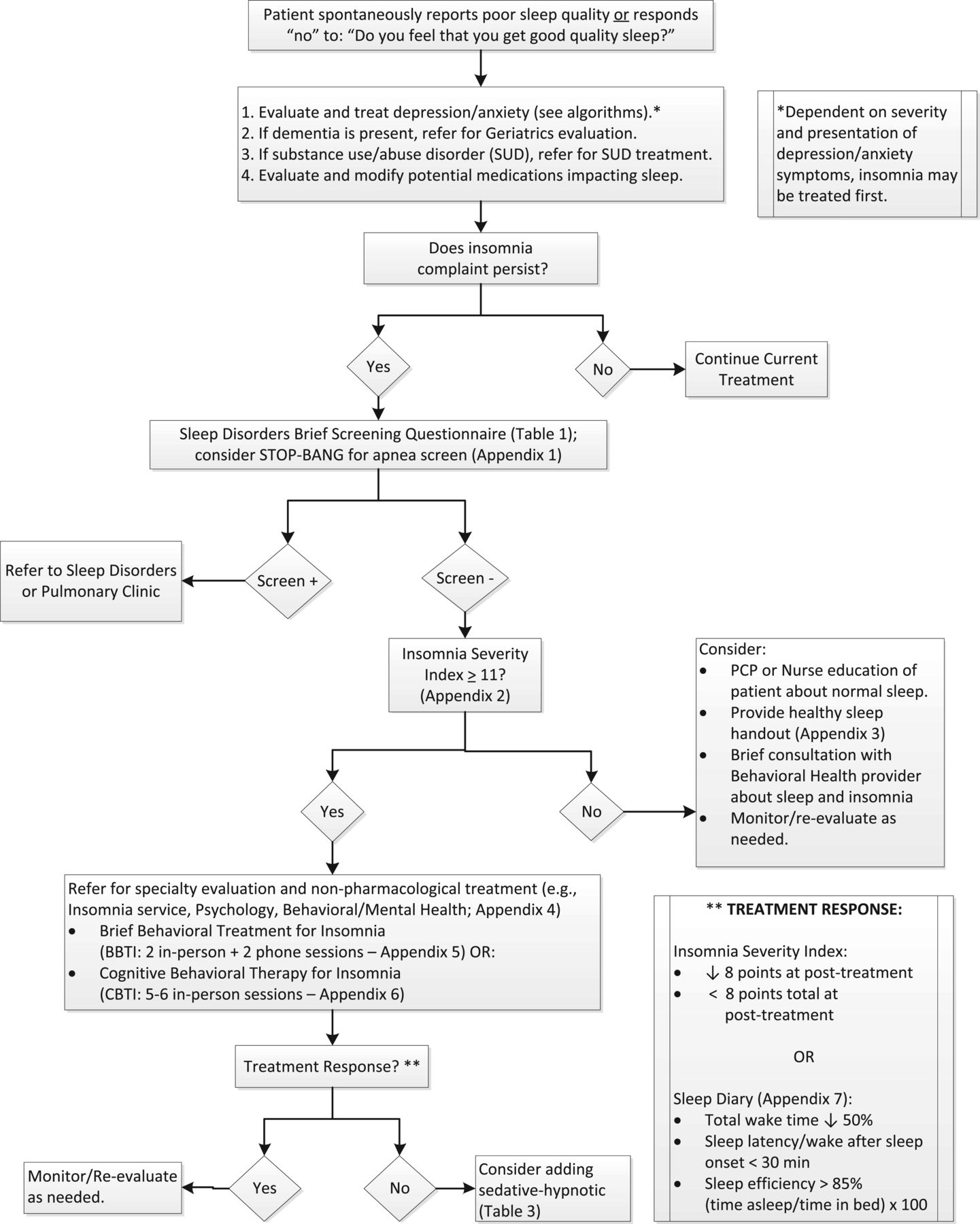
Figure 1

Table 2

Table 3 A detailed description of the modified Delphi process used to create the algorithm (Figure 1), the table providing the rationale for the algorithm components (Table 2), and the medication table (Table 3) are provided in the series overview. [29] The expert panel team leader (ADB) drafted the initial algorithm and supportive tables based upon a comprehensive review of the literature and knowledge of insomnia treatments. The expert panel, which consisted of two health psychologists with expertise in behavioral sleep medicine (ADB, AG) and a sleep medicine physician (DJB), refined the algorithm and accompanying tables before receiving feedback from the primary care panel, as described previously. [29]
Case Presentation
Insomnia Evaluation
The patient is a 66–year-old male veteran with a 20–year history of insomnia reported as secondary to chronic shoulder, low back, and knee pain. He used only over-the-counter analgesics and had declined a referral to the pain clinic in the past. He reported difficulty with maintaining sleep, often waking due to pain, with difficulty returning to sleep; symptoms occurred at least 4 nights per week. During the day, he reported fatigue, decreased motivation to complete tasks, and poor concentration. Estimated total sleep time ranged from 2–6 hours, usually no more than 2–3 hours at a time, with his bedtime from 10:30 PM–11:30 PM and wake time ranging from 3:30 AM–6:00 AM. Pre-bedtime activities included watching television, talking with his wife, smoking cigarettes, and taking sleep medication (trazodone, 50 mg). He reported minimal sleep onset latency, usually less than 10 minutes, but reported wake after sleep onset of 45 minutes or longer; after each nighttime awakening and about once a week, he was unable to return to sleep. On some nights during wake after sleep onset periods, he stayed in bed, and others he paced around the house, smoked a cigarette, read, watched baseball, or drank a glass of milk. He reported taking late-morning or early afternoon naps 1–2 times per week following nights with an early morning awakening. The patient denied symptoms of other sleep disorders (e.g., sleep apnea) or psychiatric disorders.
Medical History, Sleep Medication, and Relevant Substance Use
The patient’s medical history is significant for insomnia, osteoarthritis, lung nodule, benign prostatic hypertrophy, contact dermatitis, erectile dysfunction, acne, tobacco use disorder, and anger issues. He reported currently taking nightly trazodone 50 mg, which only occasionally helped to reduce the frequency of nighttime awakenings from 2–3 per night to 0–1 and did not help reduce early morning awakenings. He also reported a history of using flurazepam for several months (early 1990s), which he reported as helpful in improving his sleep. He also reported drinking large quantities of coffee each morning (twelve 6-oz cups [“a pot”]), but avoided caffeine in the afternoon due to concern it would interfere with nighttime sleep. He smoked one pack of cigarettes per day and half “a joint” of marijuana 2–4 times per week before bed, which he reported as helpful. Alcohol use was minimal, only a few times per year, and he denied ever drinking alcohol to assist with sleep.
Initial Clinical Course
The patient completed an insomnia evaluation, including week-long sleep diaries at baseline and throughout treatment [30], and five sessions of cognitive behavioral therapy for insomnia (CBTI) with a psychologist. When treatment began, he reported difficulty with maintaining sleep due to pain and frequent early morning awakenings. Treatment goals were: reduce wake after sleep onset, increase sleep quality, and reduce coffee consumption (morning) and cigarette use (night). The first three sessions focused on restructuring the sleep-wake schedule with stimulus control and sleep restriction, and reviewing and identifying relevant sleep hygiene factors. Session 3 also introduced concepts of cognitive therapy and further titration of the sleep-wake schedule. Session 4 continued the cognitive therapy work; session 5 emphasized self-management strategies and relapse prevention.
Approach to Management

Table 1 The standard of practice for treating insomnia disorder is multicomponent CBTI. [21, 31–34] However, the first step is to identify any organic and/or noninsomnia sleep disorders that require further evaluation and treatment (e.g., sleep apnea, narcolepsy). [35] Comorbid depression, anxiety, and other psychiatric disorders, especially if severe and untreated, may need to be addressed prior to beginning an insomnia intervention. However, for patients with mild to moderate psychiatric symptoms, treatment for insomnia may occur prior to, or concurrent with, addressing comorbid symptoms. Symptoms of dementia and/or substance use disorder should also be addressed before proceeding with management of insomnia. Lastly, medications that may interfere with sleep (e.g., opioids, activating antidepressants) need to be identified and appropriately managed. In the algorithm (Figure 1), a positive screen on the Sleep Disorders Brief Screening Questionnaire (Table 1) and the STOP-BANG, a screening measure for risk of obstructive sleep apnea [36], if indicated, will lead to referral to a sleep disorders clinic or pulmonary clinic. A negative screen will lead to insomnia screening with the Insomnia Severity Index (Appendix 2; [37]), a psychometrically sound, brief screening tool and outcome measure. An Insomnia Severity Index score ≥ 11 indicates at least minimal to moderate symptoms [38] and in a clinical sample showed 97.2% sensitivity and 100% specificity with 0% false-positive rate, 2.8% false-negative rate, and 97.9% correct classification rate. [38] An Insomnia Severity Index score ≥ 11 indicates a referral is needed to a clinician for further assessment and management of insomnia.
The core components of CBTI include [28, 39]: education, stimulus control, sleep restriction, sleep hygiene, cognitive therapy, and relaxation training. Treatment is flexible and should be adapted to fit the needs of the patient; however, it is recommended to introduce stimulus control and sleep restriction as early in the course of treatment as is clinically feasible. Education provides basic facts about normal sleep versus insomnia and insomnia etiology and prognosis. Stimulus control helps to strengthen the association of sleep and the sleep environment: avoid nonsleep activities in bed, go to bed only when sleepy, and do not stay in bed if awake. An important factor to consider in older adults with CLBP is how resting—lying down without the intention to sleep—can impact sleep quality. Despite its potential benefit, it is important not to rest in bed as this is in opposition to stimulus control; resting should take place outside the bedroom if possible. Sleep restriction changes the sleep-wake schedule to match actual sleep time and establishes a consistent wake time, a key variable to developing an improved sleep-wake schedule. Cognitive therapy helps the patient identify maladaptive and dysfunctional beliefs about sleep, then challenge and change those beliefs to decrease worry and arousal. Relaxation training, which may be active (e.g., progressive muscle relaxation) or passive (e.g., guided imagery, mindfulness), can help reduce physical tension and/or cognitive hyperarousal. Practicing before bed and throughout the day can help further improve sleep when combined with other components of CBTI. Sleep hygiene involves assessing and modifying behavioral and environmental variables that may impair sleep quality. Common sleep hygiene topics include: avoiding clock watching; limiting caffeine intake, especially in the afternoon and evening; timing exercise properly; and addressing precipitants of nocturia, such as dietary and substance use changes. Improving sleep hygiene alone does not significantly improve sleep but may help enhance CBTI outcomes achieved through stimulus control and sleep restriction.
Brief behavioral treatment for insomnia (BBTI; [40, 41]) is another evidence-based intervention based on the behavioral principles of CBTI, stimulus control, and sleep restriction, and is delivered in a briefer format. Treatment consists of two in-person sessions and two phone calls: session 1 (week 1, in-person) provides treatment rationale and introduces stimulus control and sleep restriction, and the patient establishes a new sleep-wake schedule; in session 2 (week 3, in-person), the patient and clinician review the sleep diary, problem-solve barriers to BBTI implementation, and adjust the sleep-wake schedule as needed; relapse prevention is also discussed during session 2. Brief phone calls during weeks 2 and 4 are used to answer patient questions, make minor adjustments to the sleep-wake schedule, and manage adherence issues.
Not all patients are good candidates for BBTI/CBTI, or adaptations to treatment are necessary. While many patients with CLBP can engage in BBTI/CBTI without adaptation, one common change to CBTI involves utilizing a more passive relaxation method, as tightening and relaxing of muscle groups (i.e., progressive muscle relaxation) may cause discomfort/pain for some patients. Furthermore, the cognitive therapy portion will likely focus, in part, on pain-sleep or pain-specific thoughts and worries. Individuals with bipolar disorder, psychotic disorders, or seizure disorders are at risk of symptom exacerbation with severe sleep restriction or marked sleep-wake routine disruption. If BBTI/CBTI is initiated, it needs to be adapted appropriately and symptoms monitored. Sleep duration < 6 hours should be avoided in these patients in order to minimize excessive daytime sleepiness and potential adverse effects of sleep loss. Patients with bipolar disorder may benefit from BBTI/CBTI that integrates aspects of interpersonal and social rhythm therapy [42], an evidence-based treatment that focuses on the bidirectional relationship between mood, life events, and daily routines. Patients with active substance use disorder/abuse should be referred for appropriate substance treatment before participating in BBTI/CBTI, as sleep disruption related to initial stages of treatment (i.e., sleep restriction) may exacerbate substance use symptoms or interfere with recovery. However, BBTI/CBTI during the recovery and remission period may be beneficial. [43, 44] Another population that may require treatment adaptation is patients with dementia. For patients that require a caregiver, the burden of establishing treatment may ultimately fall to them. [45] However, patients with mild dementia or mild cognitive impairment who are still able to manage aspects of their health independently may be able to successfully implement BBTI/CBTI. Older adults often deal with multiple chronic medical disorders and take numerous medications, many of which can impact sleep (e.g., diuretics contributing to nocturia and wakefulness). As part of the initial evaluation process, medical and psychiatric comorbidities and medications are always considered in regards to potential changes/adaptations to treatment. Any changes to medications will be a collaborative process between patient, prescribing provider, and insomnia clinician.
Access to insomnia care is a concern for many patients and providers, especially those in rural areas. Evidence-based insomnia care is most often available in urban settings. Even in large urban hospitals, providers trained in BBTI/CBTI may not be available. In the community, insomnia providers can be identified through publicly available resources such as the Society of Behavioral Sleep Medicine (http://www.behavioralsleep.org/index.php/society-of-behavioral-sleep-medicine-providers) and the Association for Behavioral and Cognitive Therapies (abctcentral.org/xFAT/). Within the Department of Veterans Affairs, therapists trained to deliver CBTI can be identified by contacting local Evidence-Based Psychotherapies Coordinators. One option for individuals who live a significant distance from a provider or are unable to travel are online CBTI programs. These programs often require a subscription or modest fee but are evidence-based and effective [46], and may significantly help increase access to care.
Patients with an Insomnia Severity Index score < 11 should be provided with information about healthy sleep behaviors and monitored/re-evaluated as needed. Patients who are not good candidates for BBTI/CBTI based on clinical presentation and/or an unwillingness to engage in behavioral therapies [35] should be referred to the appropriate specialist for consideration of pharmacological treatments.
Use of the algorithm should be a collaborative process between providers and patients to establish appropriate and realistic treatment goals. Although positive treatment response to CBTI or BBTI is common, > 70% for treatment completers [47–49], patients with unrealistic goals (e.g., no more nighttime awakenings, always feeling refreshed upon awakening) should be educated about appropriate treatment expectations. Many patients can expect to significantly reduce insomnia symptoms such as sleep onset latency and wake after sleep onset as well as increase sleep quality. Sleep onset latency and wake after sleep onset can often be reduced by approximately 50%, and sleep efficiency can improve by at least 10 percentage points, with many able to achieve sleep efficiency ≥ 85%, an indication of good sleep quality. A reduction of ≥ 8 points on the Insomnia Severity Index is also common and indicative of categorical symptom reduction (e.g., severe to moderate, moderate to mild, or mild to minimal). While total sleep time does not always increase, patients usually report improved sleep continuity and feeling more refreshed in the morning with less impairment during the day. Patient-determined expectations and goals, when realistic and feasible, are paramount and may be determined differently than sleep diary or Insomnia Severity Index outcomes. A key goal of the collaborative process is to identify the patient’s definition of treatment success and attempt to work toward that goal.
The briefer treatment, BBTI [40, 41], may be an appropriate treatment starting point for many patients, as it emphasizes the behavioral components—stimulus control and sleep restriction—of CBTI [21] and can be delivered more efficiently than CBTI. For patients who respond more slowly and/or who have higher levels of anxiety and cognitive distortions that help to maintain their insomnia, treatment can be extended and include cognitive therapy and relaxation training (where indicated). Finally, relaxation training—active or passive—can help patients reduce arousal both before bed and during the day. After treatment is complete (BBTI or CBTI), if nighttime sleep is efficient and consolidated but remains insufficient in duration, nighttime sleep can continue to be extended, or time-limited naps during the day may be appropriate as long as they do not interfere with nighttime sleep. Patients who start BBTI/CBTI on a sedative hypnotic, like the case example, can initiate treatment while still using medication but may want to consider a taper during treatment under the guidance of the prescribing physician; however, other patients may prefer to taper off their medications prior to the start of treatment.

Table 3 For patients not already using sedative hypnotic medications, if response to BBTI/CBTI is suboptimal (i.e., Insomnia Severity Index pre- to post-treatment reduction less than 8 points, sleep onset latency/wake after sleep onset reduced by less than 50%, and/or continued dissatisfaction with sleep quantity/quality), starting a sedative hypnotic medication may be indicated (Table 3). Furthermore, for patients who do not have access to BBTI/CBTI or who refuse treatment, sedative hypnotic medications may be appropriate. While medications can be effective, they should generally be considered a secondary option, as BBTI/CBTI provides patients with skills they can apply long after treatment ends and has a high rate of treatment response and a low risk profile. Furthermore, rebound insomnia may occur when medications are discontinued, which is less common following BBTI/CBTI. Many sedative hypnotics also are contraindicated in older adults per the American Geriatric Society Beers Criteria. [50] Recommendations for medication management of insomnia in older adults are provided in Table 3, including dosing guidelines, adverse effects, and precautions. Sedative hypnotics should be prescribed at the lowest effective dose for the shortest possible time, as even the nonbenzodiazepine receptor agonists have potential for habituation and tolerance in some patients. The recommendations in Table 3 are consistent with the 2015 Beers Criteria for potentially inappropriate medications for older adults. [50]
Resolution of Case
The patient met DSM-5 criteria for Insomnia Disorder [4], persistent with comorbid chronic pain (shoulder, back, knee). He completed an insomnia evaluation and five sessions of CBTI with a psychologist. His sleep diary at session 1 indicated sleep onset latency of approximately 8 minutes with 1–2 nighttime awakenings, for an average wake after sleep onset of 70 minutes per night. Average time in bed (TIB) was 6 hours 53 minutes, and average total sleep time was 5 hours 33 minutes; sleep efficiency was 80.6% ([total sleep time/time in bed] × 100). Using the general guideline of total sleep time + 30 minutes [41], his new time in bed was 6 hours, 12:00 AM–6:00 AM. He was initially reluctant to accept this schedule, as he preferred to go to bed earlier and was also hesitant to purposefully wake up prior to 6:00 AM. Through education and a collaborative discussion of sleep preferences, as well as consistent tracking of sleep behaviors (via sleep diary), by session 5, he shifted and extended his sleep schedule from 12:00 AM–6:00 AM to 10:30 PM–5:00 AM.
Following treatment, the patient’s sleep quality had improved. His sleep efficiency increased from 80.6% to 87.4%, wake after sleep onset significantly decreased (approximately 70 to 40 minutes per night), and he stopped taking nightly trazodone. He continued to experience 1–2 awakenings/night, but his ability to fall back asleep greatly improved. Furthermore, his early morning awakenings—waking at 2:00 AM–3:00 AM and being unable to return to sleep—had mostly stopped. He also no longer met criteria for insomnia per the Insomnia Severity Index [37]; from pre- to post-treatment, his score reduced to a 7 (no clinically significant insomnia) compared with a baseline score of 18 (moderate severity). In addition to improving his sleep, he was less fatigued during the day and reported fewer problems maintaining concentration. Unfortunately, he denied improvement in pain intensity.
The patient also greatly reduced coffee consumption (12 cups/day to 6–8 cups/day) and stopped smoking cigarettes before bed and during nighttime awakenings. He continued to smoke half “a joint” of marijuana approximately once per week and was encouraged to discontinue his marijuana use. The patient still napped periodically, usually if he wanted to stay up later to watch a hockey or baseball game, but he was mindful of the impact of daytime naps on his sleep and wake drive. He also maintained realistic expectations of additional improvements post-treatment, which focused on further reducing wake after sleep onset and caffeine intake and continuing to avoid cigarettes before bed. At a follow-up appointment 5 months post-treatment, he continued to deny clinically significant symptoms of insomnia (Insomnia Severity Index = 9). He also continued to report improved daytime functioning despite no reduction in pain.
Summary
Insomnia is highly prevalent and potentially disabling among older adults with CLBP, and they should be regularly assessed and treated appropriately for comorbid insomnia. The recommended first-line treatments are nonpharmacological—BBTI/CBTI. In the case example, treatment was guided by the patient’s initial sleep complaints that were measured using prospective sleep diaries [30], which resulted in a new sleep-wake schedule. Further adjustments were made based on his adherence to recommended behavioral changes and his sleep-wake preferences. The case presentation provides an example of patient and clinician working collaboratively to find a sleep-wake schedule that fits the patient’s preferences but still results in good-quality sleep. As is typical of BBTI/CBTI, his total sleep time did not improve, but sleep quality and sleep efficiency improved and subjective symptoms per the Insomnia Severity Index decreased. While no sedative hypnotic medication was added to the treatment plan (the patient was already prescribed trazodone 50 mg), for patients who do not respond adequately to BBTI/CBTI, are not good treatment candidates, or refuse treatment, medications may be indicated. However, like with BBTI/CBTI, use of medications needs to include a discussion of realistic treatment outcomes and risks and benefits of the medications, including potential side effects. While not conclusive, there is evidence that improving sleep can reduce pain symptoms [51–54], and treating insomnia and other sleep complaints should be an integral part of managing CLBP as one method to improve functioning and quality of life.
Key Points
Insomnia can contribute to pain and disability in older adults with CLBP;
thus, it should be screened routinely.Before referring patients for insomnia evaluation and treatment,
potentially offending medications should be modified.Special populations that may require a customized approach to insomnia
management include those with mood disorders, substance use, and
dementia; these patients should be comanaged by appropriate specialists.BBTI or CBTI are the recommended first choices for treatment.
Prescription medications should only be considered if there is
an inadequate response to nonpharmacological approaches.A decision to start a hypnotic medication in older adults with CLBP should
be preceded by a discussion that highlights the fact that hypnotics are
often associated with adverse effects. Older adults should be
educated specifically about the increased risk of falls
and residual sedation the next morning.
Acknowledgments
The authors thank Dave Newman for his thoughtful review of the manuscript.
Funding sources:
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service.
Disclosure and conflicts of interest:
The contents of this report do not represent the views of the Department of Veterans Affairs or the U.S. government. The authors have no conflicts of interest to report.
References:
Marin R, Cyhan T, Miklos W. Sleep disturbance in patients with chronic low back pain. Am J Phys Med Rehabil 2006;85(5):430–5
Bahouq H, Allali F, Rkain H, Hmamouchi I, Hajjaj-Hassouni N. Prevalence and severity of insomnia in chronic low back pain patients. Rheumatol Int 2013;33:1277–81
Alsaadi SM, McAuley JH, Hush JM, Maher CG. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J 2011;20:737–43
Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
Kelly GA, Blake C, Power CK, O'Keefe D, Fullen BM. The association between chronic low back pain and sleep: A systematic review. Clin J Pain 2009;27(2):169–81.
Agmon M, Armon G. Increased insomnia symptoms predict the onset of back pain among employed adults. PLoS One 2014;9(8):e103591
Menefee LA, Frank ED, Doghramji K, et al. Self-reported sleep quality and quality of life for individuals with chronic pain conditions. Clin J Pain 2000;16(4):290–7
Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain 2013;14(12):1539–52
Boakye PA, Olechowski C, Rashiq S, et al. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin J Pain 2015;32:327–36.
Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004;8:119–32
Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep 1999;22(suppl 2):S379–85
Skevington SM, Carse MS, Williams AC. Validation of the WHOQOL-100: Pain management improves quality of life for chronic pain patients. Clin J Pain 2001;17(3):264–75
Wesensten NJ, Balkin TJ. Cognitive sequelae of sustained operations. In: Kennedy CH, Moore J, eds. Military Neuropsychology. New York: Springer Publishing Co; 2010:297–320.
Leong IY, Farrell MJ, Helme RD, Gibson SJ. The relationship between medical comorbidity and self-rated pain, mood disturbance, and function in older people with chronic pain. J Gerontol A Biol Sci Med Sci 2007;62(5):550–5
Gaskin DJ, Richard P.
The Economic Costs of Pain in the United States
Journal of Pain 2012 (Aug); 13 (8): 715–724Stoller M. Economic effects of insomnia. Clin Ther 1994;16: 873–97; discussion 854
Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009;32:55–64
Parrish JM. Pharmacological treatment of insomnia. In: Pagel JF, Pandi-Perumal SR, eds. Primary Care Sleep Medicine. Totawa, NJ: Humana Press; 2007:47–60.
Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain 2002;18(6):355–65
Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012;11(CD007407):1–109.
Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998-2004). Sleep 2006;29:1398–414
Wilson S, Nutt D, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol 2010;24:1577–601
Bossio M. Opioid analgesics for chronic pain. Adv Nurse Pract 2006;14(12):25–9
Pagel JF. Medications and their effects on sleep. Prim Care 2005;32:491–509
Dimsdale JE, Norman DN, DeJardin D, Wallace MS. The effects of opioids on sleep architecture. J Clin Sleep Med 2007;3(1):33–6
Panagiotou I, Mystakidou K. Non-analgesic effects of opioids: Opioid's effects on sleep (including sleep apnea). Curr Pharm Des 2012;18(37):6025–33
Riedel B. Sleep hygiene. In: Lichstein KL, Morin CM, eds. Treatment of Late-Life Insomnia. Thousand Oaks: SAGE; 2000.
Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine report. Sleep 2006;29(11):1415–9
Weiner DK.
Deconstructing Chronic Low Back Pain in the Older Adult -
Shifting the Paradigm from the Spine to the Person
Pain Med 2015; 16 (5): 881–885Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302
Wilson KG, Eriksson MY, Joyce LD, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain 2002;18(2):77–83
Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions. JAMA Intern Med 2015;175:1461–72
Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Ann Intern Med 2015;163(3):191–204
Manber R, Friedman L, Siebern AT, et al. Cognitive Behavioral Therapy for Insomnia in Veterans: Therapist Manual. Washington, DC: U.S. Department of Veterans Affairs; 2014.
Winkelman JW. Clinical practice. Insomnia disorder. N Engl J Med 2015;373(15):1437–44
Chung F, Subramanyam R, Liao P, et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth 2012;108(5):768–75.
Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307
Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8
Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008;4:487–504
Buysse DJ, Germain A, Moul D, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med 2011;171(10):887–95
Troxel WM, Germain A, Buysse DJ. Clinical management of insomnia with brief behavioral treatment (BBTI). Behav Sleep Med 2012;10:266–79
Harvey AG, Soehner AM, Kaplan KA, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized clinical trial. J Consult Clin Psychol 2015;83(3):564–77
Arnedt JT, Conroy D, Rutt J, et al. An open trial of cognitive-behavioral treatment for insomnia comorbid with alcohol dependence. Sleep Med 2007;8(2):176–80
Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: A randomized controlled pilot trial. Behav Res Ther 2011;49:227–33
Shub D, Darvishi R, Kunik ME. Non-pharmacologic treatment of insomnia in persons with dementia. Geriatrics 2009;64(2):22–6
Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: A systematic review and meta-analysis. Psychother Psychosom 2012;81:206–16
McCrae C, Bramoweth A, Williams J, Roth A, Mosti C. Impact of cognitive behavioral treatment for insomnia on health care utilization and costs. J Clin Sleep Med 2014;10(2):127–35
Trockel M, Karlin BE, Taylor CB, Manber R. Cognitive behavioral therapy for insomnia with veterans: Evaluation of effectiveness and correlates of treatment outcomes. Behav Res Ther 2014;53:41–6
Germain A, Richardson R, Stocker R, et al. Treatment for insomnia in combat-exposed OEF/OIF/OND military veterans: Preliminary randomized controlled trial. Behav Res Ther 2014;61:78–88
American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63(11):2227–46
Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009 15;5(4):355–62
Vitiello MV, McCurry SM, Shortreed SM, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain 2014;155(8):1547–54
Drewes AM, Nielsen KD, Hansen B, et al. A longitudinal study of clinical symptoms and sleep parameters in rheumatoid arthritis. Rheumatology 2000;39:1287–9
Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep 2012;35(12):1667–72
National Institutes of Health. NIH State-of-the-Science Conference on Manifestations and Management of Chronic Insomnia. Bethesda, MD; 2005:1–105
Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc 2009;57(5):761–89
Means MK, Lichstein KL, Epperson MT, Johnson CT. Relaxation therapy for insomnia: Nighttime and day time effects. Behav Res Ther 2000;38(7):665–78
Morin CM, Benca R. Chronic insomnia. The Lancet 2012;379:1129–41
Clinical Pharmacology [Internet]. Tampa, FL: Gold Standard, Inc.; 2016. Available from: www.clinicalpharmacology.com (accessed October 2014).
Roth AJ, McCall WV, Liguori A. Cognitive, psychomotor and polysomnographic effects of trazodone in primary insomniacs. J Sleep Res 2011;20(4):552–8
Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep 2011;34(10):1433–42
Lankford A, Rogowski R, Essink B, et al. Efficacy and safety of doxepin 6 mg in a four-week outpatient trial of elderly adults with chronic primary insomnia. Sleep Med 2012;13(2):133–8
Aslan S, Isik E, Cosar B. The effects of mirtazapine on sleep: A placebo controlled, double-blind study in young healthy volunteers. Sleep 2002;25(6):677–9
Brandt NJ, Piechocki JM. Treatment of insomnia in older adults: Re-evaluating the benefits and risks of sedative hypnotic agents. J Gerontol Nurs 2013;39(4):48–54.
Liu J, Wang LN. Ramelteon in the treatment of chronic insomnia: Systematic review and meta-analysis. Int J Clin Pract 2012;66(9):867–73
Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: A prospective placebo-controlled study. Sleep 2012;35(11):1551–7
U.S. Food and Drug Administration. Questions and Answers: Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). 2014. Available at:
http://www.fda.gov/drugs/drugsafety/ucm334041.htm
(accessed October 2015).Valente KD, Hasan R, Tavares SM, Gattaz WF. Lower doses of sublingual Zolpidem are more effective than oral Zolpidem to anticipate sleep onset in healthy volunteers. Sleep Med 2013;14(1):20–3
Roth T, Krystal A, Steinberg FJ, Singh NN, Moline M. Novel sublingual low-dose zolpidem tablet reduces latency to sleep onset following spontaneous middle-of-the-night awakening in insomnia in a randomized, double-blind, placebo-controlled, outpatient study. Sleep 2013;36(2):189–96
Ancoli-Israel S, Krystal AD, McCall WV, et al. A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. Sleep 2010;33(2): 225–34
Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: Results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26(7):793–9
Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T, ZOLONG Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: A 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep 2008;31(1):79-90
Staner L, Danjou P, Luthringer R. A new sublingual formulation of zolpidem for the treatment of sleep-onset insomnia. Expert Rev Neurother 2012;12(2): 141–53

Return to LOW BACK PAIN
Since 8-30-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |