

Low Back Pain: Guidelines for the Clinical
Classification of Predominant Neuropathic,
Nociceptive, or Central Sensitization PainThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain Physician. 2015 (May); 18 (3): E333–346 ~ FULL TEXT
Adri Apeldoorn, PhD, Hank Hallegraeff, PhD, Jacqui Clark, MSc, PT,
Rob Smeets, MD, PhD, Annaleen Malfliet, MSc, PT, Enrique L. Girbes, MSc, PT,
Margot De Kooning, MSc, Kelly Ickmans, PhD, and Jo Nijs, PhD.
Pain in Motion Research Group;
Departments of Human Physiology and Rehabilitation Sciences,
Faculty of Physical Education & Physiotherapy,
Vrije Universiteit Brussel, Belgium.
BACKGROUND: Low back pain (LBP) is a heterogeneous disorder including patients with dominant nociceptive (e.g., myofascial low back pain), neuropathic (e.g., lumbar radiculopathy), and central sensitization pain. In order to select an effective and preferably also efficient treatment in daily clinical practice, LBP patients should be classified clinically as either predominantly nociceptive, neuropathic, or central sensitization pain.
OBJECTIVE: To explain how clinicians can differentiate between nociceptive, neuropathic, and central sensitization pain in patients with LBP.
STUDY DESIGN: Narrative review and expert opinion
SETTING: Universities, university hospitals and private practices
METHODS: Recently, a clinical method for the classification of central sensitization pain versus neuropathic and nociceptive pain was developed. It is based on a body of evidence of original research papers and expert opinion of 18 pain experts from 7 different countries. Here we apply this classification algorithm to the LBP population.
RESULTS: The first step implies examining the presence of neuropathic low back pain. Next, the differential diagnosis between predominant nociceptive and central sensitization pain is done using a clinical algorithm.
LIMITATIONS: The classification criteria are substantiated by several original research findings including a Delphi survey, a study of a large group of LBP patients, and validation studies of the Central Sensitization Inventory. Nevertheless, these criteria require validation in clinical settings.
CONCLUSION: The pain classification system for LBP should be an addition to available classification systems and diagnostic procedures for LBP, as it is focussed on pain mechanisms solely.
KEYWORDS: Chronic pain, neuroscience, diagnosis, clinical reasoning, examination, assessment
From the FULL TEXT Article:
Introduction
Despite extensive global research efforts, chronic pain remains a challenging issue for clinicians and a huge socio-economic problem. Within the chronic pain population, low back pain (LBP) is one of the most prevalent musculoskeletal disorders, affecting 70% – 85% of the adult population at some point in life. [1] Twelve months after the onset of LBP, 45% – 75% of patients still experience pain [2], accounting for major expenses in health care and disability systems. [1]
Nociceptive pain is defined as pain arising from actual or threatening damage to non-neural tissue and is due to the activation of nociceptors [3], or as pain attributable to the activation of the peripheral receptive terminals of primary afferent neurons in response to noxious chemical, mechanical, or thermal stimuli. [4] For clinical purposes, the term nociceptive pain can be used when pain is proportional to nociceptive input, and it was designed to contrast with neuropathic pain. The latter is defined as pain caused by a primary lesion or disease of the somatosensory nervous system. [3]
Within the LBP population, lumbar radiculopathy is a common type of lumbar neuropathic pain, while myofascial tissue (i.e., thoracolumbar fascia) [5] and some lumbar ligaments [6] contain nociceptors capable of generating nociceptive pain. Both nociceptive and neuropathic pain can be classified as “specific LBP” when there is a clear patho-anatomical diagnosis. However, a precise patho-anatomical diagnosis cannot be given in approximately 85% of LBP patients [7], resulting in the label “non-specific low back pain.” Imaging findings like lumbar osteoarthritis or (small) disc lesions often do not account for the symptoms experienced by LBP patients [8–10] hence, they cannot be categorized per se as having primarily nociceptive pain.
Modern pain neuroscience has advanced our understanding about pain, including the role of central sensitization (CS) in amplifying pain experiences. CS is defined as“an amplification of neural signaling within the central nervous system that elicits pain hypersensitivity” [11], “increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input” [3], or “an augmentation of responsiveness of central neurons to input from unimodal and polymodal receptors”. [12] Although one might say that these definitions differ substantially, they all point to the same underlying neurophysiological mechanism of increased neuronal response to stimuli in the central nervous system (i.e., central hyperexcitability). The definitions originate from laboratory research, but the awareness that the concept of CS should be translated to the clinic is growing. [13, 14]
CS encompasses various related dysfunctions within the central nervous system, all contributing to altered (often increased) responsiveness to a variety of stimuli like mechanical pressure, chemical substances, light, sound, cold, heat, stress, and electricity. [13] Such dysfunctions of the central nervous system include altered sensory processing in the brain [15], malfunctioning of descending anti-nociceptive mechanisms [16, 17], increased activity of nociceptive facilitatory pathways, and enhanced temporal summation of second pain or wind-up. [18, 19] In addition, the pain (neuro)matrix is overactive in the case of CS pain, with increased brain activity in areas known to be involved in acute pain sensations (insula, anterior cingulate cortex, and prefrontal cortex) as well as in regions not involved in acute pain sensations (various brain stem nuclei, dorsolateral frontal cortex, and parietal associated cortex). [20]
An increasing number of studies have examined the role of CS in patients with LBP, and the findings are equivocal. [21] Some studies have demonstrated exaggerated pain responses after sensory stimulation of locations outside the painful region, while others did not report differences between LBP patients and healthy patients. [21] However, studies analyzing brain structure and function in relation to (experimentally induced) pain have provided evidence for altered central nociceptive processing in subgroups of patients with chronic LBP. [21]
Evidence of several studies in chronic LBP suggests that CS is present in a subgroup of the LBP population. [22–24] This potentially impacts upon clinical practice, as LBP patients with a predominant CS pain type require treatment targeted at the central nervous system rather than the lower back. [14, 21, 25] Hence, in order to select an effective and preferably also efficient treatment in daily clinical practice, LBP patients should be classified clinically as experiencing predominantly either nociceptive, neuropathic, or CS pain. [22, 26]
Recently, a clinical method for classifying any pain as either predominant CS pain, neuropathic, or nociceptive pain was developed, based on a body of evidence from original research papers and expert opinion of 18 pain experts from 7 different countries. [27] Here we apply this classification algorithm to the LBP population, and explain how clinicians can differentiate clinically between predominant nociceptive, neuropathic, and CS pain in their LBP patients.
Examining the Presence of Neuropathic Low Back Pain as the First Step
Chronic lumbar radicular pain is the most common neuropathic pain syndrome which affects 20% to 35% of patients with LBP. [28] People with neuropathic LBP often experience higher levels of pain, disability, anxiety, depression, and reduced quality of life as compared to nociceptive LBP. [22, 29] Following identification of red flags, excluding the possibility of neuropathic LBP is often the first step in clinical practice. [30, 31] Guidelines have been published for the classification of neuropathic pain. [32, 33] The criteria specify that a lesion or disease of the nervous system (either central or peripheral) is identifiable and that pain is limited to a “neuroanatomically plausible” distribution. The neuropathic pain criteria preclude the use of the term “neuropathic pain” for people with diffuse or widespread pain and nervous system sensitization (i.e., CS pain), as the latter is free of a history of a lesion or disease of the nervous system and is typically characterized by a pain distribution that that is not neuroanatomically plausible. [27]
Box 1 illustrates how clinicians can examine the presence of neuropathic LBP and includes detailed history taking and physical testing. These are important parts of the screening of the examination of any LBP patient, and might even reveal rare causes of neuropathic pain in long lasting LBP (e.g., entrapment neuropathy of the L1–L2 dorsal ramus over the iliac crest [34]). Other causes of neuropathic LBP are more common like radiculitis (i.e., inflammation of one or more nerve rout[s]), resulting in pain radiating along the corresponding dermatome. Hence, clinicians should be able to identify such patients with radiculitis using the questions provided in Box 1.
It is important to highlight the issue of sensory dysfunction for the differential diagnosis between neuropathic and CS LBP. Sensory testing can be of importance for the diagnosis of neuropathic pain, although it should always be combined with diagnostic procedures confirming or refuting the nervous system lesion or disease. [32, 33] While in neuropathic LBP the location of the sensory dysfunction should be neuroanatomically logical, it is spread in non-segmentally related areas of the body in CS LBP. Clinical examination in CS LBP typically reveals increased sensitivity at sites segmentally unrelated to the primary source of nociception. [13, 27] A study of 377 patients with sciatica revealed that selfreported sensory loss (assessed through history taking) doubled the odds of having nerve root compression, and tripled the odds of having disc herniation (35). However, the diagnostic accuracy of history taking in general for predicting the presence of lumbosacral nerve root compression or disc herniation on magnetic resonance imaging (MRI) in patients with sciatica was rather poor [35], underscoring the need for combining history taking with a more comprehensive screening, including clinical tests.
Following the screening criteria outlined in Box 1 will either result in establishing or excluding neuropathic pain as an underlying cause of the patient’s LBP. Although the presence of neuropathic pain does not exclude the options of CS LBP 2 options remain if neuropathic pain is excluded: predominant nociceptive or CS LBP. Neuropathic pain may be characterized or accompanied by sensitization; peripheral and central (segmentally related) pain pathways can become hyperexcitable in patients with neuropathic pain. [39, 40] Such overlap illustrates that LBP patients can have both neuropathic and CS pain.

Figure 1 In addition, lumbar radiculopathy is a typical example of neuropathic LBP, but if treated surgically can also develop towards post-surgical nociceptive or (more likely) CS pain. In specific cases of non-responders to conservative treatment and a negative evolution, surgery is a recommended, evidence-based treatment for lumbar disc herniation with radiculopathy. [41, 42] However, a substantial portion (23% – 28%) of patients develops chronic back +/- leg pain following surgical treatment of lumbar radiculopathy. [41] In such cases, neuropathic pain remains possible: removing the mechanical pressure on the nerve(s) does not per se guarantee restoration of its complete function. The underlying mechanisms of neuropathic pain might have established itself, resulting in long-term neuropathic pain, or mechanical pressure has caused irreversible damage to the root. In such cases it is expected that the post-surgical pain distribution and related signs/symptoms still comply with the diagnostic criteria proposed for neuropathic pain (Figure 1). If not, the post-surgical pain is unlikely to be of neuropathic nature, leaving clinicians with the options of nociceptive and CS pain.
Differentiating Predominant Nociceptive and Central Sensitization
Low Back Pain Using a Classification Algorithm
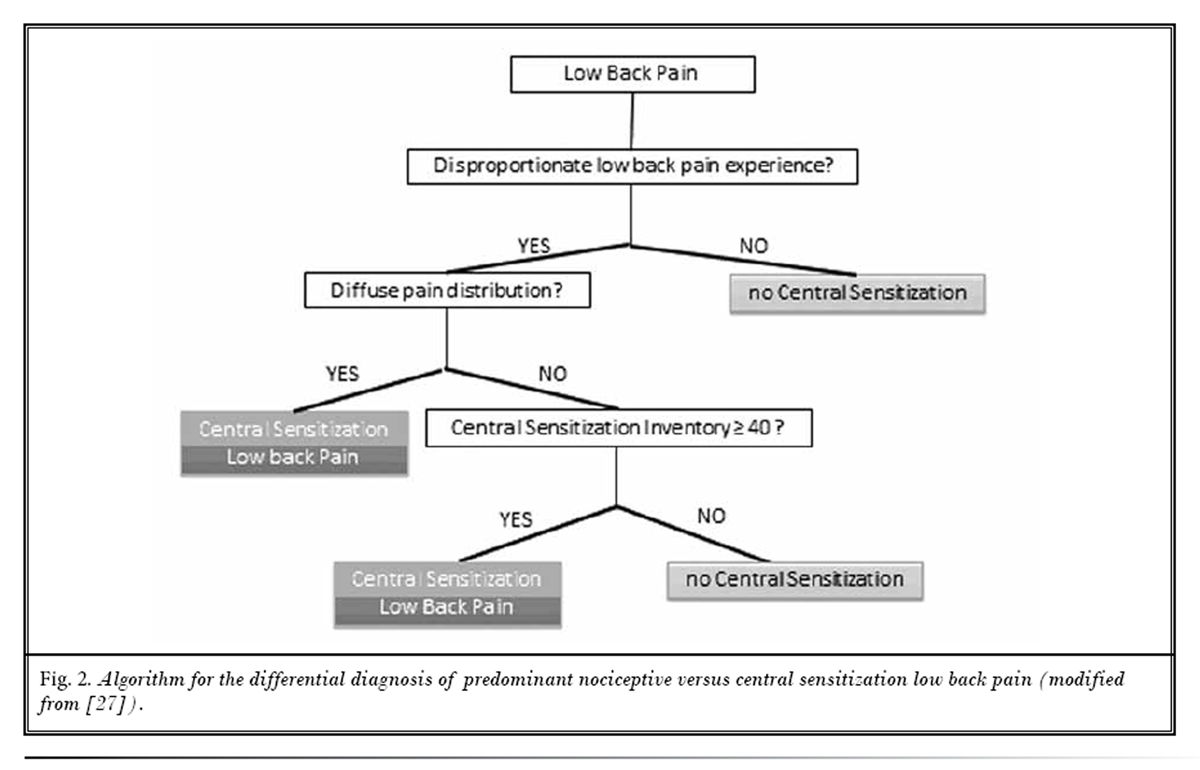
Figure 2 To differentiate predominant nociceptive and CS LBP, clinicians are advised to use the algorithm presented in Figure 2. The algorithm guides the clinician through the screening of 3 major classification criteria, each of which is explained below.
Criterion 1: Low Back Pain Experience Disproportionate to the Nature and Extent of Injury or Pathology [27]
Per definition, CS is characterized by “an amplification of neural signaling within the central nervous system that elicits pain hypersensitivity” (11) and “augmented responsiveness of central nervous system neurons to their normal or subthreshold afferent input”. [3, 12] These overlapping definitions imply that CS pain is disproportionate to the nature and extent of injury or pathology, making it a go-no-go criterion for CS pain.
Applied to the LBP population, for complying with this first criterion the severity of the LBP must be disproportionate to the nature and extent of injury or pathology (i.e., tissue damage or structural impairments which might cause nociceptive LBP). This contradicts nociceptive LBP, where the severity of the LBP is more or less proportionate to the nature and extent of the injury or pathology. Indeed, a Delphi study including 103 clinical experts revealed that “clear, proportionate mechanical/anatomical nature to aggravating and easing factors” and “clear, consistent and proportionate mechanical/anatomical pattern of pain reproduction on movement/mechanical testing of target tissues” were the criteria most strongly suggestive of nociceptive pain, while “disproportionate, non-mechanical, unpredictable pattern of pain provocation in response to multiple/non-specific aggravating/easing factors,” and “disproportionate, inconsistent, non-mechanical/nonanatomical pattern of pain provocation in response to movement/mechanical testing” were most suggestive of “central pain”. [4]
In addition, a multi-center study of 464 LBP patients identified “disproportionate, nonmechanical, unpredictable pattern of pain provocation in response to multiple/non-specific aggravating/easing factors” as the strongest predictor of CS in patients with LBP. [23] However, in absence of a gold standard for CS pain, the clinicians participating in the study used their own expert judgement for classifying LBP patients into the 3 groups (peripheral neuropathic, nociceptive, or CS LBP). [23]
For screening this first criterion, it is necessary to assess the patient’s amount of injury, pathology, and objective dysfunctions capable of generating nociceptive input in the lumbopelvic region. This includes imaging techniques for identifying such nociceptive sources (e.g., X-rays, CT scan, and NMRI), but also the clinical examination. The latter is important for identifying movement dysfunctions in the lower back and pelvic joints [43, 44], increased tension and/or myofascial trigger points in the lumbopelvic muscles [45], etc. The lumbopelvic region includes a large number of tissues capable of generating nociceptive input, including intervertabral discs [41, 46–49], muscles [50, 51], fascia [5, 52], bone [53], facet joints [46, 47, 54–56], sacroiliac joints [46, 47, 57, 58], symphysis pubis joint, ligaments [6], and joint capsules [55] (e.g., facet joint capsules contain nociceptors [54]), etc.
Next, the amount of injury, pathology, and objective dysfunctions capable of generating nociceptive input in the lumbopelvic region is weigthed with the patient’s subjective LBP experience (i.e., the self-reported LBP). In case imaging findings and the clinical examination hardly identify potential sources of lumbar nociception, the presence of disabling pain will suffice for fulfilling this criterion. However, in many (if not all) patients with LBP the clinical examination and/or imaging reveals some type of potential nociceptive source, which makes thorough clinical reasoning necessary for weighting the nociceptive input with the experienced pain. This includes taking into account all personal and environmental factors.
Such clinical reasoning includes1) focusing on the patient’s current health status (i.e., at the time he/ she comes to see the clinician); and
2) interpreting the amount of injury, pathology, and objective dysfunctions in light of the evidence favoring or refuting its clinical importance in patients with LBP.Injured tissue might have lead to nociception in the (sub)acute phase, but once healed it is unlikely to serve as a continuous or current source of nociceptive input.
When interpreting the amount of injury, pathology, and objective dysfunctions, clinicians should be aware that not all potential nociceptive sources are of clinical importance for LBP patients. This is illustrated by imaging findings of lumbar osteoarthritis, which are very poorly related to functional status in patients with LBP [59] or even the presence of LBP. [60] In fact, up to 47% of older people without LBP show evidence of lumbar facet joint osteoarthritis on CT assessment. [60] Spinal degeneration features like intervertebral disc narrowing, facet joint osteoarthritis, and spondylolysis are commonly seen on CT assessment of the lumbar spine, but the only degenerative feature associated with self-reported LBP is spinal stenosis. [61] Severe facet joint osteoarthritis (especially if several facet joints are affected) is associated with back pain in community-based older adults. [60]
MRI findings of annular tears or Schmorl’s nodes are unrelated to LBP. [62] That same study showed that the presence of intervertebral disc herniation or intervertebral disc degeneration doubled the chance of having LBP. [62] Still, the available evidence suggest that only Modic type 1 changes and intense, extensive zygapophyseal edematous changes are relatively correlated with LBP. [63] Modic type 1 changes refer to vertebral endplate changes with an edematous appearance, hypointense on T1–weighted images and hyperintense on T2–weighted images, with enhancement after gadolinium injection. [63]
Similarly, although the available evidence suggests that paraspinal muscles are significantly smaller in chronic LBP patients and on the symptomatic side of patients with chronic unilateral LBP [64], the density of paraspinal muscles like the multifidus and erector spinae is unrelated to LBP. [65] This brings us to the issue of myofascial tissues as a candidate source of (ongoing) nociception in patients with LBP. In addition to muscle nociceptors, animal research has recently established the muscle fascia as a candidate source of nociception [5, 52], but human studies are currently limited to experimental pain induction in asymptomatic people. [5] The pain associated with myofascial trigger points is thought to arise from a hypersensitive nodule in a taut band of the skeletal muscle [66], and they are capable of activating muscle nociceptors. [67]
Upon sustained noxious stimulation, myofascial trigger points may even result in primary hyperalgesia. [68] Indeed, in the vicinity of myofascial trigger points the tissue differs from normal muscle tissue by its lower pH levels (i.e., more acid), increased levels of substance P, calcitonin gene-related peptide, tumour necrosis factor-α, and interleukine-1β, each of which has its role in increasing pain sensitivity. [69] Sensitised muscle nociceptors are more easily activated and may respond excessively to normally innocuous and weak stimuli such as light pressure or muscle movement. [67, 69]
In the case of myofascial trigger points, the pathophysiology appears to be in line with evidence from clinical studies: the number of active myofascial trigger points in patients with non-specific LBP is associated with self-reported pain intensity [51], but more studies are required to confirm these findings. In addition, serious concerns are raised regarding the reliability of trigger point palpation in low back muscles. [70, 71] Still, at this point myofascial trigger points are candidate peripheral sources of nociception in patients with LBP.
Taken together, the weighting of the identified current sources of nociception with the self-reported pain and disability can result in a number of outcomes:
The patient with LBP presents insufficient evidence of injury, pathology, or objective dysfunctions capable of causing the self-reported pain. This would imply that the LBP patient fulfills this first out of 3 criteria for CS LBP. At this point, the patient may have predominant CS pain, but the clinician needs to proceed with screening of the remaining criteria (Figure 2) before making a conclusion.
There is evidence of injury, pathology, or objective dysfunctions capable of causing back pain, but not enough nociceptive input for explaining the pain experienced by this LBP patient. Again, this would imply that the patient fulfills this first out of 3 criteria for CS LBP. The patient may have predominant CS LBP, and the clinician must proceed with screening of the remaining criteria (Fig. 2).
If the LBP experienced by the patient is not considered disproportionate as there is evidence of injury, pathology, or objective dysfunctions which justify the self-reported pain and disability, CS can be ruled out at this point.
Criterion 2: Neuroanatomically Illogical Pain Pattern [27]
This criterion is related to the issue of a neuroanatomically plausible pain pattern: a neuroanatomically illogical pain pattern is present when the LBP patient presents with a pain distribution that is not neuroanatomically plausible for the presumed sources of (lumbar) nociception. For screening this criterion, a thorough assessment and interpretation of the patient’s self-reported pain distribution, in light of the identified possible sources of nociception, is required. Examples of pain distribution patterns that fulfill this criterion are bilateral pain/mirror pain (i.e., a symmetrical pain pattern), pain varying in (anatomical) location, large pain areas with a non-segmental (i.e., neuroanatomically illogical) distribution, widespread pain, and/or allodynia/ hyperalgesia outside the segmental area of (presumed) primary nociception. [27] Referred pain patterns can be either neuroanatomically logical (e.g., when the referred pain pattern stays within one or 2 neighboring segmental areas related to the source of nociception) or illogical.
As is the case with the first criterion, this second is supported by a Delphi study on clinical indicators of nociceptive versus neuropathic and central pain, showing that “widespread, non-anatomical distribution of pain” obtained up to 96% consensus level agreement among expert clinicians as a clinical indicator of central pain. [4] Also in a study of 464 LBP patients, “non-segmental/ diffuse areas of tenderness on palpation” was identified as one of the 4 key predictors of CS LBP versus peripheral neuropathic and nociceptive LBP [23], even though this finding should be interpreted in light of the limitation discussed above.
Assessing the pain distribution in LBP patients relies on thorough questioning and asking the patient to complete a body chart (e.g., the Margolis pain drawing [72] is a reliable method in chronic pain patients [73]). Even after additional training, myofascial trigger points examination has limited reliability to assess referred pain patterns in LBP patients. [70] Internal lumbar disc disruption, lumbar facet joint pain, and sacroiliac joint pain each have a local (non-diffuse) pain distribution. [46] Midline LBP increases the probability of lumbar internal disc disruption and reduces the probability of symptomatic facet joint pain or sacroiliac joint pain, while isolated paramidline LBP increases the probability of symptomatic facet or sacroiliac joint pain, but mildly reduces the likelihood of lumbar internal disc disruption. [46]
Still, lumbar intervertebral discs are capable of generating leg pain that extends below the knee; the pain pattern then originates proximally and progresses distally. [48] Sacroiliac joint dysfunction generates an area of buttock hyperaesthesia extending approximately 10 cm caudally and 3 cm laterally from the posterior superior iliac spine [58], which can be applied successfully to diagnosing sacroiliac joint dysfunction in patients. [58] Finally, it is advocated to use classical movement tests (e.g., lumbar flexion and extension) for examining whether the pain distribution changes in response to lumbar movements/joint loading. Patients with CS LBP will typically present with an inconsistent pain response to lumbar movements/joint loading. [13]
According to the recently proposed classification method [27], if neuropathic LBP is excluded and criteria 1 and 2 are both met, the classification of predominant CS LBP can be established. In the case where neuropathic LBP is excluded, and only the first (disproportionate LBP) but not the second criterion is met, further screening of criterion 3 is required (Fig. 2).
Criterion 3: Hypersensitivity of Senses Unrelated to the Musculoskeletal System [27]
CS LBP may reflect much more than generalized hypersensitivity to pain: It may be characterized by an increased responsiveness to a variety of stimuli, including but not limited to mechanical pressure. [74, 75] For instance, patients with LBP may have altered cold [76] or heat sensitivity. [77] A study showed that chronic LBP patients have not only localized (i.e., the primary area of pain) but also generalized (i.e., in an area anatomically remote from the primary area of pain, namely the forearm) cold hyperalgesia. [78] This manifestation appears to be absent in patients with acute LBP. [78] Another study reported that spinal pain patients with high mechanical pressure and thermal sensitivity showed worse clinical outcome for pain intensity. [77] This finding supports the clinical importance of sensory hyperexcitability in some LBP patients.
In line with this, it is important to understand that research has informed us that long-term opioid use can decrease thermal but not pain sensitivity in LBP patients [79], and that gender, fear avoidance beliefs, and pain catastrophizing are associated with thermal pain sensitivity in chronic LBP patients. [80] Also a recent systematic literature review and meta-analysis has shown that sensory hypersensitivity does not seem to play a major role in the pain and disability reported by patients with spinal pain. [81] Taken together, it is currently unclear what the exact value of cold and heat hyperalgesia (assessment) in LBP patients is, but its presence might point to CS.
Given the overall hyper-responsiveness of central nervous system neurons, CS may explain the altered sensitivity to many environmental (bright light, cold/ heat, sound/noise, weather, stress, food [82]) or even chemical stimuli (odors, pesticides, medication), characteristic of those with CS LBP. Weather conditions do not account for new-onset LBP [83], but research findings also indicate that weather changes might have an important role in fluctuation of pain among individuals experiencing musculoskeletal pain, including those with LBP. [84]
For assessing sensory hypersensitivity in patients with LBP, clinicians can use quantitative sensory testing (QST). The required equipment is available in many specialized pain centers. However, a recent systematic literature review and meta-analysis concluded that QST-derived pain threshold is a poor marker of CS in patients with spinal pain. [81] Many QST protocols are available and require further study, and its wider use may be hampered by its costs, complexity, and timeconsuming nature.
Other less expensive and less time-consuming options are available for routine clinical practice. First, clinicians can question LBP patients with suspected CS for new-onset hypersensitivity to bright light, sound, smell, and hot or cold sensations. [13, 85] However, the authors are unaware of studies examining the clinimetric properties of such questioning. A second more valid option appears to the part A of the Central Sensitization Inventory (CSI) [86], which assesses symptoms common to CS, with total scores ranging from 0 to 100 and a recommended and validated cutoff score of 40. [87, 88]
An increasing number of studies support the clinimetric properties of the CSI for assessing self-reported signs and symptoms of CS in chronic pain patients. [86–88] The cutoff of 40 on the CSI allows correct identification of over 82% of CS pain patients, but the chances of false-positives are relatively high [88], which supports our approach of combining this measure with a more comprehensive examination for identification of predominant CS LBP.
Discussion
The classification criteria presented here apply the recently established clinical classification criteria for CS pain [27] to the LBP population. Those classification criteria for CS pain are based on a body of evidence from original research papers, interpreted by 18 pain experts from 7 different countries. [27] The application of the classification criteria to LBP patients as presented here is substantiated by the findings of a Delphi survey [4], a study of a large group of LBP patients (n = 464) [23], validation studies of the CSI (including its ability to discriminate between CS and non-CS pain patients) [86–88] as well as several original research findings in the field of LBP research that support parts of its framework (please refer to the references included in the text above). Nevertheless, the classification algorithm for differentiating neuropathic from predominant nociceptive and CS LBP requires validation in clinical settings, including examination of its clinical applicability. In addition, the classification algorithm currently lacks “objective” criteria, and there is little proof for an (semi-)objective biomarker for CS LBP (e.g., QST measurements are not advocated for establishing CS in spinal pain patients [81]).
Classification systems for chronic LBP have been criticized as they don’t consider the multiple and interacting dimensions (i.e., psychological or movement dimensions) involved in the lived experience of people with LBP. [89] Given the variety of classification systems currently available for LBP [90–94], one might argue that the last thing we need is another one. However, the present classification system for differentiating neuropathic, nociceptive, and CS LBP builds on the available “pain-mechanism based classification” system for LBP [23, 24, 38] and the classification criteria for CS pain. [27]
It should be an addition to available classification systems for LBP, as it is focussed on pain mechanisms solely. For instance, in patients classified as nociceptive LBP, further subgrouping based on imaging findings, movement dysfunctions, and psychosocial or contextual factors will be required to direct treatment and improve outcomes. [95] Clinicians should not become fanatic supporters of one classification system for LBP (including the one presented in this paper), but incorporate in their clinical reasoning the multiple dimensions of LBP (including pain mechanisms), in order to better assess and treat people with LBP. [89]
One might consider including the presence of maladaptive psychological features as a predictor of CS LBP, as was suggested by the study by Smart et al. [23] Indeed, “cognitive emotional sensitization” refers to the modulation of brain-orchestrated descending pain inhibition/ facilitation by factors like pain hypervigilance, anxiety, depressive feelings, catastrophizing, illness beliefs, and somatization. [96] There is substantial evidence for the role of such psychological features in LBP [80, 97–99], but also in nociceptive and neuropathic LBP [100–104], suggesting that they have poor discriminative ability between the 3 pain types within the LBP population.
This makes sense when one considers the fact that all pain is in the brain [105], regardless of its mechanistic nature (i.e., being either nociceptive, neuropathic, or of central nature). All pain implies activation of the brain circuitry known as the pain (neuro)matrix, including brain activity in regions responsible for cognitive-emotional and affective processing of sensory input (i.e., amygdala, prefrontal cortex, anterior cingulate cortex, insula etc.). Still, it is advocated to include a thorough psychological screening in all patients with LBP, regardless of its mechanistic nature. This is important for identifying important treatment goals, and should include assessing maladaptive psychological features and illness behavior. The Waddell score, consisting of 8 non-organic or behavioral signs for measuring illness behavior in patients with LBP, is a reliable tool with satisfactory construct validity. [106, 107] Studies examining how the Waddell score varies across LBP patients with neuropathic, CS and nociceptive pain seem warranted.
Further to this reasoning is the option of including a fourth pain type for classification of patients with LBP, namely predominant psychogenic pain. Having such a fourth pain type might be useful to other pain populations besides LBP. For identifying predominant psychogenic LBP in clinical practice, clinicians need to exclude the options of predominant nociceptive, neuropathic, or CS pain. If none of these 3 pain types appear to dominate the patient’s clinical picture and the patient presents with a high Waddell score or other objective evidence of maladaptive psychological features and illness behaviour (e.g., pain catastrophizing combined with pain hypervigilance, depressive thoughts, and maladaptive pain coping style like avoidance behavior), then classification of predominant psychogenic LBP might be warranted.
Although LBP patients fulfilling the criteria for classifying their LBP as CS pain can have (relevant) nociception, it implies that central mechanisms rather than peripheral (lumbar) factors are dominating the clinical picture. Patients classified as having CS LBP may require specific treatment targeting the mechanisms underlying the hyperexcitability of the central nervous system rather than treatments targeted at the lumbar spine. A variety of treatment strategies target specifically pathophysiological mechanisms known to be involved in CS pain; i.e., they hold – at least theoretically – the capacity to desensitize the central nervous system. Such treatments include pharmacological options [25], electrotherapy targeting the brain (i.e., transcranial magnetic stimulation) [25], exercise therapy (108), stress management/neurofeedback training [25], cognitive behavioral therapy [14], virtual reality (25), transcutaneous electrical nerve stimulation [25], cranial electrotherapy stimulation [25], and pain neuroscience education. [14] Some of the treatments listed here, including exercise therapy and electrotherapy, have peripheral as well as central effects.
Most treatment options target the brain (topdown approach) rather than peripheral nociceptive input (bottom-up). This appears to be a rational choice, especially if one considers CS to be the dominant feature in the LBP patient. However, the clinical picture of LBP patients is often mixed with some evidence of (limited) peripheral nociceptive input combined with evidence of CS. For these patients the question arises whether successful treatment of peripheral input will diminish (or even resolve) CS as well.
From the available literature it is concluded that limited evidence in selected chronic pain populations supports treatment strategies that eliminate peripheral nociceptive input for the effective management of CS pain. [14] Hence, treatment of predominant CS pain (including CS LBP), should be oriented to the brain (i.e., top-down strategies). However, this conclusion is not based on studies with LBP patients, underscoring the need for further research in this area.
Acknowledgments
Anneleen Malfliet is a PhD research fellow of the Agency for Innovation by Science and Technology (IWT) – Applied Biomedical Research Program (TBM), Belgium. Jo Nijs is holder of a Chair funded by the European College for Decongestive Lymphatic Therapy, The Netherlands.
References:
Becker A, Held H, Redaelli M, Strauch K , Chenot JF, Leonhardt C., Keller S, Baum E, Pfingsten M, Hildebrandt J, Basler HD, Kochen MM, Donner-Banzhoff N. Low back pain in primary care: Costs of care and prediction of future health care utilization. Spine (Phila Pa 1976) 2010; 35:1714-1720.
Hestbaek L, Leboeuf-Yde C, Manniche C.
Low Back Pain: What Is The Long-term Course?
A Review of Studies of General Patient Populations
European Spine Journal 2003 (Apr); 12 (2): 149–165Merskey H. NBatITFoT. Part III: Pain terms, a current list with definitions and notes on usage. In: Merskey H.NBatITFoT (ed). Classification of Chronic Pain. Second Edition. IASP Press, Seattle, USA, 1994, pp 209-214.
Smart KM, Blake C, Staines A, Doody C. Clinical indicators of “nociceptive”, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Manual Therapy 2010; 15:80-87.
Schilder A, Hoheisel U, Magerl W, Benrath J, Klein T, Treede RD. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 2014; 155:222-231.
Tsao H, Tucker KJ, Coppieters MW, Hodges PW. Experimentally induced low back pain from hypertonic saline injections into lumbar interspinous ligament and erector spinae muscle. Pain 2010; 150:167-172.
Airaksinen O, Brox JI, Cedraschi C, et al.
COST B13 Working Group on Guidelines for Chronic Low Back Pain Chapter 4.
European Guidelines for the Management of Chronic Nonspecific Low Back Pain
European Spine Journal 2006 (Mar); 15 Suppl 2: S192–300Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, Peterson AM, Firestone L. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. NeuroImage 2002; 16:158-168.
van Tulder M, Becker A, Bekkering T, et al;
On behalf of the COST B13 Working Group on Guidelines for the Management of Acute Low Back Pain in Primary Care.
Chapter 3. European Guidelines for the Management of Acute Nonspecific Low Back Pain in Primary Care
European Spine Journal 2006 (Mar); 15 Suppl 2: S169–191Peters ML, Vlaeyen JW, Weber WE. The joint contribution of physical pathology, pain-related fear and catastrophizing to chronic back pain disability. Pain 2005; 113:45-50.
Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011; 152:S2-S15.
Meyer RA, Campbell IT, Raja SN. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R (eds). Textbook of Pain. 3rd ed. Churchill Livingstone, Edinburgh, 1995, pp 13-44.
Nijs J, Van Houdenhove B, Oostendorp RA. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Manual Therapy 2010; 15:135-141.
Nijs J, Malfliet A, Ickmans K, Baert I, Meeus M. Treatment of central sensitization in patients with “unexplained” chronic pain: An update. Expert Opinion on Pharmacotherapy 2014;15:1671-1683.
Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. European Journal of Pain (London, England) 2008; 12:1078-1089.
Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control- like effect): Its relevance for acute and chronic pain states. Current Opinion in Anaesthesiology 2010; 23:611-615.
Meeus M, Nijs J, Van de Wauwer N, Toeback L, Truijen S. Diffuse noxious inhibitory control is delayed in chronic fatigue syndrome: An experimental study. Pain 2008; 139:439-448.
Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: A multi-method study. The Journal of Headache and Pain 2008; 9:295-300.
Raphael KG, Janal MN, Anathan S, Cook DB, Staud R. Temporal summation of heat pain in temporomandibular disorder patients. Journal of Orofacial Pain 2009; 23:54-64.
Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: Findings from functional imaging studies. Cell Mol Life Sci 2009; 66:375-390.
Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: Fact or myth? The Clinical Journal of Pain 2013; 29:625-638.
Smart KM, Blake C, Staines A, Doody C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with ‘nociceptive’, ‘peripheral neuropathic’ and ‘central sensitisation’ pain. The discriminant validity of mechanisms-based classifications of low back (+/-leg) pain. Manual Therapy 2012; 17:119-125.
Smart KM, Blake C, Staines A, Thacker M, Doody C. Mechanisms-based classifications of musculoskeletal pain: Part 1 of 3: Symptoms and signs of central sensitisation in patients with low back (+/- leg) pain. Manual Therapy 2012; 17:336-344.
Smart KM, Blake C, Staines A, Thacker M, Doody C. Mechanisms-based classifications of musculoskeletal pain: Part 3 of 3: Symptoms and signs of nociceptive pain in patients with low back (+/- leg) pain. Manual Therapy 2012; 17:352-357.
Nijs J, Meeus M, Van Oosterwijck J, Roussel N, De Kooning M, Ickmans K, Matic M. Treatment of central sensitization in patients with ‘unexplained’ chronic pain: what options do we have? Expert Opinion on Pharmacotherapy 2011; 12:1087-1098.
Smart KM, Blake C, Staines A, Doody C. The discriminative validity of “nociceptive,” “peripheral neuropathic,” and “central sensitization” as mechanismsbased classifications of musculoskeletal pain. The Clinical Journal of Pain 2011; 27:655-663.
Nijs J, Torres-Cueco R, van Wilgen CP, Girbes EL, Struyf F, Roussel N,Van Oosterwijck J, Daenen L, Kuppens K, Vanderweeën L, Hermans L, Beckwée D, Voogt L, Clark J, Moloney N, Meeus M. Applying modern pain neuroscience in clinical practice: Criteria for the classification of central sensitization pain. Pain Physician 2014; 17:447-457.
Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Current Pain and Headache Reports 2009; 13:185-190.
Beith ID, Kemp A, Kenyon J, Prout M, Chestnut TJ. Identifying neuropathic back and leg pain: A cross-sectional study. Pain 2011; 152:1511-1516.
Waddell G. The back pain revolution. Churchill Livingstone, Edinburgh, 1998, p 438.
Koes BW, van Tulder MW, Ostelo R, Kim Burton A, Waddell G. Clinical guidelines for the management of low back pain in primary care: An international comparison. Spine (Phila Pa 1976) 2001; 26:2504- 2513; discussion 13-14.
Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008; 70:1630-1635.
Haanpää M TR. Diagnosis and classification of neuropathic pain. Pain Clinical Updates 2010; XVII.
Berthelot JM, Delecrin J, Maugars Y, Caillon F, Prost A. A potentially underrecognized and treatable cause of chronic back pain: Entrapment neuropathy of the cluneal nerves. The Journal of Rheumatology 1996; 23:2179-2181.
Verwoerd AJ, Peul WC, Willemsen SP, Koes BW, Vleggeert-Lankamp CL, El Barzouhi A, Luijsterburg PA, Verhagen AP. Diagnostic accuracy of history taking to assess lumbosacral nerve root compression. Spine J 2014; 14:2028-2037.
Murphy DR, Hurwitz EL, Gerrard JK et al.
Pain Patterns and Descriptions in Patients with Radicular Pain:
Does the Pain Necessarily Follow a Specific Dermatome?
Chiropractic & Osteopathy 2009 (Sep 21); 17 (1): 9Taylor C, Coxon A, Watson P, Greenough C. Do L5 and S1 nerve root compressions produce radicular pain in a dermatomal pattern? Spine (Phila Pa 1976) 2013; 38:995-998
Smart KM, Blake C, Staines A, Thacker M, Doody C. Mechanisms-based classifications of musculoskeletal pain: Part 2 of 3: Symptoms and signs of peripheral neuropathic pain in patients with low back (+/- leg) pain. Manual Therapy 2012; 17:345-351.
Smith PA. BDNF: No gain without pain? Neuroscience 2014; 96: 239-249.
Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plasticity2013; 2013:429815.
Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, Cho CH, DePalma MJ, Dougherty P, Fernand R, Ghiselli G, Hanna AS, Lamer T, Lisi AJ, Mazanec DJ, Meagher RJ, Nucci RC, Patel RD, Sembrano JN, Sharma AK, Summers JT, Taleghani CK, Tontz Jr. WL, Toton JF. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 2014; 14:180-191.
Watters WC, 3rd, McGirt MJ. An evidence- based review of the literature on the consequences of conservative versus aggressive discectomy for the treatment of primary disc herniation with radiculopathy. Spine J 2009; 9:240-257.
Spinelli BA, Wattananon P, Silfies S, Talaty M, Ebaugh D. Using kinematics and a dynamical systems approach to enhance understanding of clinically observed aberrant movement patterns. Man Ther 2014; 20:221-226.
Elgueta-Cancino E, Schabrun S, Danneels L, Hodges P. A clinical test of lumbopelvic control: Development and reliability of a clinical test of dissociation of lumbopelvic and thoracolumbar motion. Manual Therapy 2014; 19:418-424.
Ramsook RR, Malanga GA. Myofascial low back pain. Current Pain and Headache Reports 2012; 16:423-432.
Depalma MJ, Ketchum JM, Trussell BS, Saullo TR, Slipman CW. Does the location of low back pain predict its source? PM & R 2011; 3:33-39.
Laplante BL, Ketchum JM, Saullo TR, DePalma MJ. Multivariable analysis of the relationship between pain referral patterns and the source of chronic low back pain. Pain Physician 2012; 15:171-178.
O’Neill CW, Kurgansky ME, Derby R, Ryan DP. Disc stimulation and patterns of referred pain. Spine (Phila Pa 1976) 2002; 27:2776-2781.
Bogduk N, Aprill C, Derby R. Lumbar discogenic pain: State-of-the-art review. Pain Medicine (Malden, Mass) 2013; 14:813-836.
Malanga GA, Cruz Colon EJ. Myofascial low back pain: A review. Physical Medicine and Rehabilitation Clinics of North America 2010; 21:711-724.
Iglesias-Gonzalez JJ, Munoz-Garcia MT, Rodrigues-de-Souza DP, Alburquerque- Sendin F, Fernandez-de-Las-Penas C. Myofascial trigger points, pain, disability, and sleep quality in patients with chronic nonspecific low back pain. Pain Medicine (Malden, Mass) 2013; 14:1964-1970.
Taguchi T, Yasui M, Kubo A, Abe M, Kiyama H, Yamanaka A, Mizumura K. Nociception originating from the crural fascia in rats. Pain 2013; 154:1103-1114.
Sudhakar P, Sharma AR, Bhushan SM, Ranadhir G, Narsimuhulu G, Rao VV. Efficacy of SPECT over planar bone scan in the diagnosis of solitary vertebral lesions in patients with low back pain. IJNM 2010; 25:44-48.
Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. The Journal of Bone and Joint Surgery American Volume 2006; 88:63-67.
Cavanaugh JM, Ozaktay AC, Yamashita HT, King AI. Lumbar facet pain: Biomechanics, neuroanatomy and neurophysiology. Journal of Biomechanics 1996; 29:1117-1129.
Manchikanti L, Pampati V, Fellows B, Bakhit CE. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician 1999; 2:59-64.
Fortin JD, Aprill CN, Ponthieux B, Pier J. Sacroiliac joint: Pain referral maps upon applying a new injection/arthrography technique. Part II: Clinical evaluation. Spine (Phila Pa 1976) 1994; 19:1483-1489.
Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: Pain referral maps upon applying a new injection/arthrography technique. Part I: Asymptomatic volunteers. Spine (Phila Pa 1976) 1994; 19:1475-1482.
Ashraf A, Farahangiz S, Pakniat Jahromi B, Setayeshpour N, Naseri M. Correlation between degree of radiologic signs of osteoarthritis and functional status in patients with chronic mechanical low back pain. MJMS 2014; 21:28-33.
Suri P, Hunter DJ, Rainville J, Guermazi A, Katz JN. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthritis and Cartilage / OARS, Osteoarthritis Research Society 2013; 21:1199-1206.
Kalichman L, Kim DH, Li L, Guermazi A, Hunter DJ. Computed tomographyevaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. Spine J 2010; 10:200-208.
Cheung KM, Karppinen J, Chan D, Ho DW, Song YQ, Sham P, Cheah KS, Leong JC, Luk KD. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009; 34:934-940.
Ract I, Meadeb JM, Mercy G, Cueff F, Husson JL, Guillin R. A review of the value of MRI signs in low back pain. Diagnostic and Interventional Imaging 2014; 96:239-249.
Fortin M, Macedo LG. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: A systematic review with a focus on blinding. Physical Therapy 2013; 93:873-888.
Kalichman L, Hodges P, Li L, Guermazi A, Hunter DJ. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J 2010; 19:1136-1144.
Nijs J, Van Houdenhove B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: Application of pain neurophysiology in manual therapy practice. Man Ther 2009; 14:3-12.
Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: An application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther 2008; 12:371-384.
Cagnie B, Dewitte V, Barbe T, Timmermans F, Delrue N, Meeus M. Physiologic effects of dry needling. Current Pain and Headache Reports 2013; 17:348.
Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM,Gerber LH. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Archives of Physical Medicine and Rehabilitation 2008; 89:16-23.
Hsieh CY, Hong CZ, Adams AH, Platt KJ, Danielson CD, Hoehler FK, Tobis JH. Interexaminer reliability of the palpation of trigger points in the trunk and lower limb muscles. Archives of Physical Medicine and Rehabilitation 2000; 81:258-264.
Nice DA, Riddle DL, Lamb RL, Mayhew TP, Rucker K. Intertester reliability of judgments of the presence of trigger points in patients with low back pain. Archives of Physical Medicine and Rehabilitation 1992; 73:893-898.
Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain 1986; 24:57-65.
Margolis RB, Chibnall JT, Tait RC. Testretest reliability of the pain drawing instrument. Pain 1988; 33:49-51.
Farasyn A, Meeusen R. The influence of non-specific low back pain on pressure pain thresholds and disability. European Journal of Pain (London, England) 2005; 9:375-381.
Meeus M, Roussel NA, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: An experimental study. J Rehabil Med 2010; 42:884-890.
O’Sullivan P, Waller R, Wright A, Gardner J, Johnston R, Payne C,Shannon A, Ware B, Smith A. Sensory characteristics of chronic non-specific low back pain: A subgroup investigation. Manual Therapy 2014; 19:311-318.
Coronado RA, Bialosky JE, Robinson ME, George SZ. Pain sensitivity subgroups in individuals with spine pain: Potential relevance to short-term clinical outcome. Physical Therapy 2014; 94:1111-1122.
Hubscher M, Moloney N, Rebbeck T, Traeger A, Refshauge KM. Contributions of mood, pain catastrophizing and cold hyperalgesia in acute and chronic low back pain - a comparison with pain-free controls. Clin J Pain 2014; 30:886-893.
Wang H, Fischer C, Chen G, Weinsheimer N, Gantz S, Schiltenwolf M. Does long-term opioid therapy reduce pain sensitivity of patients with chronic low back pain? Evidence from quantitative sensory testing. Pain Physician 2012; 15: S135-S143.
George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain 2007; 8:2-10.
Hubscher M, Moloney N, Leaver A, Rebbeck T, McAuley JH, Refshauge KM. Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. Pain 2013; 154:1497-1504.
Geha P, Dearaujo I, Green B, Small DM. Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain 2014; 155:712-722.
Steffens D, Maher CG, Li Q, Ferreira ML, Pereira LS, Koes BW, Latimer J. Effect of wheather on back pain: Results from a case-crossover study. Arthritis Care Res (Hoboken) 2014; 66:1867-1872.
Salek KM, Mamun MA, Parvin N, Ahmed SM, Khan MM, Rijvi AN, Rahman MH, Khasru MR, Akther A, Rahman M, Islam S, Emran A. Fluctuation of pain by weather change in musculoskeletal disorders. MMJ 2011; 20:645-651.
Nijs J T-CR, van Wilgen CP, Lluch Girbés E, Struyf F, Roussel N, Van Oosterwijck J, Daenen L, Kuppens K, Vanderweeën L, Hermans L, Beckwée D, Voogt L, Clark J, Moloney N, Meeus M. Applying modern pain neuroscience in clinical practice: Criteria for the classification of central sensitization pain. Pain Physician 2014; in press.
Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ,Perez Y, Gatchel RJ. The development and psychometric validation of the central sensitization inventory. Pain Practice 2012; 12:276-285.
Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, Gatchel RJ. The central sensitization inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 2013; 14:438-445.
Neblett R, Hartzell MM, Cohen H, Mayer TG, Williams M, Choi Y, Gatchel RJ. Ability of the Central Sensitization Inventory to identify central sensitivity syndromes in an outpatient chronic pain sample. Clin J Pain 2014; 31:323-332.
Rabey M, Beales D, Slater H, O’Sullivan P. Multidimensional pain profiles in four cases of chronic non-specific axial low back pain: An examination of the limitations of contemporary classification systems. Man Ther 2015; 20:138-147.
Apeldoorn AT, Ostelo RW, van Helvoirt H, Fritz JM, Knol DL, van Tulder MW, Lankhorst GJ. A randomized controlled trial on the effectiveness of a classification- based system for subacute and chronic low back pain. Spine (Phila Pa 1976) 2012; 37:1347-1356.
Dankaerts W, O’Sullivan PB, Burnett AF, Straker LM. The use of a mechanismbased classification system to evaluate and direct management of a patient with non-specific chronic low back pain and motor control impairment – a case report. Manual Therapy 2007; 12:181-191.
Dankaerts W, O’Sullivan PB, Straker LM, Burnett AF, Skouen JS. The inter-examiner reliability of a classification method for non-specific chronic low back pain patients with motor control impairment. Manual Therapy 2006; 11:28-39.
O’Sullivan P. Classification of lumbopelvic pain disorders – why is it essential for management? Manual Therapy 2006; 11:169-170.
Kilpikoski S, Airaksinen O, Kankaanpaa M, Leminen P, Videman T, Alen M. Interexaminer reliability of low back pain assessment using the McKenzie method. Spine (Phila Pa 1976) 2002; 27:E207-E214.
Slater SL, Ford JJ, Richards MC, Taylor NF, Surkitt LD, Hahne AJ. The effectiveness of sub-group specific manual therapy for low back pain: A systematic review. Manual Therapy 2012; 17:201-212.
Brosschot JF. Cognitive-emotional sensitization and somatic health complaints. Scandinavian Journal of Psychology 2002; 43:113-121.
Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain 1999; 80:329-339.
van Vuuren BJ, van Heerden HJ, Becker PJ, Zinzen E, Meeusen R. Fear-avoidance beliefs and pain coping strategies in relation to lower back problems in a South African steel industry. European Journal of Pain (London, England) 2006; 10:233-239.
Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the pain catastrophizing scale: Invariant factor structure across clinical and non-clinical populations. Pain 2002; 96:319-324.
Chaichana KL, Mukherjee D, Adogwa O, Cheng JS, McGirt MJ. Correlation of preoperative depression and somatic perception scales with postoperative disability and quality of life after lumbar discectomy. J Neurosurgery Spine 2011; 14:261-267.
Lebow R, Parker SL, Adogwa O, Reig A, Cheng J, Bydon A, McGirt MJ. Microdiscectomy improves pain-associated depression, somatic anxiety, and mental well-being in patients with herniated lumbar disc. Neurosurgery 2012; 70:306- 311; discussion 11.
van Wilgen CP, Stewart R, Patrick Stegeman PT, Coppes M, van Wijhe M. Fear of movement in pre-operative patients with a lumbar stenosis and or herniated disc: Factor structure of the Tampa scale for kinesiophobia. Manual Therapy 2010; 15:593-598.
Voorhies RM, Jiang X, Thomas N. Predicting outcome in the surgical treatment of lumbar radiculopathy using the Pain Drawing Score, McGill Short Form Pain Questionnaire, and risk factors including psychosocial issues and axial joint pain. Spine J 2007; 7:516-524.
Sinikallio S, Aalto T, Airaksinen O, Herno A, Kroger H, Viinamaki H. Depressive burden in the preoperative and early recovery phase predicts poorer surgery outcome among lumbar spinal stenosis patients: A one-year prospective followup study. Spine (Phila Pa 1976) 2009; 34:2573-2578.
Wand BM, Parkitny L, O’Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley GL. Cortical changes in chronic low back pain: Current state of the art and implications for clinical practice. Manual Therapy 2011; 16:15-20.
Apeldoorn AT, Bosselaar H, Blom- Luberti T, Twisk JW, Lankhorst GJ. The reliability of nonorganic sign-testing and the Waddell score in patients with chronic low back pain. Spine (Phila Pa 1976) 2008; 33:821-826.
Apeldoorn AT, Ostelo RW, Fritz JM, van der Ploeg T, van Tulder MW, de Vet HC. The cross-sectional construct validity of the Waddell score. Clin J Pain 2012; 28:309-317.
Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012; 15: S205-S213.

Return to LOW BACK PAIN
Return to LOW BACK GUIDELINES
Return to CLINICAL PREDICTION RULE
Since 3-26-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |