

Low Back Pain: The Potential Contribution of
Supraspinal Motor Control and ProprioceptionThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Neuroscientist 2019 (Dec); 25 (6): 583–596 ~ FULL TEXT
OPEN ACCESS Michael Lukas Meier, Andrea Vrana, and Petra Schweinhardt
Integrative Spinal Research,
Department of Chiropractic Medicine,
University Hospital Balgrist,
Zurich, Switzerland.
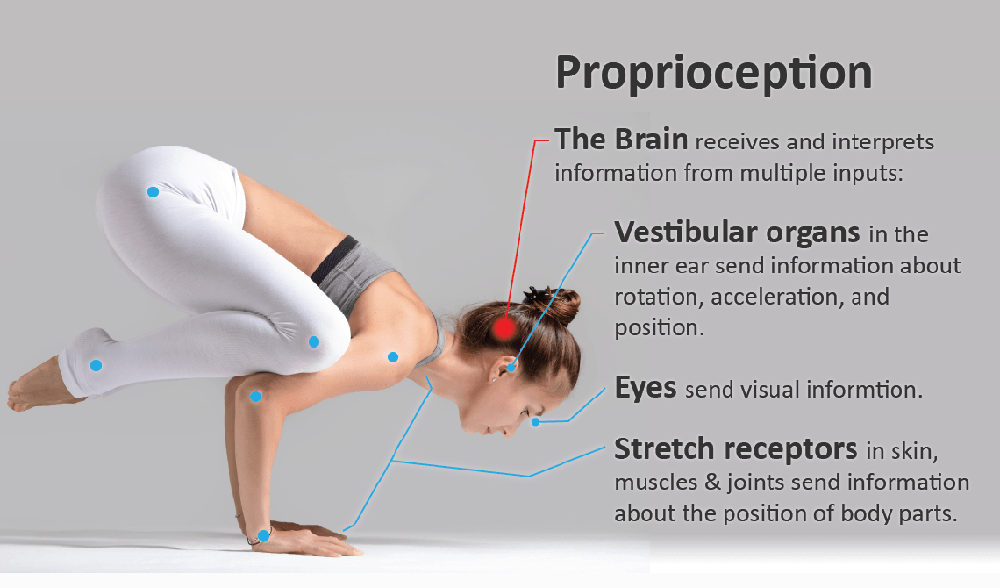
Motor control, which relies on constant communication between motor and sensory systems, is crucial for spine posture, stability and movement. Adaptions of motor control occur in low back pain (LBP) while different motor adaption strategies exist across individuals, probably to reduce LBP and risk of injury. However, in some individuals with LBP, adapted motor control strategies might have long-term consequences, such as increased spinal loading that has been linked with degeneration of intervertebral discs and other tissues, potentially maintaining recurrent or chronic LBP. Factors contributing to motor control adaptations in LBP have been extensively studied on the motor output side, but less attention has been paid to changes in sensory input, specifically proprioception.
Furthermore, motor cortex reorganization has been linked with chronic and recurrent LBP, but underlying factors are poorly understood. Here, we review current research on behavioral and neural effects of motor control adaptions in LBP. We conclude that back pain-induced disrupted or reduced proprioceptive signaling likely plays a pivotal role in driving long-term changes in the top-down control of the motor system via motor and sensory cortical reorganization. In the outlook of this review, we explore whether motor control adaptations are also important for other (musculoskeletal) pain conditions.
KEYWORDS: chronic pain; low back pain; motor control; motor cortex; muscle spindle; proprioception; somatosensory cortex
From the Full-Text Article:
Introduction
Low back pain (LBP) is extremely common with a lifetime prevalence around 75% to 84% [Thiese and others 2014] and is globally among the health conditions with the highest numbers of years lived with disability [Vos and others 2017]. In most instances of LBP, no underlying pathology can be identified [Maher and others 2016], resulting in the unfortunate diagnosis of “non-specific LBP” (nsLBP). An acute episode of LBP spontaneously resolves in one third of the patients within the first 3 months; however, about 65% of the patients still experience LBP 1 year after LBP onset [Itz and others 2013]. Consequently, recurrent or chronic LBP (LBP persisting for 12 weeks or more) is a common problem, with an enormous individual, economic and societal burden [Hoy and others 2014; van Tulder and others 2006]. Therefore, advancing the understanding of factors contributing to the chronification of LBP is a research priority [Hartvigsen and others 2018]. Among factors such as genetic, physical and psychosocial features, adaptions of motor control likely play a significant role in chronic or recurrent LBP [Hodges and others 2013] because they are associated with several important factors contributing to LBP chronification, including increased spinal tissue strains due to potential loss of trunk control and enhanced trunk muscle co-contraction, resulting in muscle fatigue [Madeleine 2010; van Dieën and others 2018b]. Both factors, loss of trunk control and enhanced muscle co-contraction, have been linked with sustained mechanical loading on spinal tissues, conceivably potentiating degeneration of intervertebral discs and other tissues [Lotz and Chin 2000; Paul and others 2013; Urban and Roberts 2003; van Dieën and others 2018b].
The overarching hypothesis of this review is that motor control adaptions induced by acute LBP play an important role in the chronification of LBP. Following a short introduction to human motor control and proprioception, we summarize the findings on motor control adaptions in LBP on the behavioral and neural level, including (supra-)spinal and psychological contributions. We integrate new research suggesting a powerful role of reduced paraspinal proprioceptive input for the top-down control of cortical sensorimotor circuits, probably associated with neuroplastic changes. The resulting cortical reorganization would potentially explain persistent and dysfunctional motor control adaptions associated with LBP chronification.
Motor Control and Proprioception
Motor control is responsible for spine posture, stability and movement and arises from a constant interplay between motor outputs to effectors (e.g., paraspinal muscles) and sensory inputs (e.g., proprioception) on various levels of the nervous system [Hodges and others 2013; Riemann and Lephart 2002]. As described in Panjabi’s model of spinal stability, appropriate motor control of the trunk relies on the interplay of the passive (osteoligamentous spinal structures), active (muscles) and control subsystems (central nervous system), with the latter integrating and coordinating sensorimotor information by exerting direct control over the active subsystem [Panjabi 1992]. Human trunk posture and movement are inherently unstable due to continuous neuromuscular noise that needs to be controlled [Willigenburg and others 2013]. As a potential cause of low back pain, clinical instability of the spine is defined as a failure of any of the three subsystems leading to adaptions of the motor control system [Hodges and others; Panjabi 2003].
Motor control adaptions are inevitably linked to adaptions in somatosensory processing as we can only precisely control what we can sense [Naito 2004]. Proprioception is the key somatosensory feedback system [Gandevia and others 2002; Sherrington 1908]. The importance of proprioception for motor control is exemplified by patients with a lack of proprioception due to, for example, large fiber neuropathy [Goble and others 2011] or loss of PIEZO2 receptor function [Chesler and others 2016]. Without visual input, these patients show impaired motor control, including deficits in coordinated movement, force control, and limb position sense [Chesler and others 2016; Lajoie and others 1996; Rothwell and others 1982; Sainburg and others 1995]. Proprioception is subserved by mechanoreceptors on deep and superficial tissues. Muscle spindles, located in the muscle belly parallel to the extrafusal muscle fibers, act as the principal proprioceptors, in addition to mechanoreceptors located in joints, ligaments, tendons, fascia, and skin [Brumagne and others 2000; Proske and Gandevia 2012]. The important role of muscle spindles in proprioception is illustrated by the observation that vibration applied to a resting muscle can produce illusions of limb movement and of displaced limb position (mediated through primary [Ia] and secondary [II] muscle afferents) [Burke and others 1976; Gilman 2002; Goodwin and others 1972; Proske and Gandevia 2012].
and electromyography (EMG) [Hodges and others 2003; Sohn and others 2013]. Adaptions in motor control are also present in acute LBP patients, reflected by alterations in the timing, magnitude and kinematics of lumbopelvic coordination [Shojaei and others 2017a; Shojaei and others 2017b; Sung and others 2015]. It has been suggested that these changes, if persistent, predispose individuals to recurrent and chronic LBP [van Dieën and others 2017]. Indeed, compelling evidence indicates altered motor control in chronic LBP patients based on, for example, spine kinematics, EMG, and center of pressure analysis [reviewed in Knox and others 2018]. Chronic LBP patients demonstrate motor control deficits during standing and sitting [Della Volpe and others 2006; Lafond and others 2009], during challenging tasks such as one-legged standing [da Silva and others 2018] or during gait and functional tasks [Christe and others 2017; Hemming and others 2017]. Furthermore, the systematic review by Knox and colleagues identified a delayed onset of muscle activity with anticipatory and compensatory postural responses to perturbations in patients with chronic LBP [Knox and others 2018]. In sum, there is substantial evidence for behavioral motor control adaptations associated with LBP.
Behavioral and Motor System Adaptions in LBP
A plethora of studies indicate strong support for motor control adaptions in LBP on a behavioral and motor system level. Experimental LBP in healthy subjects provoked adaptions of motor control that were characterized by altered balance control and trunk muscle activity, captured by changes of the center of pressure (using a foot plate]
Nevertheless, the literature is relatively inconsistent with respect to the nature of these motor control adaptions. For example, in chronic LBP, spine kinematic patterns during movement indicated a more rigid spine [Christe and others 2016], in line with clinical observations of a stronger coupling between thoracolumbar segments during movement and generally less variability of trunk movement in chronic LBP [Elgueta-Cancino and others 2014; Moseley and Hodges 2006]. Furthermore, using large-array surface EMG during a muscle fatigue exercise, chronic LBP patients showed variability in trunk muscle activity that increased less over time compared to healthy subjects, suggesting fewer degrees of freedom regarding trunk muscle recruitment configurations [Abboud and others 2014]. However, the opposite pattern of higher variability in trunk movements in chronic LBP has also been observed [Silfies and others 2009; van Dieën and others 2018b; Vogt and others 2001]. A review on lumbar extensor muscle recruitment in acute, subacute and chronic LBP patients highlighted high and task-dependent variability in trunk muscle activation patterns between and probably within individuals [van Dieën and others 2003].
Similar to the observed variability in trunk movement and related muscle activation patterns in individuals with LBP, an analysis of the literature on motoneuron excitability at cortical and spinal sites during pain indicated inconsistent findings across studies [Hodges and Tucker 2011]. Cortically and spinally mediated changes of motoneuron excitability during pain and nociception has been distinguished by simultaneous recording of, for example, the Hoffman reflex (H-reflex) and motor evoked potentials (MEPs) at, for example, biceps or erector spinae muscles [Hodges and Tucker 2011; Le Pera and others 2001; Strutton and others 2005] or by combining transcranial magnetic stimulation (TMS) at the cervicomedullary junction (to activate the axons of primary motoneurons) and at the primary motor cortex (M1). The corticospinal neural drive has been reported to be decreased in chronic LBP reflected by increased MEP thresholds and lowered EMG activity of paraspinal muscles following TMS over the vertex [Chiou and others 2014; Strutton and others 2005]. Similar findings of temporally reduced MEPs of hand and arm muscles after TMS of the primary motor cortex (M1) were reported in a study of experimental pain in healthy subjects [Farina and others 2001]. Interestingly, in this study, the reduction in MEPs appeared to be caused exclusively by supraspinal circuits because peripheral (M-wave) and spinal cord (F- and H-waves) measures of excitability were not affected. However, conflicting findings of excitability changes with pain across the motor system have been reported. MEPs, induced by TMS over M1, at hand and biceps muscles increased during painful stimulation [Del Santo and others 2007]. Furthermore, following intervertebral disc lesion in pigs (damaging the annulus fibrosis to provoke leakage of the nucleus pulposus), presumably painful, excitability of spinal circuits was slightly decreased but cortical excitability increased immediately after the lesion, revealed by recording MEPs of the multifidus muscle [Hodges and others 2009].
It is possible that some of the inconsistencies in the literature stem from response patterns that differ across specific muscles, even across different paraspinal muscles [Hodges and Tucker 2011]. Recent work has suggested a potential mechanism for increases in M1 excitability with pain: in chronic LBP, enhanced cortical excitability of M1 was linked to a maladaptive homeostatic mechanism (homeostatic plasticity), which is reflected by a general disbalance of the ratio between long-term potentials (synaptic strengthening) and longterm depression (synaptic weakening) [Thapa and others 2018]. In line with the evidence demonstrating enhanced cortical motor excitability in chronic LBP, patients failed to maintain homeostatic plasticity by showing an excessive synaptic strengthening, which was suggested as a marker of cortical reorganization.
Today, it is clear that cortical motor circuits play an important role in controlling trunk muscle excitability. For a long time, it was assumed that cortical motor control is more important for voluntary goal-directed movements compared to “automatic” processes such as postural adjustments and gait that have been associated with subcortical circuits [Deliagina and others 2008]. However, evolving evidence indicates substantial cortical motor involvement in automatic motor responses [Chiou and others 2016; Chiou and others 2018; Gandolla and others 2014; Petersen and others 2012]. In line with this, TMS mapping of surface and (intra-muscular) fine-wire EMG recordings of paraspinal muscles (multifidus and erector spinae muscles) demonstrated a high degree of functional specificity within M1, suggesting fine control of segmental motion [Tsao and others 2011a]. In individuals with chronic LBP, M1 representations (center of gravity) of the longissimus and deep multifidus muscles were observed to overlap, indicating less fine-grained (“smudging”) representations of paraspinal muscles [Tsao and others 2011b].
In addition, it has been demonstrated that plastic changes of the trunk representation in M1 were related to the severity of LBP [Schabrun and others 2017; Tsao and others 2010] and shifts in the cortical representation of trunk muscles have been associated with deficits in postural control [Tsao and others 2008]. These changes in M1 organization seem to occur very early as sustained experimental pain induced M1 reorganization after 4 days, characterized by reduced intracortical inhibition and increased facilitation [Schabrun and others 2016]. However, similar to the reported variability in behavioral findings and motoneuron excitability, changes of the M1 representation in chronic LBP show high between-subject variability [Elgueta-Cancino and others 2018], indicating different LBP phenotypes of motor adaption strategies.
Models of Motor Adaption in LBP
Several models have been proposed to predict the observed variability of motor control strategies in LBP. A relatively new model postulates a redistribution of activity within (through changes in motoneuron recruitment) and between muscles (e.g., increased and compensating activity of superficial paraspinal muscles following an injury of deep muscles), with the ultimate goal to protect tissues from further pain and injury [Hodges 2011]. In contrast to previous models postulating stereotypical inhibition or excitation of muscles during pain [Lund and others 1991; Roland 1986], the recent model accounts for the observed differences in motor adaption strategies in pain by considering complementary, additive or competitive effects on spinal and supraspinal levels [Hodges and Tucker 2011]. Moreover, this model accounts for the redundancy of the trunk motor control system that allows various muscle recruitment configurations to achieve a certain goal [Abboud and others 2018]. From a learning perspective, individual-specific redistribution of muscle activity can be explained through a reinforcement learning model [van Dieën and others 2017]. Namely, motor adaptions due to LBP are defined as the outcome of a learning process with the goal to minimize a weighted sum of costs composed of, for example, muscle activity costs, metabolic costs, or costs associated with movement-related pain and loss of control. The feeling of having control over trunk movement (reward, positive reinforcement) or the reduction of costs (e.g., movement-related pain, negative reinforcement) will lead to the acquisition of new muscle activation patterns [van Dieën and others 2017].
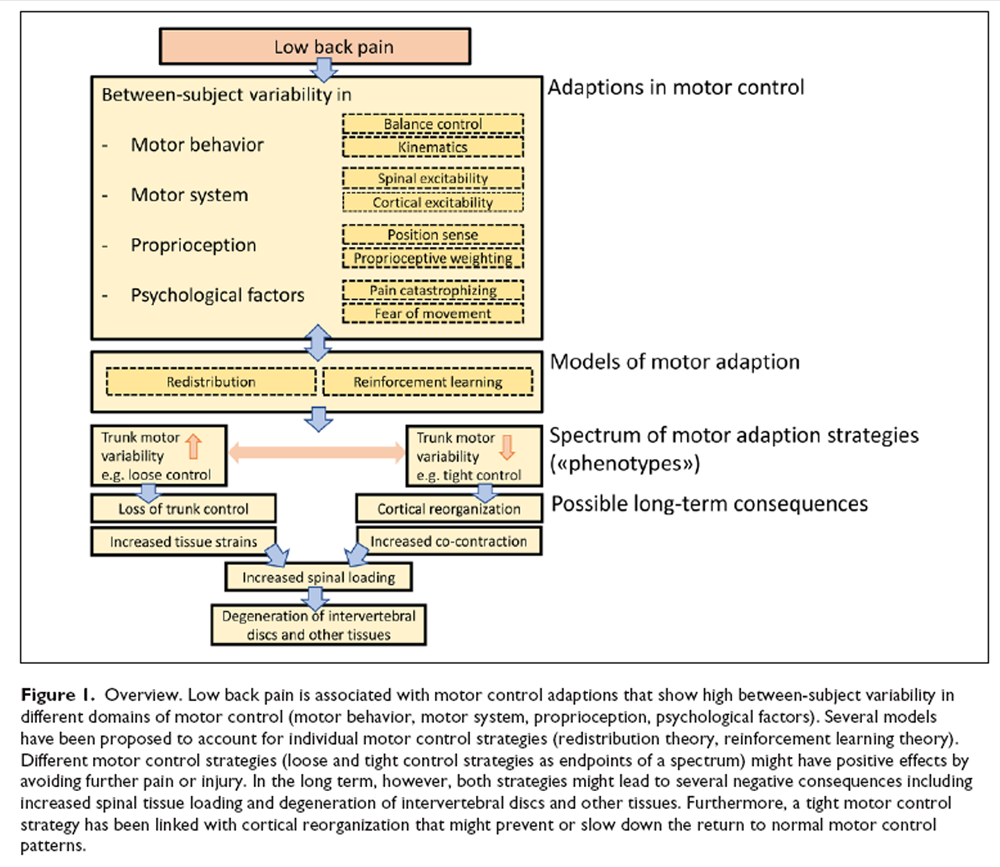
Figure 1 Two LBP phenotypes representing the opposite ends of a spectrum of motor control strategies have been suggested: Some individuals with LBP show “tight” control whereas others demonstrate “loose” control over trunk movement [van Dieën and others 2018b] (Figure 1). Tight control is associated with increased trunk muscle excitability, enhanced muscle co-contraction and less trunk motor variability. In contrast, loose control over trunk movement is related to reduced muscle excitability and increased trunk motor variability [van Dieën and others 2018b]. The reinforcement learning model predicts that motor variability will initially increase at the onset of LBP to allow for sufficient degrees of freedom to adopt the least painful motor control strategy which might lead to a normal strategy when pain disappears [Hodges and others 2013; Madeleine and others 2008; Moseley and Hodges 2006]. Furthermore, increasing motor variability might prevent muscle fatigue [Madeleine 2010]. However, in the long-term, motor variability is expected to decrease due to increased costs associated with loss of trunk control and pain [van Dieën and others 2017]. This pattern of initially increased, followed by reduced motor variability, is supported by similar observations during the transition from acute to chronic neck-shoulder pain [Madeleine 2010]. This is in line with the dynamical systems theory of biological systems, which proposes that, under certain conditions, behavioral states switch to a new and stable movement pattern (with less variability) when the increase of variability reaches a critical point, reflected by a highly unstable system [Stergiou and Decker 2011].
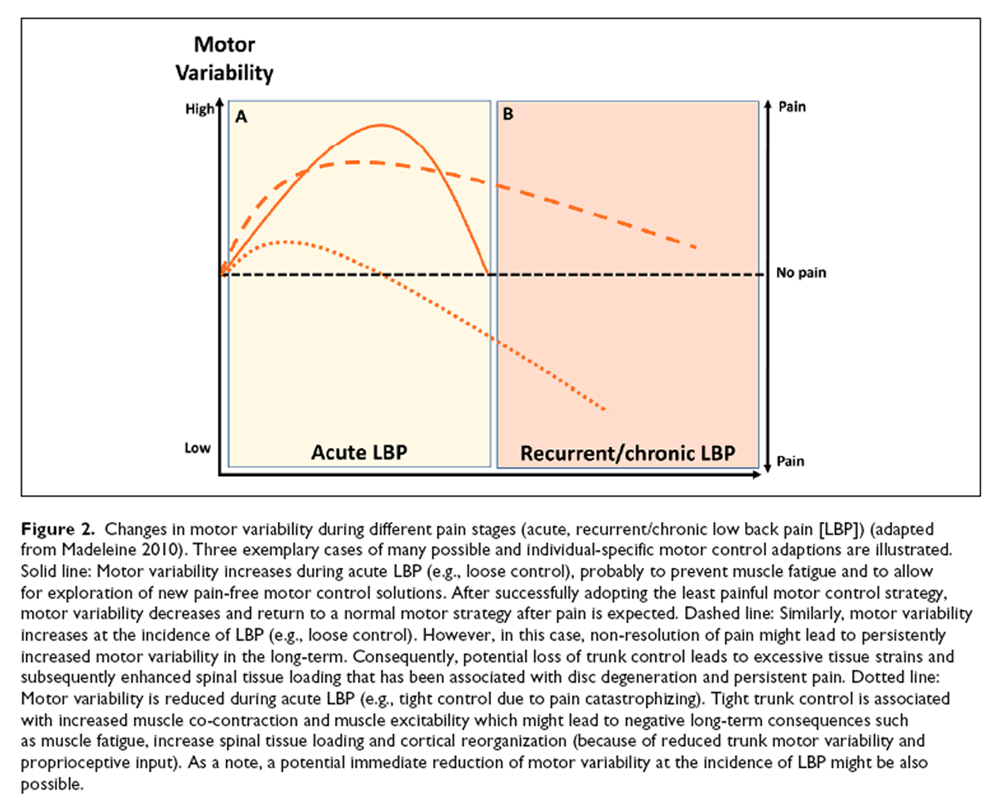
Figure 2 In the short term, motor control adaptions might have beneficial effects by avoiding further pain or injury, either through adopting a protective, stabilizing strategy to limit movements (which might be painful, tight control) or through applying a destabilizing strategy (loose control) to limit muscle force exertion and related costs such as metabolic costs, fatigue, and tissue loading [Ross and others 2017; van Dieën and others 2017] (Figure 2A). Furthermore, a loose control strategy might allow exploring alternative and pain-free trunk motor control solutions. However, prolonged motor control adaptions due to LBP have been linked with permanently increased loading on spinal tissues that either can be triggered by excessive tissue strains due to loss of muscular control (loose control) or by enhanced muscle co-contraction (tight control) [van Dieën and others 2018b] (Figure 2B). Excessive mechanical loading has been associated with disruption of the intervertebral disc structure [Adams and Roughley 2006; Urban and Roberts 2003], initiating a cascade of cell-mediated responses, including cell death [as shown in animal models; Lotz and Chin 2000], probably leading to disc degeneration that maintains and aggravates LBP [Lotz and others 1998; Paul and others 2013; van Dieën and others 2018b]. Furthermore, reduced proprioceptive input (e.g., due to decreased trunk motor variability) has been suggested to contribute to cortical neuroplastic changes that might affect the organizational structure in sensorimotor cortices and top-down trunk motor control [van Dieën and others 2017].
This would provide an explanation for the observed changes in M1 motor maps, however, a direct link between motor control strategies and M1 organization has not yet been established [Elgueta-Cancino and others 2018; Goossens and others 2018]. Furthermore, it is currently unclear when and to which extent such alterations in M1 motor maps are caused by impairments on the “input side,” that is, the processing of paraspinal somatosensory inputs. As described above, somatosensory input is an essential component of the motor control system and particularly proprioceptive impairment can contribute substantially to motor control dysfunction [Borich and others 2015; Riemann and Lephart 2002; Rosenkranz and others 2008]. In the following, we therefore summarize findings on proprioception in LBP on a behavioral level, followed by a discussion of potential cortical alterations induced by altered proprioceptive input from paraspinal muscles.
Proprioceptive Impairments in LBP
On a behavioral level, LBP patients have been shown to have impaired trunk proprioception. Individuals with chronic LBP showed lower acuity for detecting changes in trunk position [Lee and others 2010] and demonstrated significantly higher trunk repositioning errors during flexion of the back compared with pain-free individuals [Newcomer and others 2000]. Similar to the existing literature on motor behavior and motor system adaptions in LBP, studies on proprioceptive function in LBP show some heterogeneity [Hodges and others 2013]. A recent systematic review and meta-analysis on lumbar proprioception in LBP concluded that LBP patients indeed show impairments in lumbar proprioception compared to painfree individuals for active joint repositioning sense (JRS) and detection threshold of passive motion in sitting position [Tong and others 2017]. No effects were found for active JRS in standing or passive JRS in sitting [Tong and others 2017], probably because different proprioceptive tests differ with regard to the required motor skills and memory processes. In addition, using a force plate to analyze postural sway on stable and unstable support surfaces, vibratory stimulation of the triceps surae, tibialis anterior, and paraspinal muscles revealed an altered proprioceptive weighting in chronic LBP patients characterized by an increased weighting of ankle proprioception relative to trunk muscle proprioception [Brumagne and others 2008; Claeys and others 2011]. Interestingly, in a prospective study, a more ankle-steered proprioceptive weighting has been identified as a risk factor for the development of mild LBP in young individuals [Claeys and others 2015]. However, the opposite result of no association between proprioceptive deficits and the development of LBP has also been reported in a study involving almost 300 subjects [Silfies and others 2007], emphasizing the need for more longitudinal research in this area.
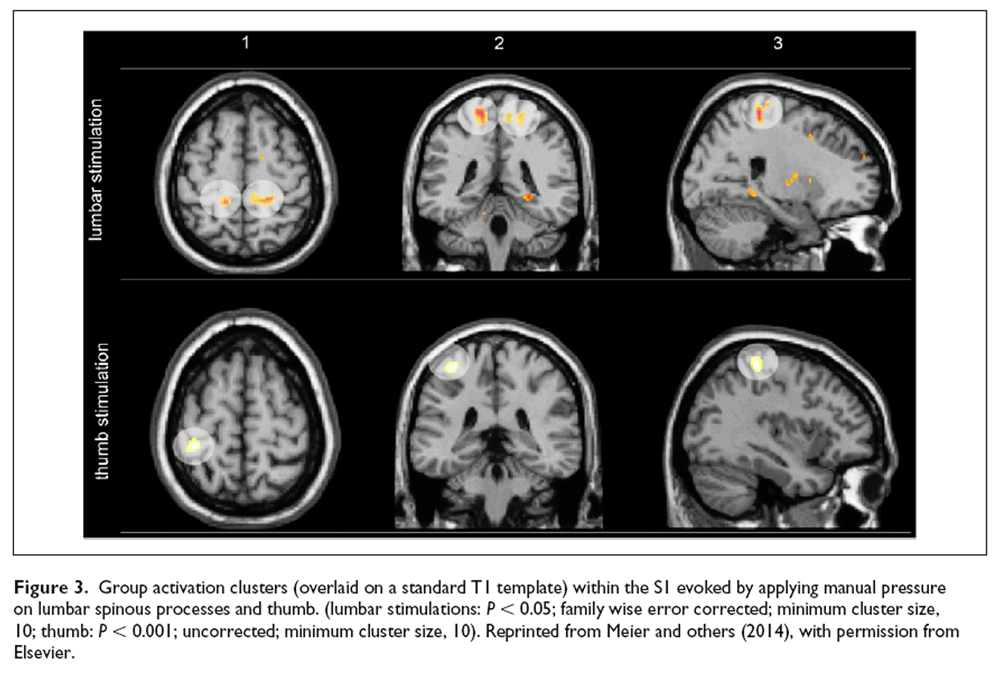
Figure 3 Proprioceptive information might be reduced or disrupted as a result of traumatic damage of tissues, muscle fatigue [Taimela and others 1999] and/or the activation of nociceptors, which consequently interferes with motor control [Thunberg and others 2002; van Dieën and others 2018b]. Persistent nociception leads to an enhanced activation of the sympathetic nervous system [Nijs and others 2012], which directly innervates muscle spindles and modulates their discharge [Radovanovic and others 2015]. Thus, it is conceivable that (physical or emotional) stress associated with sympathetic nervous system activation might depress the information flow from muscle spindles, leading to deterioration of proprioceptive information flow across the spinocortical axis. This might represent a mechanism contributing to observed impairments in trunk proprioception in LBP patients. Yet, little is known about the cortical representation of paraspinal somatosensory (in particular proprioceptive) inputs to the primary somatosensory cortex (S1) which play an essential role in accurate motor output [Borich and others 2015; Riemann and Lephart 2002].
Cortical Targets of Somatosensory and Proprioceptive Input
The proprioceptive input axis to cortical targets has been investigated in studies using muscle vibration on limbs, primarily resulting in activation of primary sensorimotor cortices contralateral to the stimulation site [Goble and others 2012; Kavounoudias and others 2008; Naito and others 2007]. However, evidence about the cortical representation of paraspinal somatosensory inputs is scant. In his pioneering work [Penfield 1947], Penfield identified the hip and the shoulder on the convexity of the postcentral gyrus and drew the back between these two areas on the sensory Homunculus. In 2018, intracortical stimulation in humans of BA1 in S1 identified the representations of the thorax and abdomen to lie indeed between hip and shoulder [Roux and others 2018] but the cortical somatotopic representation of paraspinal proprioceptive input along the thoracolumbar axis is still unclear and needs further investigation. We first attempted to “map” the lower back on a cortical level by applying manual pressure stimuli on three lumbar segments [Boendermaker and others 2014; Meier and others 2014]. These studies revealed primarily activation patterns in medial parts of S1 (Figure 3) and the secondary somatosensory cortex. However, because manual pressure likely activated several types of mechanoreceptors in different tissues, the resulting cortical activation is not specifically attributable to proprioceptive input. Furthermore, previous studies investigating the sensory representation of the back did not consider the heterogeneity of the S1 landscape: S1 consists of four distinct cytoarchitectonic areas, namely Brodmann areas (BA) 3a, 3b, 1, and 2, of which each includes a full somatotopic representation of the contralateral body [Martuzzi and others 2014; Powell and Mountcastle 1959].
Animal and human studies have revealed that body parts are represented at distinct positions in these four subareas where BA 3a receives proprioceptive information from muscles and joints and BA 3b, 1, and 2 process signals from the skin [Iwamura and others 1993; Martuzzi and others 2014; Naito 2004; Yamada and others 2016]. Nevertheless, the notion of segregated cortical channels for proprioceptive and tactile input has recently been challenged by research showing that BA 3a as well as 3b respond to both types of input, tactile and proprioceptive [Kim and others 2015]. Thus, the organizational structure of somatosensory input from the back in S1, in particular of proprioceptive input, is still not entirely clear. What is clear is that S1 reorganization can lead to dysfunctions in motor output and motor learning [Borich and others 2015] and, as detailed above, that LBP is associated with impaired proprioception. It is therefore plausible that degraded paraspinal proprioceptive feedback is causally linked to impairments in motor control in LBP via neuroplastic S1 changes [van Dieën and others 2017]. Therefore, systematic cortical mapping of paraspinal proprioceptive input will be essential for a better understanding of its role in aberrant sensorimotor integration and related potential maladaptive cortical plasticity in chronic LBP [Makin and Bensmaia 2017; Massé-Alarie and Schneider 2016].
Impairments of Somatosensory Input and Cortical Reorganization in LBP
In line with the notion that somatosensory input might be a powerful driver of motor adaption and cortical reorganization, sensory training using vibratory (i.e., stimulation of muscle spindle afferents using vibration frequencies around 80 Hz) stimulation of the affected hand muscles in people with musician’s dystonia reshaped the cortical sensorimotor organization toward a more differentiated pattern that was associated with improved hand motor control [Rosenkranz and others 2009]. Similarly, applying vibration to erector spinae muscles in chronic LBP patients significantly enhanced trunk motor control [Boucher and others 2015]. A shifted sensory representation in S1 of tactile input from the back was observed more than 20 years ago in a small group of chronic LBP patients by magnetencephalography [Flor and others 1997]. As discussed above, S1 (re)organization of proprioceptive input from paraspinal muscle spindles is likely to be more important pathophysiologically for the chronification of LBP than that of tactile input [Beaudette and others 2016].
The “Sensorial” Nature of M1: A Model of Cortical Reorganization in Chronic LBP?
Based on the “optimal control” model that uses internal models based on sensory feedback, M1 has been traditionally thought to send descending and `pure’ motor commands (through forward connections) to peripheral effectors to produce the desired movement [Genewein and Braun 2012; Wolpert and Kawato 1998]. However, it has been hypothesized that the brain recruits an alternative approach to handle more complex muscle recruitment patterns involved in complex and redundant systems such as trunk motor control [Adams and others 2013; Borich and others 2015]. In this “active inference” account of M1, M1 is suggested to model the proprioceptive consequences of motorneuron activity rather than to simply issue motor commands [Adams and others 2013]. The firing of alpha motoneurons would be determined by the comparison at the level of the spinal cord between descending proprioceptive predictions from M1 and the proprioceptive input from muscle spindle afferents to produce the desired (predicted) movement trajectory. If a prediction error is present, the discharge of alpha motoneurons is adapted until the prediction error is zero [Adams and others 2013].
Simultaneously, gamma motor neurons optimize the sensitivity of the muscle spindles. The proprioceptive information resulting from muscle activity is transmitted to the sensorimotor cortex (sensory reafference) for predictive coding: backward projections from M1 to S1 subserve proprioceptive predictions while forward projections from S1 to M1 convey potential prediction errors that report the difference between sensory information and prediction. Potential error signals received by M1 are then used to correct its representations so that its predictions improve [Adams and others 2013]. In support of an active inference role of M1, a study using functional electrical stimulation (FES; provokes proprioceptive signaling through sensory fiber stimulation that creates the impression of muscle extension), functional magnetic resonance imaging and dynamic causal modelling demonstrated that the M1 output and the neural communication between M1 and S1 were sensitive to artificially altered proprioceptive input during constant movement patterns [Gandolla and others 2014]. More specifically, during FES-induced alterations of proprioceptive signaling, a facilitatory effect on the intrinsic connectivity of M1 to S1 was observed (that was absent without FES), which was suggested to reflect the updating of sensory predictions sent to spinal motoneurons. In monkeys, M1 stimulation activated the biceps or triceps muscles differentially dependent on the degree of flexion of the monkey’s arm, further supporting a role of M1 efferents in conveying proprioceptive predictions [Graziano 2006].
An active inference role of M1 might have important implications regarding motor control adaptions in the presence of increased proprioceptive prediction errors that might originate from reduced/disrupted proprioceptive input, probably triggered by nociceptive input [Nijs and others 2012; Thunberg and others 2002], enhanced activation of the sympathetic nervous system [Radovanovic and others 2015], muscle fatigue [Taimela and others 1999], or reduced trunk motor variability [van Dieën and others 2017]. In principle, the central nervous system (CNS) can minimize prediction errors in two ways:(1) It can attempt to adapt the proprioceptive input by initiate/changing movements or redistribute muscle activity, therefore fulfilling proprioceptive predictions by spinal circuits or
(2) it can match its proprioceptive predictions (from M1) to the proprioceptive information inflow
[Adams and others 2013].
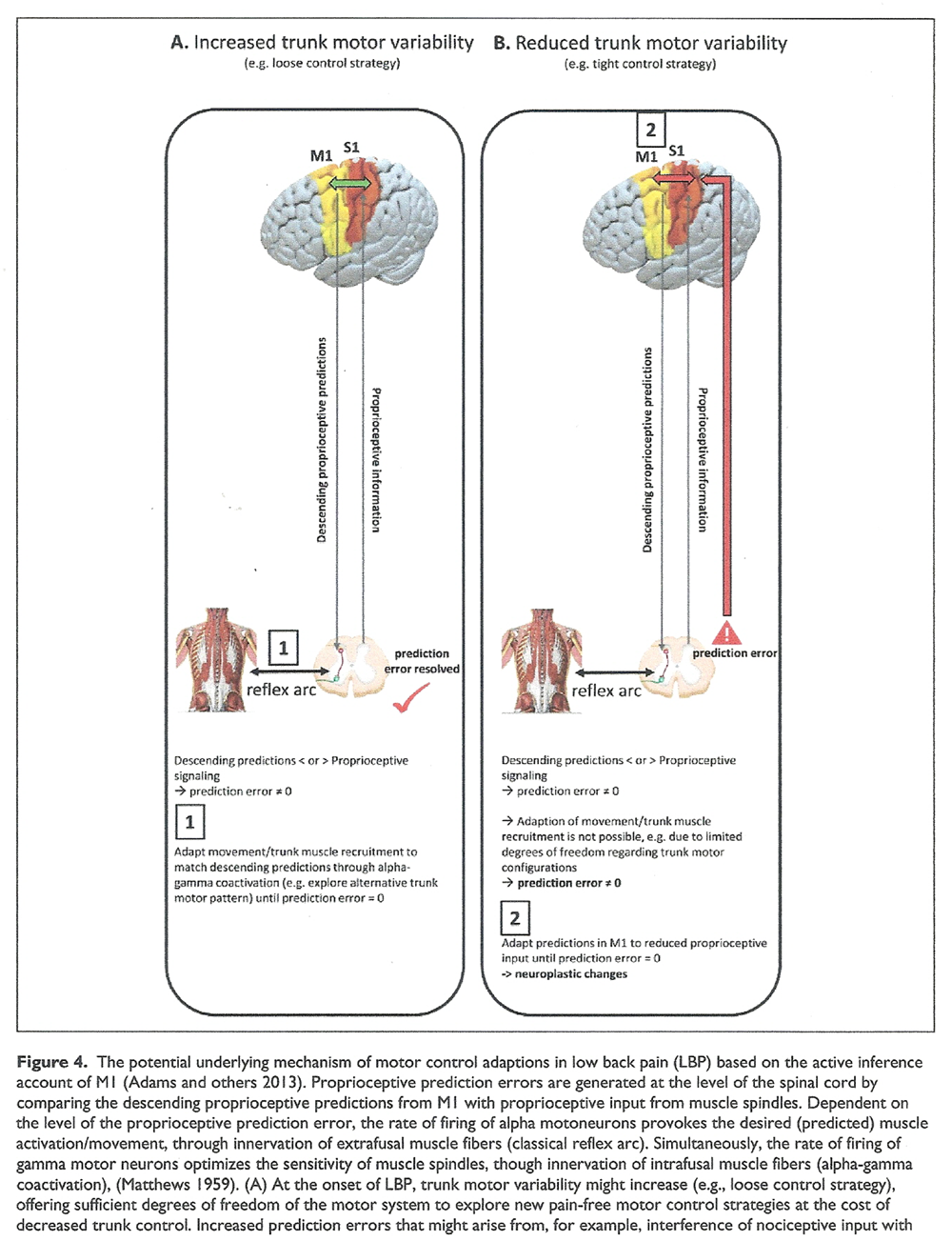
Figure 4

Figure 4 Text Generally, the CNS aims to prevent passing the prediction error to supraspinal circuits (and therefore avoiding a correction) as the spinal circuity ought to resolve any mismatch between descending predictions and afferent feedback using local reflex arcs [Adams and others 2013]. In the beginning, i.e. in acute LBP, the CNS might be able to suppress increasing proprioceptive prediction errors through movement/redistributing muscle activity and increasing trunk motor variability to explore alternative trunk motor recruitment patterns (Figutre 4A).
However, this approach might be limited when motor solutions become less variable, as shown during the transition from acute to recurrent or chronic LBP. In addition, adopting a tight control strategy might additionally limit trunk motor variability. Consequently, in this case, the CNS might be forced to minimize proprioceptive prediction errors by matching the descending predictions from M1 to the reduced or disrupted proprioceptive input, probably provoking neuroplastic adaptions in the long-term (Figure 4B). This might provide an explanation for cortical sensorimotor reorganization associated with a stable and more rigid but unfavorable motor control pattern, potentially leading to sustained increases in spinal loading, degeneration of spinal tissues, and muscle fatigue.
Psychological Factors Contributing to the Adaptions of Motor Control in LBP
Finally, considering recent theories about motor adaption from a learning perspective, the weighting of costs associated with LBP might not only be driven by nociceptive input or pain but also by pain-related cognitions [van Dieën and others 2017]. Deficits in motor control may be amplified or even be induced/generated by cognitiveemotional factors such as anticipation or fear of pain [Langevin and Sherman 2007; Tucker and others 2012]. In line with this, changes in motor unit discharge (recorded with fine-wire electrodes in the quadriceps muscle) during anticipation of pain were similar to changes provoked by experimental activation of nociceptors [Tucker and others 2012]. Furthermore, in chronic LBP, fear of pain has been shown to alter mechanical properties of the spine such as trunk stiffness [Karayannis and others 2013].
Moreover, it has been shown that individuals with high levels of pain catastrophizing tend to adopt a tight motor control strategy when a painful stimulus is applied, whereas those with low levels demonstrated a loose motor control strategy [Ross and others 2017]. In line with this, in healthy subjects, it has further been observed that negative pain cognitions are associated with a reduction in variability of postural strategies and stiffening of the spine (similar to those observed in chronic LBP) that outlasted the experimental pain [Moseley and Hodges 2006; Moseley and others 2004]. Therefore, sustained tightening of the trunk due to ongoing anticipation of pain constitutes an important factor that might contribute to the persistence of altered (tight) motor control, possibly leading to recurrent and chronic LBP in the long term due to increased spinal tissue loading, reduced paraspinal proprioceptive input and cortical reorganization.
Conclusions and Outlook
Research in the past two decades has provided important evidence how motor control adaptions in LBP might contribute to pain chronification through effects on spinal tissue loading, associated itself with degeneration of intervertebral discs and other tissues. However, the underlying biological and psychosocial interactions are still poorly understood and seem to vary across individuals, reflected in the modest effect sizes of motor control exercises, spurring a call for personalized interventional therapies [van Dieën and others 2018a]. Yet, to unleash the full potential of personalized treatments, more basic research on motor adaptions in LBP is mandatory, especially when considering the evolving evidence of cortical circuits in driving motor control adaptions during the course of LBP. Complementary findings from behavioral and neuroimaging studies underscore the prominent role of aberrant sensory processing in LBP.
By integrating novel research on the active inference account of M1, we propose a potential mechanism how proprioceptive impairments in LBP might ultimately force the CNS to change its sensorimotor cortical organization. Although the existence of different LBP phenotypes of motor control adaptions needs further validation, a tight motor control strategy (and probably maladaptive pain-related cognitions) might be more prone to cortical reorganization (compared with loose control) because of the more strongly reduced trunk motor flexibility limiting the ability of the CNS to increase motor variability and exploration of new motor control patterns. Further research efforts are necessary to clarify the functional relevance of cortical reorganization in chronic LBP: Does it simply represent an epiphenomenon of motor control adaption or is it causally related to the occurrence of recurrent and chronic LBP by reinforcing non-reversible motor control patterns? Neuroimaging might help to reveal the potential role of cortical markers (in particular, changes in cortical mapping of altered paraspinal proprioceptive input) of motor control adaptions at different stages of LBP by incorporating biomechanical (e.g., spine kinematics) and psychosocial (e.g., fear of movement-related pain) measures.
Interestingly, altered motor control patterns have been reported in other musculoskeletal pain condition, including neck pain [Meisingset and others 2015] and knee osteoarthritis [Tawy and others 2018]. Thus, it is possible that the mechanisms potentially contributing to the chronification of LBP discussed in this review might be important in musculoskeletal pain in general. Moreover, motor control adaptions might occur with pain irrespective of the tissue type initially involved [Schilder and others 2012]. It is then thinkable that, perhaps via similar mechanisms as described here, pain that initially was not musculoskeletal in nature leads to a secondary musculoskeletal pain problem. This would of course aggravate and complicate the clinical presentation of the patient, once more indicating the importance of advancing our understanding of the intricate interplay of pain and the motor system.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Andrea Vrana is generously supported by ChiroSuisse and the Foundation for the Education of Chiropractors in Switzerland.
References:
Abboud J, Daneau C, Nougarou F, Dugas C, Descarreaux M. 2018.
Motor adaptations to trunk perturbation: effects of experimental back pain and spinal tissue creep.
J Neurophysiol 120(4):1591–601Abboud J, Nougarou F, Pagé I, Cantin V, Massicotte D, Descarreaux M. 2014.
Trunk motor variability in patients with non-specific chronic low back pain.
Eur J Appl Physiol 114(12):2645–54.Adams MA, Roughley PJ. 2006.
What is intervertebral disc degeneration, and what causes it?
Spine 31(18):2151–61.Adams RA, Shipp S, Friston KJ. 2013.
Predictions not commands: active inference in the motor system.
Brain Struct Funct 218(3):611–43.Beaudette SM, Larson KJ, Larson DJ, Brown SH. 2016.
Low back skin sensitivity has minimal impact on active lumbar spine proprioception and
stability in healthy adults.
Exp Brain Res 234(8):2215–26.Boendermaker B, Meier ML, Luechinger R, Humphreys BK, Hotz-Boendermaker S. 2014.
The cortical and cerebellar representation of the lumbar spine.
Hum Brain Mapp 35(8):3962–71.Borich MR, Brodie SM, Gray WA, Ionta S, Boyd LA. 2015.
Understanding the role of the primary somatosensory cortex: opportunities for rehabilitation.
Neuropsychologia 79(pt B): 246–55.Boucher J-A, Abboud J, Nougarou F, Normand MC, Descarreaux M. 2015.
The effects of vibration and muscle fatigue on trunk sensorimotor control in low back
pain patients. vPLoS One 10(8):e0135838.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. 2000.
The role of paraspinal muscle spindles in lumbosacral position sense in individuals with
and without low back pain.
Spine 25(8):989–94.Brumagne S, Janssens L, Knapen S, Claeys K, Suuden-Johanson E. 2008.
Persons with recurrent low back pain exhibit a rigid postural control strategy.
Eur Spine J 17(9):1177–84.Burke D, Hagbarth KE, Löfstedt L, Wallin BG. 1976.
The responses of human muscle spindle endings to vibration during isometric contraction.
J Physiol (Lond) 261(3): 695–711.Chesler AT, Szczot M, Bharucha-Goebel D, ?eko M, Donkervoort S, 2016.
The role of PIEZO2 in human mechanosensation.
N Engl J Med 375(14):1355–64.Chiou SY, Shih YF, Chou LW, McGregor AH, Strutton PH. 2014.
Impaired neural drive in patients with low back pain.
Eur J Pain 18(6):794–802.Chiou S-Y, Gottardi SEA, Hodges PW, Strutton PH. 2016.
Corticospinal excitability of trunk muscles during different postural tasks.
PLoS One 11(1):e0147650.Chiou S-Y, Hurry M, Reed T, Quek JX, Strutton PH. 2018.
Cortical contributions to anticipatory postural adjustments in the trunk.
J Physiol (Lond) 596(7):1295–306.Christe G, Kade F, Jolles BM, Favre J. 2017.
Chronic low back pain patients walk with locally altered spinal kinematics.
J Biomech 60:211–8.Christe G, Redhead L, Legrand T, Jolles BM, Favre J. 2016.
Multi-segment analysis of spinal kinematics during sit-to-stand in patients with
chronic low back pain.
J Biomech 49(10):2060–7.Claeys K, Brumagne S, Dankaerts W, Kiers H, Janssens L. 2011.
Decreased variability in postural control strategies in young people with
non-specific low back pain is associated with altered proprioceptive reweighting.
Eur J Appl Physiol 111(1):115–23.Claeys K, Dankaerts W, Janssens L, Pijnenburg M, Goossens N, Brumagne S. 2015.
Young individuals with a more anklesteered proprioceptive control strategy may develop
mild nonspecific low back pain.
J Electromyogr Kinesiol 25(2):329–38.da Silva RA, Vieira ER, Fernandes KBP, Andraus RA, and others. 2018.
People with chronic low back pain have poorer balance than controls in challenging tasks.
Disabil Rehabil 40(11):1294–300.Del Santo F, Gelli F, Spidalieri R, Rossi A. 2007.
Corticospinal drive during painful voluntary contractions at constant force output.
Brain Res 1128(1):91–8.Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. 2008.
Spinal and supraspinal postural networks.
Brain Res Rev 57(1):212–21.Della Volpe R, Popa T, Ginanneschi F, Spidalieri R, Mazzocchio R, Rossi A. 2006.
Changes in coordination of postural control during dynamic stance in chronic
low back pain patients.
Gait Posture 24(3):349–55.Elgueta-Cancino E, Schabrun S, Danneels L, Hodges P. 2014.
A clinical test of lumbopelvic control.
Man Ther 19(5): 418–24.Elgueta-Cancino E, Schabrun S, Hodges P. 2018.
Is the organization of the primary motor cortex in low back pain related to pain,
movement, and/or sensation?
Clin J Pain 34(3):207–16.Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, and others. 2001.
Transient inhibition of the human motor cortex by capsaicin-induced pain.
A study with transcranial magnetic stimulation.
Neurosci Lett 314(1–2): 97–101.Flor H, Braun C, Elbert T, Birbaumer N. 1997.
Extensive reorganization of primary somatosensory cortex in chronic back pain patients.
Neurosci Lett 224(1):5–8.Gandevia SC, Proske U, Stuart DG, editors. 2002.
Sensorimotor control of movement and posture.
New York, NY: Kluwer Academic/Plenum.Gandolla M, Ferrante S, Molteni F, Guanziroli E, and others. 2014.
Re-thinking the role of motor cortex: context-sensitive motor outputs?
Neuroimage 91:366–74.Genewein T, Braun DA. 2012.
A sensorimotor paradigm for Bayesian model selection.
Front Hum Neurosci 6:291.Gilman S. 2002.
Joint position sense and vibration sense: anatomical organisation and assessment.
J Neurol Neurosurg Psychiatry 73(5):473–7.Goble DJ, Coxon JP, van Impe A, Geurts M, Doumas M, and others. 2011.
Brain activity during ankle proprioceptive stimulation predicts
balance performance in young and older adults.
J Neurosci 31(45):16344–52.Goble DJ, Coxon JP, van Impe A, Geurts M, van Hecke W, and others. 2012.
The neural basis of central proprioceptive processing in older versus younger adults:
an important sensory role for right putamen.
Hum Brain Mapp 33(4):895–908.Goodwin GM, McCloskey DI, Matthews PB. 1972.
The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions
of movement and by the effects of paralysing joint afferents.
Brain 95(4):705–48.Goossens N, Rummens S, Janssens L, Caeyenberghs K, Brumagne S. 2018.
Association between sensorimotor impairments and functional brain changes in patients
with low back pain: a critical review.
Am J Phys Med Rehabil 97(3):200–11.Graziano M. 2006.
The organization of behavioral repertoire in motor cortex.
Annu Rev Neurosci 29:105–34.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J et al.
What Low Back Pain Is and Why We Need to Pay Attention
Lancet. 2018 (Jun 9); 391 (10137): 2356–2367
This is the second of 4 articles in the remarkable Lancet Series on Low Back PainHemming R, Sheeran L, van Deursen R, Sparkes V. 2017.
Nonspecific chronic low back pain: differences in spinal kinematics in subgroups
during functional tasks.
Eur Spine J 27(1):163–70.Hodges PW. 2011.
Pain and motor control: from the laboratory to rehabilitation.
J Electromyogr Kinesiol 21(2):220–8.Hodges PW, Cholewicki J, van Dieën JH. 2013.
Spinal control.
Edinburgh, Scotland: Elsevier.Hodges PW, Galea MP, Holm S, Holm AK. 2009.
Corticomotor excitability of back muscles is affected by intervertebral disc lesion in pigs.
Eur J Neurosci 29(7):1490–500.Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. 2003.
Experimental muscle pain changes feedforward postural responses of the trunk muscles.
Exp Brain Res 151(2):262–71.Hodges PW, Tucker K. 2011.
Moving differently in pain: a new theory to explain the adaptation to pain.
Pain 152(3 suppl):S90–8.Hoy, D., L. March, P. Brooks, F. Blyth, A. Woolf, et al. (2014).
The Global Burden of Low Back Pain: Estimates from the Global Burden of Disease 2010 study
Ann Rheum Dis. 2014 (Jun); 73 (6): 968–974Itz CJ, Geurts JW, van Kleef M, Nelemans P.
Clinical Course of Non-specific Low Back Pain:
A Systematic Review of Prospective Cohort Studies Set in Primary Care
European Journal of Pain 2013 (Jan); 17 (1): 5–15Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O. 1993.
Rostrocaudal gradients in the neuronal receptive field complexity in the finger region
of the alert monkey’s postcentral gyrus.
Exp Brain Res 92(3):360–8.Karayannis NV, Smeets RJEM, van den Hoorn W, Hodges PW. 2013.
Fear of movement is related to trunk stiffness in low back pain.
PLoS One 8(6):e67779.Kavounoudias Roll JP, Anton JL, Nazarian B, Roth M, Roll R. 2008.
Proprio-tactile integration for kinesthetic perception: an fMRI study.
Neuropsychologia 46(2):567–75.Kim SS, Gomez-Ramirez M, Thakur PH, Hsiao SS. 2015.
Multimodal interactions between proprioceptive and cutaneous signals
in primary somatosensory cortex.
Neuron 86(2):555–66.Knox MF, Chipchase LS, Schabrun SM, Romero RJ, Marshall PWM. 2018.
Anticipatory and compensatory postural adjustments in people with low back pain:
a systematic review and meta-analysis.
Spine J. Epub Jun 12.Lafond D, Champagne A, Descarreaux M, Dubois J-D, Prado JM, Duarte M. 2009.
Postural control during prolonged standing in persons with chronic low back pain.
Gait Posture 29(3):421–7.Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, and others. 1996.
Gait of a deafferented subject without large myelinated sensory fibers below the neck.
Neurology 47(1):109–15.Langevin H.M., Sherman K.J.
Pathophysiological Model for Chronic Low Back Pain Integrating Connective Tissue
and Nervous System Mechanisms
Medical Hypotheses 2007 (Jan); 68 (1): 74–80Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, and others. 2001.
Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain.
Clin Neurophysiol 112(9):1633–41.Lee AS, Cholewicki J, Reeves NP, Zazulak BT, Mysliwiec LW. 2010.
Comparison of trunk proprioception between patients with low back pain and healthy controls.
Arch Phys Med Rehabil 91(9):1327–31.Lotz JC, Chin JR. 2000.
Intervertebral disc cell death is dependent on the magnitude and
duration of spinal loading.
Spine 25(12):1477–83.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. 1998.
Compression-induced degeneration of the intervertebral disc:
an in vivo mouse model and finite-element study.
Spine 23(23):2493–506.Lund JP, Donga R, Widmer CG, Stohler CS. 1991.
The painadaptation model: a discussion of the relationship between
chronic musculoskeletal pain and motor activity.
Can J Physiol Pharmacol 69(5):683–94.Madeleine P. 2010.
On functional motor adaptations: from the quantification of motor strategies
to the prevention of musculoskeletal disorders in the neck-shoulder region.
Acta Physiol (Oxf) 199(suppl 679):1–46.Madeleine P, Mathiassen SE, Arendt-Nielsen L. 2008.
Changes in the degree of motor variability associated with experimental and chronic
neck-shoulder pain during a standardised repetitive arm movement.
Exp Brain Res 185(4):689–98.Maher C, Underwood M, Buchbinder R. 2016.
Non-specific low back pain.
Lancet 389(10070):736–47Makin TR, Bensmaia SJ. 2017.
Stability of sensory topographies in adult cortex.
Trends Cogn Sci 21(3):195–204.Martuzzi R, van der Zwaag W, Farthouat J, Gruetter R, Blanke O. 2014.
Human finger somatotopy in areas 3b, 1, and 2: a 7T fMRI study using a natural stimulus.
Hum Brain Mapp 35(1):213–26.Massé-Alarie H, Schneider C. 2016.
Revisiting the corticomotor plasticity in low back pain: challenges and perspectives.
Healthcare (Basel) 4(3):E67Matthews PB. 1959.
A study of certain factors influencing the stretch reflex of the decerebrate cat.
J Physiol (Lond) 147(3):547–64.Meier ML, Hotz-Boendermaker S, Boendermaker B, Luechinger R, Humphreys BK. 2014.
Neural responses of posterior to anterior movement on lumbar vertebrae:
a functional magnetic resonance imaging study.
J Manipulative Physiol Ther 37(1):32–41.Meisingset I, Woodhouse A, Stensdotter A-K, Stavdahl Ø, and others. 2015.
Evidence for a general stiffening motor control pattern in neck pain:
a cross sectional study.
BMC Musculoskelet Disord 16:56.Moseley GL, Hodges PW. 2006.
Reduced variability of postural strategy prevents normalization of motor changes
induced by back pain: a risk factor for chronic trouble?
Behav Neurosci 120(2):474–6.Moseley GL, Nicholas MK, Hodges PW. 2004.
Does anticipation of back pain predispose to back trouble?
Brain 127(pt 10):2339–47.Naito E. 2004.
Sensing limb movements in the motor cortex: how humans sense limb movement.
Neuroscientist 10(1):73–82.Naito E, Nakashima T, Kito T, Aramaki Y, Okada T, Sadato N. 2007.
Human limb-specific and non-limb-specific brain representations during kinesthetic
illusory movements of the upper and lower extremities.
Eur J Neurosci 25(11):3476–87.Newcomer KL, Laskowski ER, Yu B, Johnson JC, An KN. 2000.
Differences in repositioning error among patients with low back pain compared
with control subjects.
Spine 25(19):2488–93.Nijs J, Daenen L, Cras P, Struyf F, Roussel N, Oostendorp RAB. 2012.
Nociception affects motor output: a review on sensory-motor interaction with focus
on clinical implications.
Clin J Pain 28(2):175–81.Panjabi MM. 1992.
The stabilizing system of the spine. Part I.
Function, dysfunction, adaptation, and enhancement.
J Spinal Disord 5(4):383–9; discussion 397.Panjabi MM. 2003.
Clinical spinal instability and low back pain.
J Electromyogr Kinesiol 13(4):371–9.Paul CPL, Schoorl T, Zuiderbaan HA, Zandieh Doulabi B, and others. 2013.
Dynamic and static overloading induce early degenerative processes in caprine
lumbar intervertebral discs.
PLoS One 8(4):e62411.Penfield. 1947.
Some observations on the cerebral cortex of man.
Proc R Soc Lond B Biol Sci 134(876):329–47.Petersen TH, Willerslev-Olsen M, Conway BA, Nielsen JB. 2012.
The motor cortex drives the muscles during walking in human subjects.
J Physiol (Lond) 590(10):2443–52.Powell TP, Mountcastle VB. 1959.
The cytoarchitecture of the postcentral gyrus of the monkey Macaca mulatta.
Bull Johns Hopkins Hosp 105:108–31.Proske U, Gandevia SC. 2012.
The proprioceptive senses: their roles in signaling body shape, body position
and movement, and muscle force.
Physiol Rev 92(4):1651–97.Radovanovic D, Peikert K, Lindström M, Domellöf FP. 2015.
Sympathetic innervation of human muscle spindles.
J Anat 226(6):542–8.Riemann BL, Lephart SM. 2002.
The sensorimotor system, part I: the physiologic basis of functional joint stability.
J Athl Train 37(1):71–9.Roland MO. 1986.
A critical review of the evidence for a pain-spasm-pain cycle in spinal disorders.
Clin Biomech (Bristol, Avon) 1(2):102–9.Rosenkranz K, Butler K, Williamon A, Cordivari C, Lees AJ, Rothwell JC. 2008.
Sensorimotor reorganization by proprioceptive training in musician’s dystonia
and writer’s cramp.
Neurology 70(4):304–15.Rosenkranz K, Butler K, Williamon A, Rothwell JC. 2009.
Regaining motor control in musician’s dystonia by restoring sensorimotor organization.
J Neurosci 29(46):14627–36.Ross GB, Sheahan PJ, Mahoney B, Gurd BJ, Hodges PW, Graham RB. 2017.
Pain catastrophizing moderates changes in spinal control in response to
noxiously induced low back pain.
J Biomech 58:64–70.Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. 1982.
Manual motor performance in a deafferented man.
Brain 105 (pt 3):515–42.Roux F-E, Djidjeli I, Durand J-B. 2018.
Functional architecture of the somatosensory homunculus detected by electrostimulation.
J Physiol (Lond) 596(5):941–56.Sainburg RL, Ghilardi MF, Poizner H, Ghez C. 1995.
Control of limb dynamics in normal subjects and patients without proprioception.
J Neurophysiol 73(2):820–35.Schabrun SM, Christensen SW, Mrachacz-Kersting N, Graven-Nielsen T. 2016.
Motor cortex reorganization and impaired function in the transition to
sustained muscle pain.
Cereb Cortex 26(5):1878–90.Schabrun SM, Elgueta-Cancino EL, Hodges PW. 2017.
Smudging of the motor cortex is related to the severity of low back pain.
Spine 42(15):1172–8.Schilder JCM, Schouten AC, Perez RSGM, Huygen FJPM, Dahan A, and others. 2012.
Motor control in complex regional pain syndrome: a kinematic analysis.
Pain 153(4):805–12.Sherrington CS. 1908.
The integrative action of the nervous system; with a new foreword by the
author & a bibliography of his writings.
London, England: Constable.Shojaei I, Salt EG, Hooker Q, van Dillen LR, Bazrgari B. 2017a.
Comparison of lumbo-pelvic kinematics during trunk forward bending and backward return
between patients with acute low back pain and asymptomatic controls.
Clin Biomech (Bristol, Avon) 41:66–71.Shojaei I, Vazirian M, Salt EG, van Dillen LR, Bazrgari B. 2017b.
Timing and magnitude of lumbar spine contribution to trunk forward bending and
backward return in patients with acute low back pain.
J Biomech 53:71–7.Silfies SP, Bhattacharya A, Biely S, Smith SS, Giszter S. 2009.
Trunk control during standing reach: a dynamical system analysis of movement strategies
in patients with mechanical low back pain.
Gait Posture 29(3):370–6.Silfies SP, Cholewicki J, Reeves NP, Greene HS. 2007.
Lumbar position sense and the risk of low back injuries in college athletes:
a prospective cohort study.
BMC Musculoskelet Disord 8:129.Sohn MK, Lee SS, Song HT. 2013.
Effects of acute low back pain on postural control.
Ann Rehabil Med 37(1):17–25.Stergiou N, Decker LM. 2011.
Human movement variability, nonlinear dynamics, and pathology:
is there a connection?
Hum Mov Sci 30(5):869–88.Strutton PH, Theodorou S, Catley M, McGregor AH, Davey NJ. 2005.
Corticospinal excitability in patients with chronic low back pain.
J Spinal Disord Tech 18(5):420–4.Sung W, Abraham M, Plastaras C, Silfies SP. 2015.
Trunk motor control deficits in acute and subacute low back pain are not
associated with pain or fear of movement.
Spine J 15(8):1772–82.Taimela S, Kankaanpää M, Luoto S. 1999.
The effect of lumbar fatigue on the ability to sense a change in lumbar position.
A controlled study.
Spine 24(13):1322–7.Tawy GF, Rowe P, Biant L. 2018.
Gait variability and motor control in patients with knee osteoarthritis
as measured by the uncontrolled manifold technique.
Gait & Posture 59:272–7.Thapa T, Graven-Nielsen T, Chipchase LS, Schabrun SM. 2018.
Disruption of cortical synaptic homeostasis in individuals with chronic low back pain.
Clin Neurophysiol 129(5):1090–6.Thiese MS, Hegmann KT, Wood EM, Garg A, Moore JS, and others. 2014.
Prevalence of low back pain by anatomic location and intensity in an occupational population.
BMC Musculoskelet Disord 15:283.Thunberg J, Ljubisavljevic M, Djupsjöbacka M, Johansson H. 2002.
Effects on the fusimotor-muscle spindle system induced by intramuscular injections
of hypertonic saline.
Exp Brain Res 142(3):319–26.Tong MH, Mousavi SJ, Kiers H, Ferreira P, Refshauge K, van Dieën J. 2017.
Is there a relationship between lumbar proprioception and low back pain?
A systematic review with meta-analysis.
Arch Phys Med Rehabil 98(1):120–36.e2.Tsao H, Danneels LA, Hodges PW. 2011a.
Individual fascicles of the paraspinal muscles are activated by discrete
cortical networks in humans.
Clin Neurophysiol 122(8):1580–7.Tsao H, Danneels LA, Hodges PW. 2011b.
ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain.
Spine 36(21):1721–7.Tsao H, Galea MP, Hodges PW. 2008.
Reorganization of the motor cortex is associated with postural control deficits
in recurrent low back pain.
Brain 131(pt 8):2161–71.Tsao H, Galea MP, Hodges PW. 2010.
Driving plasticity in the motor cortex in recurrent low back pain.
Eur J Pain 14(8):832–9.Tucker K, Larsson A-K, Oknelid S, Hodges P. 2012.
Similar alteration of motor unit recruitment strategies during the anticipation
and experience of pain.
Pain 153(3):636–43.Urban JPG, Roberts S. 2003.
Degeneration of the intervertebral disc.
Arthritis Res Ther 5(3):120–30.van Dieën JH, Flor H, Hodges PW. 2017.
Low-back pain patients learn to adapt motor behavior with adverse secondary consequences.
Exerc Sport Sci Rev 45(4):223–9.van Dieën JH, Reeves NP, Kawchuk G, van Dillen L, Hodges PW. 2018a.
Analysis of motor control in low-back pain patients: a key to personalized care?
J Orthop Sports Phys Ther. Jun 12 Epub.van Dieën JH, Reeves NP, Kawchuk G, van Dillen L, Hodges PW. 2018b.
Motor control changes in low-back pain: divergence in presentations and mechanisms.
J Orthop Sports Phys Ther. Jun 12 Epubvan Dieën JH, Selen LPJ, Cholewicki J. 2003.
Trunk muscle activation in low-back pain patients, an analysis of the literature.
J Electromyogr Kinesiol 13(4):333–51.van Tulder M, Becker A, Bekkering T, Breen A, Carter T, Gil del Real MT.
European Guidelines for the Management of Acute Nonspecific Low Back Pain in Primary Care
European Spine Journal 2006 (Mar); 15 Suppl 2: S169–191Vogt L, Pfeifer K, Portscher And M, Banzer W. 2001.
Influences of nonspecific low back pain on three-dimensional lumbar spine
kinematics in locomotion.
Spine 26(17):1910–9.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, and others. 2017.
Global, regional, and national incidence, prevalence, and years lived with disability
for 328 diseases and injuries for 195 countries, 1990-2016:
a systematic analysis for the Global Burden of Disease Study 2016.
Lancet 390(10100):1211–59.Willigenburg NW, Kingma I, Hoozemans MJM, van Dieën JH. 2013.
Precision control of trunk movement in low back pain patients.
Hum Mov Sci 32(1):228–39.Wolpert DM, Kawato M. 1998.
Multiple paired forward and inverse models for motor control.
Neural Netw 11(7–8):1317–29.Yamada H, Yaguchi H, Tomatsu S, Takei T, Oya T, Seki K. 2016.
Representation of afferent signals from forearm muscle and cutaneous nerves in the
primary somatosensory cortex of the macaque monkey.
PLoS One 11(10):e0163948.
Return to LOW BACK
Return to CHIROPRACTIC SUBLUXATION
Since 11-05-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |