

The Effect of Spinal Manipulation on Brain Neurometabolites
in Chronic Nonspecific Low Back Pain Patients:
A Randomized Clinical TrialThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Irish Journal of Medical Science 2020 (May); 189 (2): 543–550 ~ FULL TEXT
OPEN ACCESS Daryoush Didehdar, Fahimeh Kamali, Amin Kordi Yoosefinejad, Mehrzad Lotfi
Department of Physical Therapy,
School of Rehabilitation Sciences,
Shiraz University of Medical Sciences,
Shiraz, Iran.
BACKGROUND: In patients with chronic nonspecific low back pain (NCLBP), brain function changes due to the neuroplastic changes in different regions.
AIM: The current study aimed to evaluate the brain metabolite changes after spinal manipulation, using proton magnetic resonance spectroscopy (1H-MRS).
METHODS: In the current study, 25 patients with NCLBP aged 20-50 years were enrolled. Patients were randomly assigned to lumbopelvic manipulation or sham. Patients were evaluated before and 5 weeks after treatment by the Numerical Rating Scale (NRS), the Oswestry Disability Index (ODI), and 1H-MRS.
RESULTS: After treatment, severity of pain and functional disability were significantly reduced in the treatment group vs. sham group (p < 0.05). After treatment, N-acetyl aspartate (NAA) in thalamus, insula, dorsolateral prefrontal cortex (DLPFC) regions, as well as choline (Cho) in the thalamus, insula, and somatosensory cortex (SSC) regions, had increased significantly in the treatment group compared with the sham group (p < 0.05). A significant increase was further observed in NAA in thalamus, anterior cingulate cortex (ACC), and SCC regions along with Cho metabolite in thalamus and SCC regions after treatment in the treatment group compared with the baseline measures (p < 0.05). Also, a significant increase was observed in Glx (glutamate and glutamine) levels of thalamus (p = 0.03). There was no significant difference in terms of brain metabolites at baseline and after treatment in the sham group.
CONCLUSION: In the patient with low back pain, spinal manipulation affects the central nervous system and changes the brain metabolites. Consequently, pain and functional disability are reduced.
KEYWORDS: 1H-MRS; Low back pain; Spinal manipulation
From the Full-Text Article:
Introduction
Nonspecific chronic low back pain (NCLBP) is common disease in the lumbar area without any neurologic signs and specific reasons. [1] NCLBP accounts for 90% of the chronic low back pain (CLBP) cases. [2, 3] This disorder, which is associated with pain and disability, negatively affects the patient’s quality of life, productivity, and occupation. [2, 4] In chronic pains, after recovering from the early damage, pain is still perceived, demonstrating the role of changes in the function of the central nervous system(CNS). [5] According to the Tracey theory, pain is transformed from acute to chronic due to hypersensitivity of pain-processing network in CNS. [6] Hardy (1950) stated that chronic pain and hyperalgesia occurred following the initial damage due to increased CNS activity and its sensitization. [7] Therefore, chronic pain and prolonged sensory impairment following an injury is owing to the increased CNS excitability involving pain or sensitization. [5, 7] In addition, studies have shown that CLBP causes central sensitization [8, 9], and patients with CLBP are hypersensitive to painful stimuli due to neuroplastic changes. [10] However, in patients with NCLBP, following a physical activity, the blood flow increases in regions associated with pain matrix (SSC, insula, and frontal cortex) Moreover, in NCLBP patients compared with healthy individuals, neurochemical metabolites decrease in the DLPFC, thalamus, and orbitofrontal cortex. [2, 11–15]
To treat and resolve issues associated with NCLBP, medical treatments and physiotherapy are recommended. Their purpose is to reduce pain and disability caused by NCLBP. [10, 16, 17] Spinal manipulation is a successful, cost-effective, and non-invasive treatment for NCLBP. [16, 18] Researches have confirmed the effect of manipulation on function improvement and pain reduction in patients with NCLBP. [19, 20] The neurophysiological mechanism of manipulation refers to the ability of the central nervous system to modulate sensory information. [21]
To evaluate the effect of spinal manipulation on patients’ brain with NCLBP, modern imaging techniques, such as functional magnetic resonance imaging (fMRI), transcranial magnetic stimulation (TMS), and 1H-MRS, are used. [22] 1H-MRS is a non-invasive technique that measures the level of metabolites such as glutamate, glutamine, N-acetyl aspartate (NAA), creatine (Cr), myo-inositol, and choline (Cho) in the living human brain. [23] The NAA metabolite is synthesized in neurons and reflects the density of the neurons, and its reduction indicates the loss of the neuronal function. [24] Cho metabolite represents the health of neuron membranes, and its changes reflect upon the nervous system impairment. [24] Glutamate and glutamine (Glx) is the main excitatory neurotransmitter of the brain, binding to both ionotropic and metabotropic receptors that play an important role in pain. [24]
Studies have indicated neurometabolite changes in different regions of the brain (related to pain matrix area) in patients with chronic low back pain. [13, 15, 22, 25, 26] Certain studies suggest that neurobiological changes play important roles in the manifestation of chronic pain and its treatment. [13, 15, 22, 25–27]
There is limited knowledge about the neurobiological changes in pain-processing regions associated with chronic pain following manipulation therapy; accordingly, the aim of this study was to determine the effect of spinal manipulation therapy on the neurometabolites of the brain and neurochemical changes in neuronal surfaces using the 1H-MRS technique as a precise, reliable [28], and preferred method in patients with NCLBP. We hypothesized that the brain metabolite change after treatment by the spinal manipulation.
Materials and methods
Study design

Figure 1 The current randomized, double-blind, clinical trial study (IRCT20150923024149N15) was conducted on patients randomly divided into treatment and sham groups (Figure 1) (Consort follow chart). Written informed consent was obtained from all the participants.
Randomization
After public advertisement, 85 patients with low back pain, referring to clinics affiliated or non-affiliated to Shiraz University of Medical Sciences, were selected by simple sampling method. Out of 85 patients, 60 were excluded since they did not meet the inclusion criteria. Twenty-five patients were randomly assigned to either treatment (n = 10, six males and four females) or sham (n = 15, eight males and seven females) based on block randomization method (each block was 5) (Fig. 1). Patients and the examiner were blind to the group allocations.
Participants
Following the approval of the study protocol by the local Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1396.139), patients with NCLBP aged 20– 50 years were enrolled based on the following inclusion criteria: pain intensity ranging from 3 to 7 units based on the Numerical Rating Scale (NRS), and those who were not treated within the last 3 months. [29, 30] Exclusion criteria were patients diagnosed with neurologic, rheumatoid, or diabetic disorders; the ones with fractures of the ribs, vertebras, and pelvis, abdominal aortic aneurysm, neoplasm, peripheral neuropathy and radiculopathy, osteoporosis, fibromyalgia, pain in other areas of the body, three or more positive results of clinical tests on the sacroiliac joint (distraction, compression, thigh thrust, Gaenslen’s sign, FABER, and sacral thrust tests), and surgical history in lumbopelvic region; and those who used analgesics.
The sample size was set to eight subjects in each group based on previous studies as well as NAA and Glx metabolite level in the brain, considering d = 0.06, α = 0.05, and β = 0.2. [22, 26] Given the possibility of withdrawal and 20% rejection rate, 10 subjects were enrolled in the treatment group, while with 45%possibility of rejection rate in the sham group, 15 subjects were recruited.
Intervention
Patients in the treatment group received three sessions (every other day) of spinal lumbar and sacroiliac joint manipulation while those in the shamgroup underwent three sessions (every other day) of positioning similar to that of the manipulation therapy on lumbar and sacroiliac joint without applying the technique.

Figure 2 Sacroiliac joint manipulation: Patient is placed in a supine position on a treatment table, while the therapist is on the opposite side of the joint to be manipulated. While the patient interlocks their fingers behind their head, the therapist turns the patient’s upper trunk toward himself. He further puts another hand on the anterior superior iliac spine (ASIS) of the patient, which is far away from him and applies a highvelocity low-amplitude thrust, while the maximal rotation of the upper trunk is obtained (Figure 2b).
Manipulation of the lumbar spine: Patient is placed in a lateral position on the treatment table, while the therapist stands in front. The therapist bends the patient upper leg and places the patient’s ankle on the popliteal region of the lower leg. Then, the therapist takes the lower arm and shoulder of the patient and pulls it forward to make the upper side of the trunk rotate and bend. Next, the therapist puts one hand on the upper and anterior part of the patient’s chest while his other hand is on the patient pelvis to maintain patient’s position. In this situation, the therapist applies a highvelocity low-amplitude thrust to the patient’s pelvis in an anterior direction (Fig. 2a).Outcome measures
Demographic information including age, gender, weight, and height of the patients was recorded. Before starting the treatment course, both groups were asked not to use any medication and treatment during the study. To draw a comparison, the level of NAA metabolites in the brain (primary outcome measure), functional disability, and pain intensity (secondary outcome measure) was measured at baseline and 5 weeks post-treatment, since brain changes require more than 5 weeks. [11]
Pain intensity
The NRS with a scoring scale of 0 to 10 was used to determine pain intensity, with 10 and 0 representing maximum and no pain, respectively. A 2-unit reduction in pain based on the NRS was considered as minimally clinical important difference (MCID). [31]
Functional disability
The Persian version of the Oswestry Low Back Pain Disability Questionnaire (ODI) was used to examine the patients’ functional disability. [32] This questionnaire has 10 items scoring from 0 to 5; the maximum disability score is 50, and the values are expressed as percentage. This questionnaire is reliable for the evaluation of post-treatment clinical changes in patients with CLBP; a 10% reduction in the reported score is considered as MCID. [33]
Measure of brain metabolites

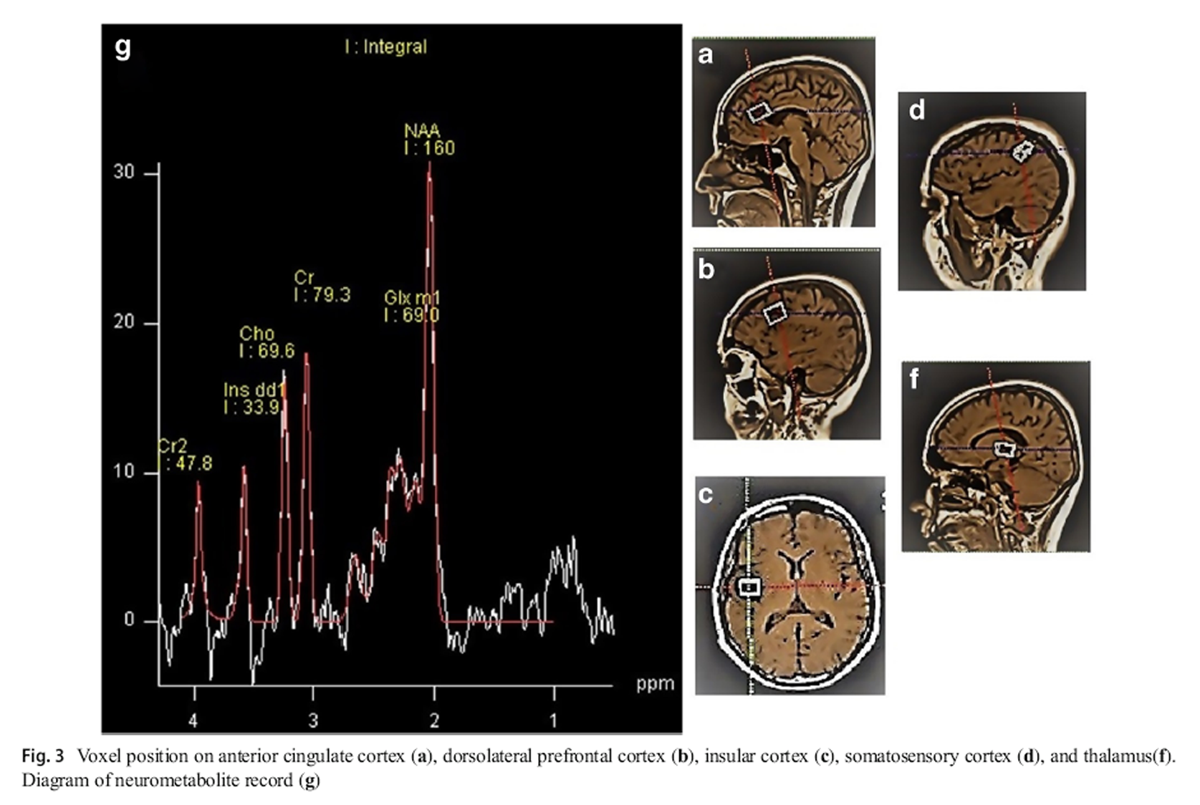
Figure 3 To recordMRS, we employed a single-voxel 1.5-T MRI scanner (Magnetron Avanto TIM, version B19, Siemens, Germany), using a point-resolved spectroscopy (PRESS) technique with pulse sequence (TE, 30 ms; TR, 1500 ms). Magnetic field homogeneity was optimized for the selected spectroscopy volume by manual shimming. The resulting peak width of water at half-maximum was 9 Hz for all voxels. The voxel 10 × 22 × 14 mm was used to define the anterior cingulate cortex (ACC) (Figure 3a), the voxel 21 × 17 × 18 mm to define the left DLPFC (Fig. 3b), the voxel 10 × 22 × 14 mm to define the left-insular cortex (Fig. 3c), the voxel 10 × 15 × 14 mm to define the left primary somatosensory cortex (Fig. 3d), and the voxel 10 × 20 × 13 mm to define the left thalamus (Fig. 3f). Accordingly, the levels of NAA, Cho, Cr metabolites, and Glx (glutamate and glutamine) were measured and compared with the level of Cr as the internal standard (Fig. 3g). [26]
Statistical analysis
Data are analyzed via the SPSS version 23 software, using non-parametric Mann-Whitney tests to compare the mean difference between the two groups and the Wilcoxon test to compare the mean before and after the treatment in each group. Data are expressed as mean and standard deviation (SD). p values of < 0.05 were considered statistically significant.
Results

Table 1 Twenty-five patients were randomly assigned to treatment and sham groups. During the study, two patients in the sham group were excluded due to usage of analgesics. At baseline (before intervention) and 5 weeks following treatment, data related to pain, functional disability, and brain neurometabolite were recorded and analyzed (Fig. 1). There was no significant difference between the groups at baseline regarding demographic characteristics (Table 1).
Pain and functional disability

Table 2 According to Table 2, there was no significant difference in NRS and ODI between the groups at baseline; 5 weeks after the treatment, the difference became significant and more than MCID. Moreover, 5 weeks after the treatment, the NRS and ODI of the treatment group were reduced by 3.6 and 16 units, respectively (which is more than MCID), while there was no MCID difference in the sham group (Table 2).
Brain metabolites
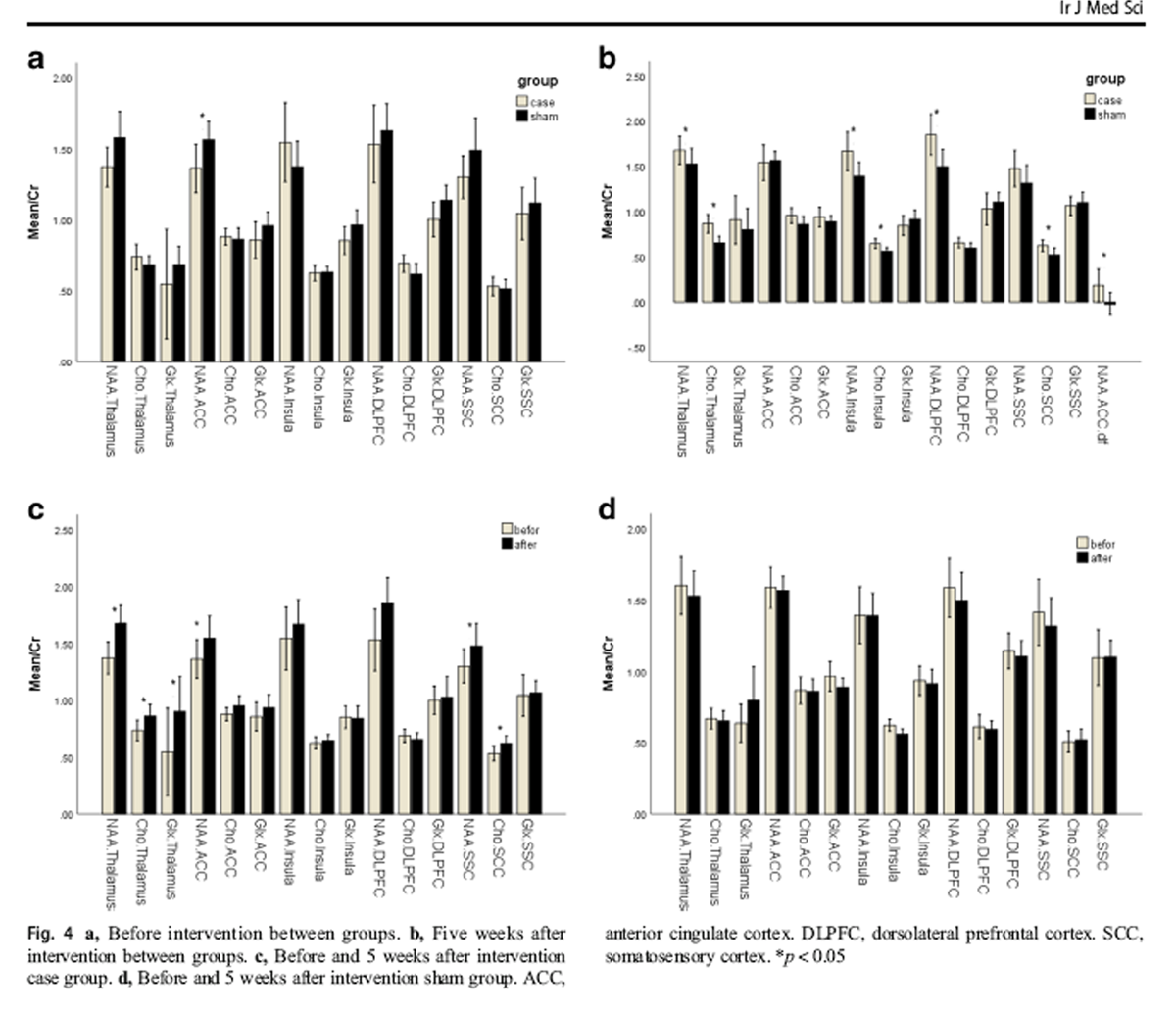
Figure 4 At baseline, the NAA level in the ACC region of the treatment group (1.36 ± 0.23) was significantly lower than that of the sham group (1.56 ± 0.23), but no significant difference was observed among other regions of the brain in terms of metabolites (Figure 4a). Five weeks after the treatment, the NAA metabolite increased in the ACC region of the treatment group (1.54 ± 0.27), with significant mean difference between the two groups (p = 0.04) (Figure 4b). In addition, 5 weeks after the treatment, the NAA metabolite in the thalamus, insula, and DLPFC, and Cho metabolite in thalamus, insula, and SSC were significantly higher in the treatment group than in the sham group (Fig. 4b).
In the treatment group, NAA metabolite significantly increased in the thalamus, ACC, and SSC and Chometabolite in thalamus and SSC regions 5 weeks after the treatment compared with the baseline measures (Fig. 4c). Furthermore, in the treatment group, Glx metabolite in thalamus significantly increased following the treatment compared with the baseline measures (Fig. 4c). The metabolites of the brain in the sham group did not undergo a significant change before and 5 weeks after the treatment (Fig. 4d).
Discussion
The results of this study demonstrated that in patients with NCLBP, 5 weeks after lumbopelvic manipulation, pain and functional disability were reduced significantly and clinically. Moreover, a significant increase of metabolite concentrations was observed (1) 5 weeks after the treatment for the case group and (2) between groups, mostly after the intervention.
In chronic pain, neurophysiologic and brain metabolite changes are an indication of neuroplasticity in CNS [5, 7, 34–36]. Additionally, chronic pain is the result of increased irritability of CNS involved in pain or sensitization. [5, 7] Furthermore, in patients with NCLBP, blood flow increases during physical activity in regions associated with pain matrix (somatosensory cortex, insula, and frontal lobe of the cortex). [11] Studies have further demonstrated that the level of neurochemical metabolites (NAA, Glx, and Cho) was decreased in DLPFC, thalamus, and orbitofrontal cortex in patients with NCLBP compared with healthy people. [2, 11–15]
The neurophysiologic mechanism of the manipulation is the result of the CNS’s ability to modulate sensory information [21, 37]. Sensory afferents from the receptors of the tissue surrounding the vertebrae are activated by spinal manipulation, affecting the transmission of painful signals and motor function from the spinal cord to the cerebral cortex. [21, 37] Studies have evaluated the effect of spinalmanipulation on the brain pain matrix regions of patients with low back pain by fMRI, showing that functional activity in these regions increased following the spinal manipulation. [38, 39] Murphy and Hawick Taylor (2007) reported that cortical processing after manipulation improved function and reduced pain. [40] Gussew et al. (2011) examined areas associated with pain matrix (i.e., thalamus, insula, and ACC) in patients with low back pain and reported that reduced levels of Glx indicated impaired glutamatergic neurotransmission due to prolonged pain perception, while decreased NAA and Cho levels reflected the impaired function of glial cells and neurons. [22]
The results of this study showed that following spinal manipulation in the treatment group, NAA was significantly elevated in thalamus, ACC, and SSC, as well as Cho in thalamus and SSC compared with the baseline measures. In addition, in the treatment group, Glx significantly increased in thalamus following spinal manipulation treatment compared with the baseline level, which was associated with decreased pain and disability. In general, spinal column dysfunction affects CNS and changes the afferents put into CNS, causing CNS plasticity following injury and pain. [41] Furthermore, spinal manipulation can alter CNS processing and sensorymotor integration; therefore, neuroplastic changes reflect a mechanism to reduce pain and improve functional ability after spinal manipulation. [41]
Studies have shown that spinal manipulation in patients with CLBP reduces pain and improves functional ability during a 3-to-6-month follow-up. [42–44] Additionally, in patients with NCLBP, lumbopelvic manipulation decreased pain and improved function with 1 to 6 months of follow-up, similar to the results obtained from other therapeutic methods, such as exercise therapy and physiotherapy. [10, 16, 17] The current study results are in line with the aforementioned studies, stating that in the treatment group, pain was reduced and functional disability was ameliorated 5 weeks after lumbopelvic manipulation compared with the baseline measures. Pain relief and improved functional disability are associated with improvement in the neurometabolites of the brain, demonstrating the impact of spinal manipulation on the brain, and accordingly, pain relief and disability reduction.
The current study was the first to investigate the metabolites of the brain following lumbopelvic manipulation in patients with Nonspecific chronic low back pain (NCLBP). The limitations of the current study were its high cost, being time-consuming, and 1.5-T magnetic field strength MRI. It is suggested that 3-T MRI be employed in future studies to measure glutamine and glutamate levels separately. Furthermore, another limitation is that we did not record psychosocial information to evaluate its relationship to changes of metabolites and pain. It is further recommended that the effect of other treatments (thermal therapy, physical therapy, exercise therapy, acupuncture) with spinal manipulation be evaluated on CNS by the 1H-MRS technique in patients with nonspecific chronic low back pain (NCLBP).
Conclusion
Spinal manipulation with effect on CNS leads to change and increase metabolite concentrations in specific brain regions, thereby, relieving pain and disability following chronic low back pain.
Acknowledgments
This article was extracted from the Physiotherapy Ph.D. thesis (1396-01-06-14881) from Shiraz University of Medical Sciences. The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Author contribution
All authors designed the study. Daryoush Didehdar collected and analyzed the data. All authors discussed the results and commented on the manuscript. All authors have carefully read and reviewed the manuscript.
References:
Kamper SJ, Apeldoorn A, Chiarotto A, Smeets R, Ostelo R, Guzman J, Van Tulder M (2015)
Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis.
Bmj 350:h444Zhao X, Xu M, Jorgenson K, Kong J (2017)
Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: a systematic review.
NeuroImage: Clin 13:33–38Koes B, Van Tulder M, Thomas S (2006)
Diagnosis and Treatment of Low Back Pain
British Medical Journal 2006 (Jun 17); 332 (7555): 1430–1434Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA (2009)
Comprehensive review of epidemiology, scope, and impact of spinal pain.
Pain physician 12(4):E35–E70Coderre TJ, Katz J, Vaccarino AL, Melzack R (1993)
Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence.
Pain 52(3):259–285Tracey I, Bushnell MC (2009)
How neuroimaging studies have challenged us to rethink: is chronic pain a disease?
J pain 10 (11):1113-1120Hardy JD, Wolff HG, Goodell H (1950)
Experimental evidence on the nature of cutaneous hyperalgesia.
J Clin Invest 29(1):115–140O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L (2007)
Generalized deep-tissue hyperalgesia in patients with chronic low-back pain.
Eur J Pain 11(4):415–420.
https://doi.org/10.1016/j.ejpain.2006.05.009Giesecke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Williams DA, Clauw DJ (2004)
Evidence of augmented central pain processing in idiopathic chronic low back pain.
Arthritis Rheum 50 (2):613-623. Doi:doi:
https://doi.org/10.1002/art.20063Castro-Sánchez AM, Lara-Palomo IC, Matarán-Peñarrocha GA, Fernández-de-las-Peñas C (2016)
Short-term effectiveness of spinal manipulative therapy versus functional technique in patients with chronic nonspecific low back pain: a pragmatic randomized controlled trial.
Spine J 16(3):302–312Kregel J, Meeus M, Malfliet A, Dolphens M, Danneels L, Nijs J, Cagnie B (2015)
Structural and functional brain abnormalities in chronic low back pain: a systematic review.
Semin Arthritis Rheum 45(2):229–237Grachev I, Fredrickson B, Apkarian A (2002)
Brain chemistry reflects dual states of pain and anxiety in chronic low back pain.
J Neural Transm 109(10):1309–1334Grachev I, Ramachandran T, Thomas P, Szeverenyi N, Fredrickson B (2003)
Association between dorsolateral prefrontal N-acetyl aspartate and depression in chronic back pain: an in vivo proton magnetic resonance spectroscopy study.
J Neural Transm 110(3):287–312Grachev ID, Fredrickson BE, Apkarian AV (2000)
Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study.
Pain 89(1):7–18Siddall PJ, Stanwell P, Woodhouse A, Somorjai RL, Dolenko B, Nikulin A, Bourne R (2006)
Magnetic resonance spectroscopy detects biochemical changes in the brain associated with chronic low back pain: a preliminary report.
Anesth Analg 102(4):1164–1168Rubinstein SM, van Middelkoop M, Assendelft WJ, de Boer MR, van Tulder MW (2011)
Spinal manipulative therapy for chronic low-back pain.
Cochrane Database Syst Rev (2):Cd008112.
DOI:DOI: https://doi.org/10.1002/14651858.CD008112.pub2Chown M, Whittamore L, Rush M, Allan S, Stott D, Archer M (2008)
A prospective study of patients with chronic back pain randomised to group exercise, physiotherapy or osteopathy.
Physiotherapy 94(1):21–28Globe, G, Farabaugh, RJ, Hawk, C et al.
Clinical Practice Guideline:
Chiropractic Care for Low Back Pain
J Manipulative Physiol Ther. 2016 (Jan); 39 (1): 1–22Hidalgo, B, Detrembleur, C, Hall, T, Mahaudens, P, and Nielens, H.
The Efficacy of Manual Therapy and Exercise for Different Stages of Non-specific Low Back Pain:
An Update of Systematic Reviews
J Man Manip Ther. 2014 (May); 22 (2): 59–74Orrock PJ, Myers SP (2013)
Osteopathic intervention in chronic non-specific low back pain: a systematic review.
BMC Musculoskelet Disord 14(1):129Haavik H, Murphy B (2012)
The Role of Spinal Manipulation in Addressing Disordered Sensorimotor Integration
and Altered Motor Control
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 768–776Gussew A, Rzanny R, Güllmar D, Scholle H-C, Reichenbach JR (2011)
1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain.
Neuroimage 54(2):1315–1323Harris RE, Clauw DJ (2012)
Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy.
Neurosci Lett 520(2):192–196.
https://doi.org/10.1016/j.neulet.2012.03.042Schmidt-Wilcke T (2015)
Neuroimaging of chronic pain.
Best Pract Res Clin Rheumatol 29(1):29–41Sharma NK, Brooks WM, Popescu AE, VanDillen L, George SZ, McCarson KE (2012)
Neurochemical analysis of primary motor cortex in chronic low back pain.
Brain sci 2(3):319–331Yabuki S, S-i K, S-i K (2013)
Assessment of pain due to lumbar spine diseases using MR spectroscopy: a preliminary report.
J Orthop Sci 18(3):363–368Lin A, Ramadan S, Stanwell P, Luu T, Celestin J, Bajwa Z, Mountford C (2010)
In vivo L-COSY MR distinguishes glutamate from glutamine and shows neuropathic pain to cause a build up of glutamine in the brain.
Proc Int Soc Magn Reson Med 18:381Plitman E, Chavez S, Nakajima S, Iwata Y, Chung JK, Caravaggio F, Kim J, Alshehri Y (2018)
Striatal neurometabolite levels in patients with schizophrenia undergoing long-term antipsychotic treatment: a proton magnetic resonance spectroscopy and reliability study.
Psychiatry Res Neuroimaging 273:16–24Kamali F, Shokri E (2012)
The effect of two manipulative therapy techniques and their outcome in patients with sacroiliac joint syndrome.
J Bodyw Mov Ther 16(1):29–35Kent P, Keating JL (2005)
Classification in nonspecific low back pain: what methods do primary care clinicians currently use?
Spine 30(12):1433–1440Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR.
Clinical Importance of Changes in Chronic Pain Intensity
Measured on an 11-point Numerical Pain Rating Scale
Pain 2001 (Nov); 94 (2): 149-158Mousavi SJ, Parnianpour M, Mehdian H, Montazeri A, Mobini B (2006)
The Oswestry disability index, the Roland-Morris disability questionnaire, and the Quebec back pain disability scale: translation and validation studies of the Iranian versions.
Spine 31(14):E454–E459Davidson M, Keating JL (2002)
A comparison of five low back disability questionnaires: reliability and responsiveness.
Phys Ther 82(1):8–24May A (2008)
Chronic pain may change the structure of the brain.
Pain 137(1):7–15Wand BM, Parkitny L, O’Connell NE, Luomajoki H.
Cortical Changes in Chronic Low Back Pain: Current State of the Art and Implications for Clinical Practice
Man Ther. 2011 (Feb); 16 (1): 15-20Alleva J, Hudgins T, Belous J, Origenes AK (2016)
Chronic low back pain.
Disease-a-Month. 9(62):330–333Pickar JG, Bolton PS.
Spinal Manipulative Therapy and Somatosensory Activation
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 785–794Pickar JG.
Neurophysiological Effects of Spinal Manipulation
Spine J (N American Spine Society) 2002 (Sep); 2 (5): 357–371Yuan W, Shen Z, Xue L, Tan W, Cheng Y, Zhan S, Zhan H (2015)
Effect of spinal manipulation on brain functional activity in patients with lumbar disc herniation.
J Zhejiang Univ Med Sci 44(2):124–130Kanovský P, Bareš M, Rektor I (2003)
The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings.
Clin Neurophysiol 114(6):981–991Taylor H. H., Murphy B.
Altered Central Integration of Dual Somatosensory Input After Cervical Spine Manipulation
J Manipulative Physiol Ther. 2010 (Mar); 33 (3): 178–188Gedin F, Dansk V, Egmar A-C, Sundberg T, Burström K (2018)
Patient-reported Improvements of Pain, Disability, and Health-related Quality of Life
Following Chiropractic Care for Back Pain - A National Observational Study in Sweden
J Bodyw Mov Ther. 2019 (Apr); 23 (2): 241–246Coulter ID, Crawford C, Hurwitz EL, Vernon H, Khorsan R, Suttorp Booth M, Herman PM.
Manipulation and Mobilization for Treating Chronic Low
Back Pain: A Systematic Review and Meta-analysis
Spine J. 2018 (May); 18 (5): 866–879Licciardone JC, Gatchel RJ, Aryal S (2016)
Recovery from chronic low back pain after osteopathic manipulative treatment: a randomized controlled trial.
J Am Osteopath Assoc 116(3):144–155
Return to LOW BACK PAIN
Return to CHIROPRACTIC SUBLUXATION
Since 12-02-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |