

Dietary Polyunsaturated Fatty Acids:
Impact on Cancer Chemotherapy and RadiationThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Alternative Medicine Review 2002 (Feb); 7 (1): 4–21 ~ FULL TEXT
Kenneth A. Conklin, MD, PhDAbstract
Preclinical studies have shown that certain polyunsaturated fatty acids may actually enhance the cytotoxicity of several antineoplastic agents and the anticancer effects of radiotherapy. These effects are possibly mediated by incorporation of the polyunsaturated fatty acids into cancer cell membranes, thus altering the physical and functional properties. In addition, certain polyunsaturated fatty acids may also reduce or prevent some of the side effects of these therapies, and administering antioxidants to prevent polyunsaturated fatty acid-induced oxidative stress may further enhance the impact of chemotherapy and radiation.
Introduction
Interpreting Reactive Oxygen Species (ROS) Mediated Mechanisms

Table 1 Interpreting the results of studies designed to assess the impact of polyunsaturated fatty acids (PUFAs) on chemotherapy and radiation is difficult because PUFAs alone can affect cancer cell growth and viability. PUFAs create oxidative stress (Table 1) in biological systems as they undergo lipid peroxidation, forming free radicals such as peroxyl and alkoxyl radicals. Although these lipid hydroperoxides are relatively short-lived, their breakdown results in the formation of secondary products of lipid peroxidation (aldehydes such as malondialdehyde and the 4-hydroxyalkenals) that are longer-lived and can attack a variety of cellular targets.
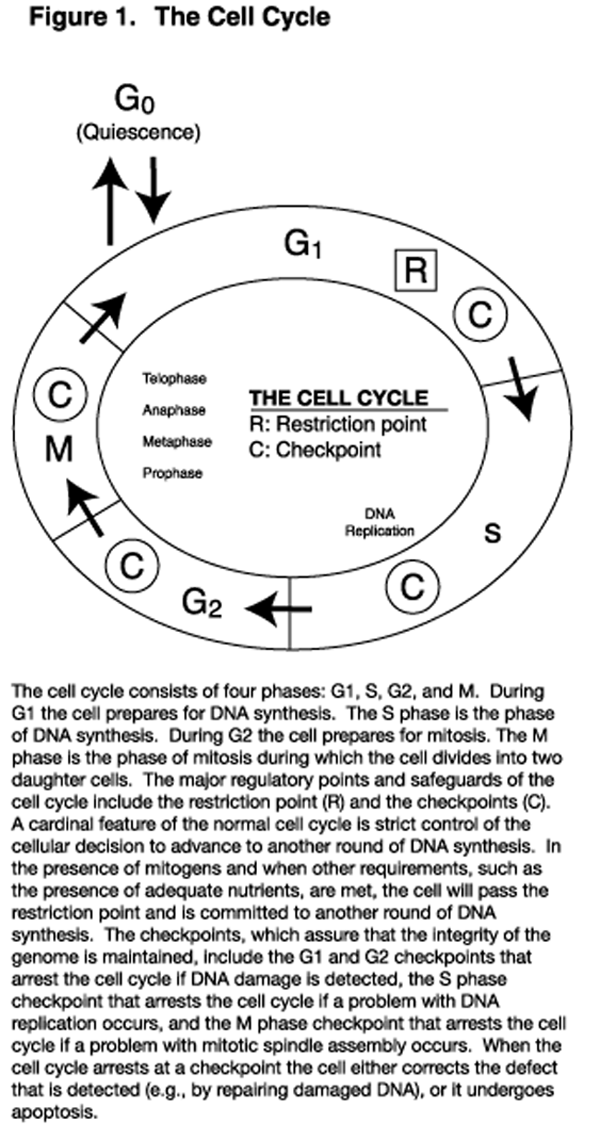
Figure 1 Low concentrations of these aldehydes affect the cell cycle (Figure 1) in ways that reduce the rate of cell proliferation. These effects include inhibiting the transition of cells from the G0 phase to the G1 phase, prolonging the G1 phase, slowing progression through the S phase by inhibiting the activity of DNA polymerases, inhibiting cell cycle progression through the restriction point, and causing arrest at cycle cell checkpoints. [1,2] These effects that retard cell cycle progression will impact proliferating cells such as those in culture and those of certain animal tissues, including neoplasms, bone marrow, and the intestinal epithelium. Whereas low-level PUFA-induced oxidative stress is cytostatic, higher levels of oxidative stress result in apoptosis (programmed cell death), and still higher levels cause cellular necrosis. [3–5]
Many investigators have demonstrated that omega-6 (n–6) and omega-3 (n–3) PUFAs including linoleic acid (LA), gamma-linolenic acid (GLA), dihommogamma-linolenic acid (DGLA), arachidonic acid (AA), alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) inhibit growth and are cytotoxic to cancer cells in vitro; [6–15] that the effects are associated with the production of lipid peroxides and aldehydes; [8–13] and that the cytotoxicity of the added PUFAs is reduced by the addition of antioxidants. [8–13] Studies with laboratory animals have also demonstrated that feeding a diet containing peroxidation products of fish oil1 [6] reduces tumor growth, and that the effect is reduced by administering antioxidants. [17,18]
However, the effects in vitro are observed at PUFA concentrations (30 microM and above in most studies) exceeding normal plasma free fatty acid (FFA) levels. PUFAs in culture medium undergo lipid peroxidation more readily than those of plasma or tissues because: (1) culture medium, compared to plasma, contains lower levels of albumin that binds FFAs [19] and sequesters iron and copper that promote lipid peroxidation; (2) culture medium generally contains fewer antioxidants than plasma; (3) PUFAs in plasma lipoproteins are protected by antioxidants within the lipoproteins; and (4) cellular PUFAs are protected from lipid peroxidation by multiple antioxidants. Additionally, growth inhibition in vitro does not necessarily correlate with the degree of lipid peroxidation [13] and antioxidants preventing lipid peroxidation in vitro do not completely reverse the effects of certain PUFAs on cell growth. [11, 12, 14]
Researchers found that administering LA without antioxidants also reverses the suppressive effects of fish oil on the growth of colon adenocarcinoma in mice. [20] Further research has found that preventing lipid peroxidation in experimental diets by the addition of antioxidants does not interfere with the growth inhibitory effects of fish oil on primary tumor growth or the development of metastases in nude mice with transplanted human breast and prostate cancer cells. [21–23] These results suggest PUFAs are cytostatic and cytotoxic in vitro and in vivo when conditions allow lipid peroxidation to occur, but that certain PUFAs in the absence of oxidative stress also have inhibitory effects on tumor cell growth.
Interpreting Results of Non-ROS Mediated Mechanisms
A mechanism whereby certain PUFAs inhibit cancer cell growth in the absence of oxidative stress is alteration of eicosanoid production. Considerable data [24–31] describes the role of AA-derived eicosanoids in processes that are necessary for or enhance tumor growth and metastasis. Animal studies show dietary supplementation with LA that elevates the generation of AA-derived eicosanoids in herbivorous rodents is associated with cancer promotion, tumor cell invasion, angiogenesis, and cancer metastasis. These effects appear to be mediated, in part, by the interaction of AA-derived eicosanoids with growth factors and oncogenes, [24, 25, 27, 28, 30, 31] and their effects on protein kinases. [26, 31] Administration of long-chain n-3 PUFAs (EPA and DHA) is associated with suppression of these processes via modulation of eicosanoid synthesis. In vitro studies, using low FFA concentrations and experimental conditions nonconducive to lipid peroxidation, support the contention that cancer cell growth is enhanced by AA-derived eicosanoids and that their effects are counteracted by long-chain n–3 PUFAs. [19, 32, 33]
It has also been shown that tumor cells produce a potent mitogenic compound from LA (13-hydroxyoctadecadienoic acid) via a lipoxygenase pathway, and that n–3 PUFAs inhibit cellular uptake of LA, thereby reducing the rate of cell proliferation. [34] Additionally, incorporation of EPA, DHA, and GLA into cancer cell membranes may alter cancer growth and metastasis by mechanisms independent of their effects on eicosanoid synthesis. Such mechanisms include alteration of surface receptors or signaling proteins of the cell membrane, initiating cell cycle arrest of apoptosis; [15] alteration of cancer cell adhesion; and enhancement of tight junction function. [35] Thus, suppressing the synthesis of AA-derived eicosanoids or alteration of cell membrane function by EPA, DHA, or GLA can alter the growth of cancer cells and potentially lead to misinterpretation of the impact of PUFAs on the effects of cytotoxic anticancer agents and radiation.
An additional factor that complicates interpretation of results of animal and human studies is the potential of fish oil to prolong survival by attenuating cancer cachexia. This effect of the long-chain n–3 PUFAs, demonstrated in laboratory animals [36, 37] and humans, [38, 40] is associated with suppression of pro- inflammatory cytokine synthesis and elaboration of acute-phase proteins. Fish oil has also been shown to enhance immune function in malnourished individuals with advanced cancer. [41] Thus, EPA and DHA supplementation during chemotherapy or radiation may prolong survival without directly altering cancer progression or the impact of therapy.
Although the above factors increase the difficulty of interpreting results from in vitro and in vivo experiments, considerable data support the contention that certain PUFAs, in addition to their inherent ability to suppress tumor cell proliferation, also enhance the response to cytotoxic chemotherapy and radiation.
In Vitro Studies

Table 2 The impact of PUFAs on the sensitivity to antineoplastic agents has been investigated in several neoplastic cell lines of laboratory animal and human origin. Two experimental designs have been employed. In the first, established cultures of cells are exposed simultaneously to a PUFA and an antineoplastic agent. Data from such experiments can be misleading since lipid peroxidation products are cytostatic and cytotoxic; and anticancer drugs that generate oxidative stress in biological systems can enhance this effect. These drugs (Table 2) include most anthracyclines (doxorubicin, epirubicin, and idarubicin, but not mitoxantrone), the epipodophyllotoxins (etoposide and teniposide), the camptothecins (topotecan and irinotecan), the platinum coordination complexes (cisplatin and carboplatin), the bleomycins, and certain alkylating agents. [42–45] Although oxidative mechanisms do not account for the antineoplastic activities of these agents, [46–48] an apparent enhancement of their cytotoxicity when they are combined with PUFAs in culture can be accounted for by the drugs' induction of oxidative stress, which enhances the oxidation of PUFA. Although some antineoplastic agents do not induce oxidative stress (taxanes such as paclitaxel and docetaxel; vincal alkaloids such as vincristine and vinblastine; antimetabolites; and purine and pyrimidine analogues), results of studies combining the agents with PUFAs may also be difficult to interpret when PUFAs are used in concentrations that are cytostatic or cytotoxic.
In the second experimental design, cells are incubated for 24–48 hours with PUFAs, resulting in the marked enrichment of their cellular membranes with the PUFA added to the culture medium. The cells are then resuspended in medium with the antineoplastic agent but without the PUFA. These studies have, in most cases, utilized drugs in clinically relevant concentrations and do not cause lipid peroxidation of cellular PUFAs. Because of cellular antioxidant systems, they are less susceptible to oxidation than are PUFAs in culture medium. Such studies more likely reflect the impact of PUFAs on the response of cancer cells to the cytotoxic action of antineoplastic agents.
Anthracyclines
Several investigators, utilizing human cervical carcinoma (HeLa) cells, [49, 50] human breast carcinoma cells, [51, 52] L1210 murine leukemia cells, [53] transformed rat fibroblasts, [54] and lymphoma cells, [55] reported doxorubicin cytotoxicity was enhanced when PUFAs were added to the culture medium. This effect was demonstrated with DHA, EPA, GLA, ALA, AA, and LA. Others reported epirubicin cytotoxicity was enhanced in the presence of GLA. [56] PUFAs were used in concentrations that reduced cell growth or viability by 10–20 percent, and in most studies the concentrations were 30 microM or greater, [49–53] which can result in cytotoxicity due to lipid peroxidation. Generation of lipid peroxidation products was demonstrated in one study. [50] Although doxorubicin was used in clinically relevant concentrations (less than or equal to 2 microM), concentrations at which its cytotoxicity is attributable to topoisomerase II inhibition, [46, 48] the greater than additive cytotoxic effects of PUFAs plus doxorubicin can be explained by enhanced lipid peroxidation of the added PUFAs instead of an increase of the drug's cytotoxic action. That enhanced lipid peroxidation was responsible, at least in part, for the results of these studies is supported by data showing:(1) the effect of PUFAs was proportional to the degree of unsaturation;
(2) the cytotoxicity of DHA plus doxorubicin was associated with the generation of lipid hydroperoxides;
(3) the combined effect of DHA plus doxorubicin was enhanced by the addition of an oxidant system;
(4) the generation of lipid hydroperoxides and the enhancement of doxorubicin cytotoxicity by the addition of DHA was abolished by vitamin E; and
(5) simultaneous exposure to DHA or GLA and mitoxantrone, an anthracycline which does not induce lipid peroxidation, did not influence the drug's cytotoxicity. [51, 55, 57]Although the above studies are inconclusive as to the impact of PUFAs on the cytotoxicity of doxorubicin, cancer cells with PUFA-enriched membranes, but suspended in medium without PUFAs during drug exposure, have been shown to exhibit increased sensitivity to doxorubicin. A substantial enhancement of doxorubicin cytotoxicity was demonstrated in L1210 murine leukemia cells, [58–60] doxorubicin-sensitive and -resistant small-cell lung carcinoma cells [61] and doxorubicin-resistant P388 murine leukemia cells [62] following membrane enrichment with DHA, and in L1210 murine leukemia cells [59] and drug-resistant human ovarian cancer cells [63] following membrane enrichment with EPA. Doxorubicin cytotoxocity was enhanced to a lesser degree in cells grown in medium containing GLA [59, 60] or ALA. [59] Importantly, doxorubicin cytotoxicity was enhanced to a greater degree by GLA and DHA when antioxidants were added to the growth medium, [62] indicating that enhancement of doxorubicin cytotoxicity following membrane enrichment with PUFAs did not involve an oxidative mechanism.
Exposure of drug-sensitive tumor cells to doxorubicin or epirubicin results in the drugs being localized primarily within the nuclei, with much smaller amounts being localized in plasma membranes, microsomes, and the cytosol. [64–68] A significant amount of doxorubicin is localized in mitochondria [67] that contain DNA (as do nuclei). Idarubicin and mitoxantrone exhibit primarily a perinuclear distribution. [68] In contrast to doxorubicin- and epirubicin-sensitive tumor cells, cells resistant to these agents not only take up far less drug, but that which is taken up is localized primarily in the cytoplasm. [64–66, 68] These results are consistent with the high affinity of anthracyclines for DNA and the drugs' antineoplastic mechanism of action (topoisomerase II inhibition) [46, 48] Drug-sensitive and -resistant neoplastic cells grown in medium containing PUFAs, and then exposed to the drugs in PUFA-free medium, exhibited enhanced uptake of doxorubicin [59–62, 67] and mitoxantrone, [67, 69] with the increase in uptake by nuclei being far greater than the increase in uptake by other cellular fractions. [67] GLA treated drug-resistant tumor cells exhibited enhanced idarubicin uptake and greater nuclear localization of mitoxantrone. [68] Additionally, the enhancement of doxorubicin uptake by different PUFAs parallels their enhancement of doxorubicin cytotoxicity. [59, 62] Thus, increased concentrations of the anthracyclines at their site of action (nuclei) most likely account for their enhanced cytotoxicity following membrane enrichment with PUFAs.
Enrichment with PUFAs alters the physical and functional properties of tumor cell membranes. Membrane fluidity increases with the degree of unsaturation of the fatty acids (FAs) in membrane phospholipids, influencing membrane permeability as well as other membrane properties. [58, 59] Doxorubicin and mitoxantrone uptake (that occurs by passive diffusion) [63, 68, 69] increased as membrane unsaturation increased following incorporation of DHA, [60, 61, 67, 69] and the uptake of doxorubicin was proportional to the degree of membrane unsaturation. [59, 62] These results suggest the increase of drug uptake and enhancement of drug cytotoxicity following membrane enrichment with PUFAs result from increased membrane fluidity. In this regard, doxorubicin-resistant cell lines, some of which exhibit reduced membrane fluidity, [70] exhibit a greater increase of drug uptake and cytotoxicity following membrane enrichment with PUFAs than do their drug-sensitive counterparts. [61–63] Similar results have been observed with idarubicin. [68] These observations are associated with incorporation of much greater amounts of PUFAs by drug-resistant cells than by drug-sensitive cells. [61] Although drug-resistance in many cell lines is accompanied by over expression of multi-drug-resistant export pumps (MDR-1 P-glycoprotein or any of several MRP proteins), incorporation of PUFAs into membrane phospholipids does not appear to influence resistance by these mechanisms. [62] Thus, although drug resistance does not correlate with the degree of membrane fluidity in all cancer cell lines, [71] enhancing fluidity by membrane enrichment with PUFAs may be a means of reducing drug resistance for some malignancies.
Cisplatin
Simultaneous exposure to PUFAs and cisplatin has been reported to enhance cisplatin cytotoxicity in HeLa cells (72 microM GLA or 33 microM EPA), [49, 50] cisplatin-sensitive human ovarian cells (18–36 microM GLA), [63] cisplatin-resistant human ovarian cells (72–144 microM GLA or 33–132 microM EPA), [63] and human neuroblastoma cells (108 microM GLA).57 However, cell growth or viability was reduced 10–20 percent by PUFAs alone in most studies, [49, 50, 63] and by 50 percent in one study. [57] In the studies utilizing HeLa cells, significant lipid peroxidation was detected. Thus, although the addition of PUFAs may enhance the drug's cytotoxic action (formation of platinum-DNA adducts and DNA interstrand cross-links), the greater than additive cytotoxic effects of PUFAs plus cisplatin observed in these studies can also be explained by the drug's enhancement of lipid peroxidation.
In contrast to the above studies that are inconclusive as to the impact of PUFAs on cisplatin cytotoxicity, cisplatin-resistant small-cell lung carcinoma cells with DHA-enriched membranes [72] and cisplatin-resistant human ovarian cancer cells with GLA- or EPA-enriched membranes [63] exhibited enhanced sensitivity to cisplatin when exposed to the drug in medium without the PUFA. In the former study, enhanced cisplatin cytotoxicity was associated with increases of the total platinum bound to DNA and of platinum-DNA adducts and DNA interstrand cross-links. The cytotoxicity of cisplatin was not influenced by PUFA enrichment of membranes in the cisplatin-sensitive counterparts of both cell lines, although DHA enrichment in the small-cell lung carcinoma cells did increase the total platinum bound to DNA and the formation of platinum-DNA adducts. The results of these studies suggest PUFAs may have a role in enhancing drug sensitivity of cisplatin-resistant cancer cells.
Alkylating Agents
Greater-than-additive cytotoxic effects of PUFAs and mitomycin C were observed in lymphoma cells (5–25 microM DHA, EPA, AA, ALA, and LA)55 and bladder cancer cells (15 microM GLA), [56] but not in neuroblastoma cells (108 microM GLA). [57] However, in the latter study, the impact of GLA may be difficult to detect since PUFAs alone reduce cell growth by more than 50 percent. In the study with lymphoma cells, the greatest effect was observed with DHA and a somewhat lesser effect with EPA. The effects of AA, ALA, or LA were similar, but less than those of DHA or EPA. Since the apparent enhancement of mitomycin C cytotoxicity paralleled the degree of unsaturation of PUFAs, and since mitomycin C generates reactive oxygen species in biological systems, the greater-than-additive cytotoxic effects of PUFAs plus mitomycin C may be due to increased lipid peroxidation induced by the drug instead of an enhancement by PUFAs of DNA alkylation by the drug.
PUFA membrane enrichment of L1210 murine leukemia cells by feeding mice a diet high in LA did not influence the pharmacokinetics of carrier-mediated melphalan transport. [60, 73] Thus, altering the PUFA composition of membranes did not influence the uptake of this antineoplastic agent by the leukemic cells.
Etoposide
Greater-than-additive cytotoxic effects of GLA plus etoposide were not observed in neuroblastoma cells. [57] although the results of the study are difficult to interpret because the GLA alone reduced cell proliferation by more than 50 percent.
Vinca Alkaloids
The simultaneous exposure to vincristine and PUFAs (in concentrations that resulted in a 10–35 percent reduction of cell proliferation) resulted in an apparent enhancement of the drug's cytotoxicity in HeLa cells (72 microM GLA or 33 microM EPA) [49, 50] and a vincristine-resistant human cervical carcinoma cell line (approximately 15 microM or 30 microM GLA, AA, EPA, or DHA). [49] Simultaneous exposure of vincristine-sensitive57,74 and -resistant74 human neuroblastoma cells to 108 microM GLA or 65 microM DHA plus vincristine resulted in a 1.5– to 2–fold increase in the drug's apparent cytotoxicity, although exposure to PUFAs alone resulted in 40–60 percent inhibition of cell growth. The sensitivity to vindesine and vinblastine in the drug-sensitive cell line increased about two-fold in the presence of GLA. [57] The uptake of vincristine was doubled by GLA, EPA, and DHA in HeLa cells, [49] and by GLA and DHA in vincristine-sensitive and -resistant neuroblastoma cells. [57,74] Although these results suggest PUFAs can enhance the uptake and cytotoxicity of vinca alkaloids, they are less than conclusive because of the high degree of cytotoxicity exhibited by PUFAs alone and high levels of lipid peroxidation products generated by PUFAs. [50, 57, 74]
Methotrexate
Growth of L1210 murine leukemia cells in mice fed diets enriched with LA enhanced membrane fluidity of tumor cells. [60, 75] This change was associated with a decrease of the Km but no change in the Vmax for the active transport of methotrexate. Thus, enhanced membrane fluidity was associated with increased affinity of the transport system for the drug (the drug concentration necessary to achieve half-maximal transport Km was decreased) although the maximum rate of transport Vmax did not change.
Purine and Pyrimidine Analogues
Simultaneous exposure of transformed rat fibroblasts to 20 microM EPA or DHA and cytosine arabinoside, 2-chloro-2'-deoxyadenosine, 5-fluorodeoxyuridine, or 7-deazaadenosine did not influence the cytotoxicity of the drugs. [54] However, in L1210 cells, 30 microM EPA or DHA enhanced the cytotoxicity of cytosine arabinoside, a drug that enters cells by facilitated diffusion.53 GLA, in a highly cytotoxic concentration (108 microM) did not influence the apparent cytotoxicity of cytosine arabinoside or 5-fluorouracil (5-FU) in human neuroblastoma cells.57
Radiation
Supplementing the culture medium of rat astrocytoma cells [76] with 15-45 microM GLA, EPA, or DHA for one day prior to, during, and for one week following gamma-irradiation enhanced the cytotoxicity of the radiation treatment. When cells were exposed to PUFAs prior to but not during or following gamma-irradiation, the radiation treatment was enhanced by GLA but not EPA or DHA. Addition of 15–45 microM GLA or 30–45 microM DHA within one hour following, but not prior to or during gamma-irradiation, also enhanced the effects of the treatment.
In contrast to astrocytoma cells, pancreatic cancer cells exhibited an enhanced response to gamma-irradiation with far lower concentrations of PUFAs. [8] Cells exposed to 30.63 microM DHA prior to irradiation exhibited an enhanced response to the treatment; whereas, exposure to 30.08 microM DHA during or following gamma-irradiation enhanced the response. Cell killing was also enhanced by exposure to 32.5 microM EPA or AA during gamma-irradiation.
In both of the above studies, the greatest impact of PUFAs on cell cytotoxicity was when the PUFA was present in the culture medium during or immediately following gamma-irradiation, with a lesser impact when cells were allowed to incorporate PUFA into their membranes prior to exposure to gamma-irradiation in PUFA-free medium. This is consistent with the high susceptibility of PUFAs in culture medium to oxidation, resulting in the formation of high levels of cytotoxic products when they are exposed to radiation-generated free radicals (compared to membrane PUFAs that are somewhat protected by cellular antioxidant systems). However, the results do suggest that membrane enrichment with PUFAs can enhance the radio sensitivity of cancer cells to gamma rays.
In contrast to the above results, membrane enrichment with PUFAs of human retinoblastoma cells (with DHA) and L1210 cells (mice fed LA) did not enhance the cytotoxicity of x-rays [77] when the cancer cells were exposed to the treatment in PUFA-free culture medium. These results, and those of the above studies, demonstrate that the degree of radio sensitization following membrane enrichment with PUFAs varies greatly among different types of cells.
Studies in Laboratory Animals
Anthracyclines
In athymic mice with MX-1 human mammary carcinoma xenografts, doxorubicin treatment resulted in greater inhibition of tumor growth when mice were fed a 10-percent fish oil (17% EPA, 11% DHA) diet instead of a 10–percent corn oil (60% LA, 30% oleic acid (OA), <1% ALA) diet. [78] In athymic mice injected with A549 human lung cancer cells, feeding a 19-percent fish oil plus 1–percent corn oil diet resulted in a decrease in the size of the tumor mass following treatment with doxorubicin; whereas, in mice fed a 20–percent corn oil diet, the growth of the tumors was simply halted by doxorubicin treatment. [79] The diets in these studies were prepared so as to prevent lipid peroxidation of the added oils. In the latter study, adding iron (a pro-oxidant) to the diet did not influence the cytotoxicity of doxorubicin in mice fed the fish oil diet; whereas, it counteracted the antitumor effect of doxorubicin in mice fed the corn oil diet. These results show that lipid peroxidation was not responsible for the cytotoxicity of doxorubicin in this experimental model, and that the enhancement of doxorubicin anti- tumor activity by fish oil did not involve an oxidative process. The results are also consistent with the conclusions of others that the anticancer mechanism of action of doxorubicin is due to topoisomerase II inhibition and does not involve free radical-induced oxidative processes. [46, 48]
In contrast to the above results, the anti-tumor effect of epirubicin in fish oil-fed rats with N-methyl nitrosourea-induced mammary tumors was reported to be enhanced by lipid peroxidation inducers and inhibited by vitamin E. [80] However, the investigators did not indicate that measures were taken to prevent lipid peroxidation in the laboratory chow to which 15–percent sardine oil was added. Thus, the results of this study may have been influenced by a diet containing lipid peroxidation products that can suppress tumor growth. [16]
In dogs with high-grade stage III and IV lymphoma, a diet high in fish oil (versus soybean oil: 55% LA, 25% OA, 5% ALA), vitamin E, and arginine increased the disease-free interval and survival time following doxorubicin treatment. [81] Although the improved response in fish oil-fed dogs may be due to enhancement of the anti- tumor activity of the drug, the fish oil diet also improved several metabolic parameters and reduced the levels of inflammatory cytokines, suggesting that attenuation of cancer cachexia may be involved.
Cisplatin
In C57BL/6J mice bearing Lewis lung carcinoma, treatment with cisplatin resulted in significantly slower tumor growth and less metastatic load when mice were fed a diet containing 4–percent fish oil plus 1–percent corn oil instead of a 5–percent soybean oil diet. When the fish oil diet was supplemented with vitamins C and E, cisplatin exhibited greater antineoplastic activity. These results support the contention that the antineoplastic action of cisplatin does not involve a free radical-induced oxidative mechanism, and that the enhancement of cisplatin antitumor activity from fish oil did not involve an oxidative process. [82]
Alkylating Agents
A 25–percent corn oil diet compared to a 5–percent corn oil diet enhanced the antitumor response to mitomycin C in athymic mice with implanted MX-1 mammary carcinoma. [83] The high corn oil diet increased the activity of tumor bioreductive enzymes that generate the electrophilic species of mitomycin C responsible for alkylation of DNA. A 10–percent fish oil diet compared to a 10–percent corn oil diet, [78] or a 20–percent fish oil plus 5–percent corn oil diet compared to a 5–percent corn oil diet, [84] also enhanced the antitumor activity of mitomycin C in the same experimental model. In the latter study the fish oil diet, like the 25–percent corn oil diet, [83] was associated with increased activity of mitomycin C bioreductive activating enzymes, accounting for the drug's enhanced cytotoxic activity. In two of these studies [83, 84] the high PUFA diets resulted in tumor oxidative stress (as shown by elevated levels of lipid peroxidation and protein oxidation) and increased activity of antioxidant enzymes including catalase and superoxide dismutase, and the enzymes that catalyze antioxidant activities involving glutathione. Thus, the elevation of mitomycin C-activating enzymes may be in response to PUFA-induced oxidative stress. Although some investigators have reported oxidative stress inhibits tumor growth in animals, [17, 18] rats fed the 25–percent corn oil diet exhibited not only a higher level of oxidative stress, but also more rapid tumor growth than did those fed the 5–percent corn oil diet. [83]
The antitumor activity of cyclophosphamide, another alkylating agent requiring bioactivation, is also greater when athymic mice with transplanted MX-1 tumor are fed a 20–percent fish oil plus 5–percent corn oil diet instead of a 5–percent corn oil diet. [85] Fish oil significantly elevated several liver and tumor cytochrome P450 isozymes that are catalysts for cyclophosphamide activation to its electrophilic alkylating form. These effects may account for the enhancement of the drug's antitumor activity. Additionally, mice fed the fish oil diet exhibited less weight loss and less acute toxicity (decreased mortality) than mice fed the corn oil diet. This may be due to the enhanced activity of liver aldehyde dehydrogenase observed in fish oil fed mice, which results in more rapid detoxification of acrolein, a toxic metabolite of cyclophosphamide.
Cytosine Arabinoside
The antitumor activity of cytosine arabinoside in rats inoculated with C10 fibrosarcoma cells was the same in animals fed a diet containing 4.5–percent DHA as it was in animals fed either a 5– or 10–percent safflower oil (80% LA, 15% OA) diet. [86] However, following treatment with cytosine arabinoside, animals fed the DHA-enriched diet, compared to a 10–percent safflower [86, 87] or a 5–percent corn oil diet, [87] exhibited higher bone marrow cellularity and a higher level of granulocyte-macrophage colony-forming units. Animals fed the DHA-enriched diet also exhibited less intestinal tract toxicity following cytosine arabinoside treatment than did the animals fed the safflower oil diets. [86] Thus, although DHA did not influence the antitumor activity of cytosine arabinoside, it may reduce the adverse effects of the drug. In both studies, the diets had very low oxidation status, which is important since oxidative stress can damage the rapidly growing cells of bone marrow and the intestinal epithelium.
Irinotecan
In athymic mice inoculated with MCF-7 human breast cancer cells, irinotecan, a topoisomerase I inhibitor, halted tumor growth when the mice were fed chow containing 7–percent corn oil. [88] In contrast, tumors regressed in response to irinotecan treatment in animals fed diets containing either 4–percent corn oil plus 3–percent fish oil or 1–percent corn oil plus 6–percent fish oil. Additionally, animals fed the 7–percent corn oil diet exhibited significant damage to the intestinal mucosa; whereas, the mucosal architecture of animals fed the fish oil diets was largely unchanged. Despite the lack of added antioxidants to the fish oil diets, oxidative stress in response to administration of irinotecan or fish oil was not detected. The results of this study suggest fish oil can enhance the antitumor activity of irinotecan and reduce the drug's dose-limiting toxicity.
Bleomycin
Therapy with bleomycin is complicated by a dose-dependent induction of interstitial pneumonitis that can progress to pulmonary fibrosis. Following intratracheal administration of bleomycin, histologic lung damage of hamsters fed a diet containing 10–percent evening primrose oil (EPO) (75% LA, 10% GLA) was less than that of hamsters fed a 10–percent corn oil diet. [89] The lung collagen content, an index of pulmonary fibrosis, was reduced in animals fed the GLA-containing diet. The GLA-containing diet resulted in a marked increase in lung phospholipid content of GLA and DGLA, as well as a moderate increase in LA and AA content. It also resulted in a marked elevation of lung prostaglandin E1 and 15-OH-DGLA, anti-inflammatory eicosanoids derived from DGLA, and a suppression of bleomycin-induced formation of leukotriene B4, a pro-inflammatory eicosanoid derived from AA. Thus, the protection from bleomycin-induced pulmonary toxicity by the EPO diet may be due to the alteration of eicosanoid metabolism.
Radiation
Administration of 3 mL/day of EPO (70% LA, 9% GLA) or an oil containing 65–percent LA, 7–percent GLA, and 2–percent EPA reduced early (erythema and moist desquamation, 6–9 weeks after irradiation) and late (erythema and dermal necrosis, 10–16 weeks after irradiation) radiation-induced skin damage in pigs following beta-irradiation. [90, 91] Skin protected from both early and late damage was observed when either oil was administered for four weeks prior to and for 16 weeks following a single dose of beta-irradiation. The two oils afforded the same degree of protection. Protection from early damage was also observed when EPO was administered for 10 weeks following, but not before, irradiation. However, when either oil was given for four weeks before, but not following, irradiation, there was no protection from skin damage. The investigators suggest that skin protection was mediated by suppression of the production of pro-inflammatory eicosanoids by the administration of EPA or GLA.
In mice with transplantable rhabdomyosarcoma administration of 10 microL of EPO (70% LA, 9% GLA) from two weeks before until four weeks following gamma-irradiation reduced the radiation-induced increase of skin blood flow and early skin damage. [92] However, EPO supplementation did not modify tumor blood flow or tumor sensitivity to radiation.
Clinical Studies
Baronzio et al [93] reported an improved response to chemotherapy and radiation in patients who received 5–7 g/day of n–3 PUFAs in combination with 2–3 g/day of unspecified antioxidants. Although these results are encouraging, it is difficult to ascribe the benefits observed to the administration of PUFAs since antioxidants may also enhance the efficacy of chemotherapy. [94] In addition to this interventional study, Boubnoux et al [95] investigated the relationship between breast adipose tissue-PUFA content of 56 patients with localized breast carcinoma and the response to three cycles of chemotherapy with mitoxantrone, vindesine, cyclophosphamide, and 5-FU (47 patients), or the same chemotherapy regimen with epirubicin in place of mitoxantrone (9 patients). Twenty-six patients had a complete or partial response to chemotherapy; whereas, the remaining patients exhibited no response or tumor progression. The level of n–3 PUFAs in adipose tissue was higher in those patients with a complete or partial response to treatment, and DHA content was significantly associated with an improved response.
PUFAs, Oxidative Stress, and Cancer Therapies
Supplementing the diet with PUFAs creates oxidative stress, reflected by reduced levels of antioxidants, e.g., vitamin E, [96] if supplementation is not accompanied by the administration of antioxidants. As noted above, oxidative stress can impact the proliferation of cancer cells by slowing cell cycle progression (prolonging the G1 phase or causing cells to enter the G0 phase) and inducing cell cycle checkpoint arrest. [1–5] Although these effects may slow cancer growth and progression, they may also reduce the cytotoxicity of chemotherapy and radiation.
Many cancer chemotherapeutic agents act only during certain phases of the cell cycle. Examples include DNA synthesis inhibitors (some purine and pyrimidine analogues) and topoisomerase inhibitors (anthracyclines, epipodophyllotoxins, and camptothecins) that act during the S-phase, and antimitotic agents (taxanes and vinca alkaloids) that act during the M-phase. Thus, halting cell cycle progression in the G1 phase or causing cells to be quiescent (G0 phase) will reduce the effectiveness of these drugs. However, drugs that exhibit phase-nonspecific activities, such as alkylating agents and platinum coordination complexes, are also more cytotoxic when cells exhibit unrestricted progression through the cell cycle than when they remain in the G1 or G0 phase. Oxidative stress, by causing checkpoint arrest that normally does not occur in cancer cells, may further impair chemotherapeutic effectiveness by allowing repair of damage caused by the drugs.
Oxidative stress has also been shown to alter the mode of chemotherapy-induced cell death, usually occurring by apoptosis following cellular damage by antineoplastic agents. [97, 98] Oxidative stress inhibits drug-induced apoptosis and results in cell death by necrosis,3–5 an effect that reduces the cytotoxicity of chemotherapeutic agents, including doxorubicin, etoposide, cisplatin, and cytosine arabinoside [99, 100] Certain antioxidants have been shown to prevent the oxidative stress-induced inhibition of apoptosis by antineoplastic agents and to enhance the drugs' cytotoxicity. [100] Thus, administering antioxidants with PUFAs during chemotherapy may enhance the effectiveness of the treatment. Antioxidant administration during chemotherapy can also reduce or prevent the development of certain side effects. [94]
As with chemotherapy, the cytotoxic effects of radiation are less when cells are in the G1 or G0 phase of the cell cycle; checkpoint arrest may lead to repair of cell damage caused by the treatment; and oxidative stress may interfere with radiotherapy-induced apoptosis. Thus, administering PUFAs during radiotherapy may enhance the effectiveness of the treatment, although concerns have been expressed because antioxidants may counteract the free radical-inducing impact of low linear energy transfer radiation (beta-radiation, gamma-radiation, and x-rays). In mice with transplanted squamous carcinoma, an exceptionally high intraperitoneal dose of vitamin E (1 g/kg dl-alpha-tocopherol) administered 30 minutes before irradiation has been shown to protect tumors from the lethal effect of x-rays. [101] However, in rats with transplanted sarcomas or hepatomas, the intramuscular injection of 50, 250, or 500 mg/kg of dl-alpha-tocopherol seven days before treatment, or 50 mg/kg injected both seven days and one day before treatment, enhanced the lethal effect of tumor irradiation; whereas, a 1–g/kg dose injected seven days before treatment resulted in no change in the tumor response. [102–104] The mechanism whereby vitamin E (500 mg/kg or less) enhanced the impact of tumor irradiation may be by enhancing blood tumor flow and oxygenation (free radical generation by radiotherapy is proportional to the oxygen tension). [24, 105–107] Additionally, studies in mice have shown that supplementation with other antioxidants, including retinol palmitate (150,000 IU/kg diet), and beta-carotene (90 mg/kg diet),108 enhanced the antitumor response of radiation therapy. Results of clinical studies also suggest administering antioxidants during radiation therapy may be beneficial. [93]
Commentary
Dietary supplementation with certain PUFAs, including EPA, DHA, and GLA (which is rapidly elongated to DGLA), may provide a means of enhancing the response to cancer therapies. Altering the physical and functional properties of tumor cell membranes by enrichment with these PUFAs may increase the response to chemotherapy and radiation, and may, to some degree, reverse the resistance of cancer cells to certain chemotherapeutic agents. Although there is a lack of clinical data to support the contention that certain PUFAs enhance the response to cancer therapies, preclinical data suggest PUFA supplementation is beneficial. Certainly, clinical studies need to be conducted to confirm the preclinical data.

Table 3 Although many effects of PUFA supplementation may enhance the impact of cytotoxic antineoplastic agents and radiation, aldehydes generated by PUFA-induced oxidative stress may reduce the efficacy of these treatments by slowing cell cycle progression, inducing cell cycle checkpoint arrest, and altering the mode of cell death in response to these treatments (Table 3). Supplementation with antioxidants may enhance the effects of PUFA administration during chemotherapy and radiation by reducing oxidative stress and the generation of aldehydes, a contention supported by the results of Yam et al, [82] which demonstrate that antioxidants added to a fish oil diet enhance the antineoplastic activity of cisplatin more than the fish oil diet alone.
Unanswered questions remain regarding the impact of antioxidants, since few clinical studies have been done, and, although a substantial amount of preclinical data supports the contention that antioxidants can improve the response to antineoplastic agents which have mechanisms of action that do not involve reactive oxygen species, [94, 109, 110] far less data is available regarding the impact of antioxidants on radiotherapy. Additionally, reactive oxygen species, generated during oxidative stress, have been implicated as downstream mediators of apoptosis. [111] If reactive oxygen species are mediators, antioxidants could interfere with apoptosis, although there is considerable evidence that reactive oxygen species are not necessary for apoptosis to occur, [112, 113] and that the generation of reactive oxygen species is a late event that occurs after cells are already committed to apoptosis. [114] Also, inhibition of caspases, cysteine proteases that carry out disassembly of the cell following proapoptotic signals, [115] by oxidative stress3 or other inhibitors, [116] interferes with drug-induced apoptosis and reduces the cytotoxicity of multiple chemotherapeutic agents. [99, 100, 116]

Figure 2 Although the mechanism whereby oxidative stress inhibits caspase activity is unclear, aldehydes, generated following the oxidation of PUFAs, are strong electrophiles that bind to nucleophilic moieties such as cysteine residues of proteins. Tetrapeptide aldehydes have been shown to be potent inhibitors of caspases. [117] Strong electrophiles such as the aldehydes resulting from PUFA oxidation may also bind to cysteine-rich extracellular domains of death receptors [118] and interfere with apoptotic signals initiated by death ligands. Although cellular damage by chemotherapeutic agents and radiation is generally considered to cause caspase activation and apoptosis by mechanisms that involve cytochrome C release from mitochondria, death receptors are implicated in apoptosis induced by certain cytotoxic agents (Figure 2). [119] Certainly, many aspects of oxidative stress as it relates to chemotherapy- and radiotherapy-induced apoptosis need to be elucidated.

Return to OMEGA-3
Return to CANCER & EFAs
Since 3-01-2002


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |