Multiple Sclerosis, An Autoimmune
Inflammatory Disease: Prospects
for its Integrative ManagementThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Alternative Medicine Review 2001 (Dec); 6 (6): 540–566 ~ FULL TEXT
Parris M. Kidd, PhDMultiple sclerosis (MS) is aptly named for the many scars it produces in the brain and spinal cord. A sometimes fatal, often debilitating disease, MS features autoimmune inflammatory attack against the myelin insulation of neurons. Thymus derived (T) cells sensitized against myelin self-antigens secrete tumor necrosis factor, cytokines, prostaglandins, and other inflammatory mediators that strip away the myelin and sometimes destroy the axons. Familial and twin inheritance studies indicate MS is mildly heritable. No single MS locus has been identified, but an HLA haplotype has been implicated. Unique geographic distribution of the disease is best attributed to some combination of vitamin D abnormality and dietary patterns. No pharmaceutical or other therapies exist that confer prolonged remission on MS, and obvious interrelationships between toxic, infectious, and dietary factors make a persuasive case for integrative management. The time-proven MS diet meticulously keeps saturated fats low, includes three fish meals per week, and eliminates allergenic foods. Dietary supplementation for MS minimally requires potent vitamin supplementation, along with the thiol antioxidants, the anti-inflammatory omega-3 fatty acids, and adaptogenic phytonutrients. Gut malabsorption and dysbiosis can be corrected using digestive enzymes and probiotics. Long-term hyperbaric oxygen therapy can slow or remit the disease. Transdermal histamine offers promise, and adenosine monophosphate may sometimes benefit. Chronic viruses and other infectious load must be aggressively treated and exercise should maintain muscle tone and balance. Early intervention with integrative modalities has the potential to make MS a truly manageable disease.
Introduction
Multiple sclerosis (MS) is an inflammatory, autoimmune, demyelinating disease of the central nervous system. It generally strikes at an early age, most often the early adult years. Its most frequent symptoms include numbness, impaired vision, loss of balance, weakness, bladder dysfunction, and psychological changes. Fatigue is an early symptom in MS, often the earliest. The disease can wax and wane for up to 30 years, but in perhaps half of all cases it steadily progresses to severe disability and premature death. [1]
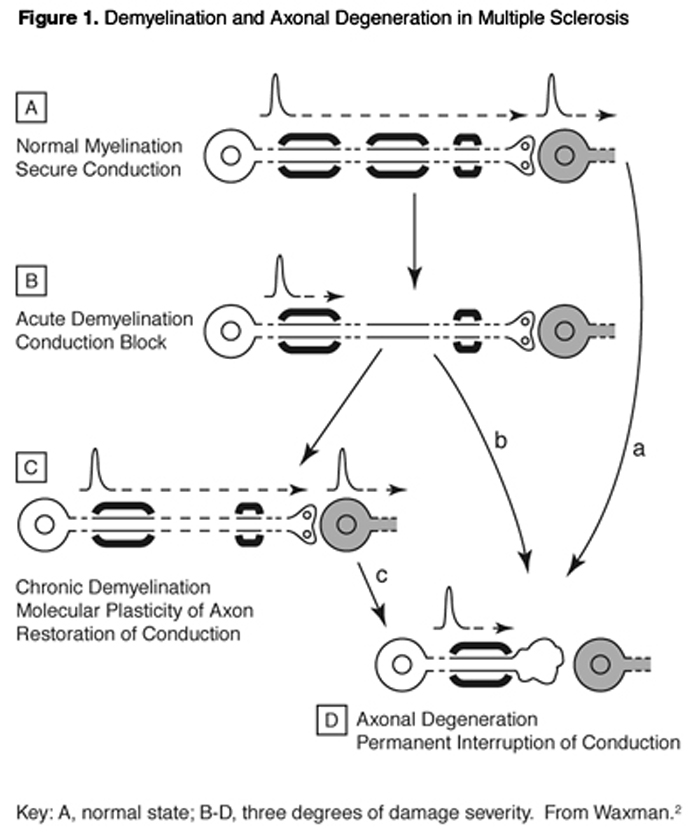
MS owes its name to the presence of multiple sclerotic (hardened) lesions in the brain and spinal cord multiple scars. The optic tract also is often involved. This disease has major autoimmune character, with T-cells and other immune effector populations entering the brain and attacking the nerve cells, stripping away their myelin insulation and sometimes destroying their axons and entire remaining structures. Principal patterns of demyelination and axonal degeneration are schematized in Figure 1.
MS is the most common cause of neurologic disability in young adults. The lesions of demyelination are histopathologically characteristic of the disease. Brain examination by MRI (magnetic resonance imaging) can accurately detect these "white matter plaques." MRI correlates well with the classic histopathology of the lesions, and is progressively a more sensitive tool for detecting the characteristic lesions of MS in situ, as compared to conventional functional evaluation.
Currently approved drug therapies for MS are highly toxic; the immunosuppressants cortisone, prednisone, methotrexate, and cytoxan are still mainstays of conventional MS management. In 1993, interferon ß-1b was approved in the United States as attack prevention therapy, [3] but this drug itself is burdened with frequent and severe adverse effects. [4] The limitations of the conventional drug therapies for MS make imperative the development of a less toxic, integrative strategy for its management.
Diagnosis, Prevalence, and Progression of MS
Multiple sclerosis is a complex disease, perhaps encompassing more than a single etiopathological entity and very likely subject to multifactorial etiology. [5] MS prevalence worldwide is estimated at one million cases; in the United States this number is 250,000- 350,000. [6] Although not generally considered life threatening, this disease kills about 3,000 people each year in the United States. [7]
MS overlaps extensively with numerous other syndromes, including acute disseminated encephalomyelitis (ADEM), Marburg’s variant of MS, recurrent optic neuritis, neuromyelitis optica (Devic’s syndrome), internuclear ophthalmoplegia, acute transverse myelitis, and cerebellar, pyramidal, and dorsal column dysfunctions. Weinshenker [3]proposed grouping all these into a pathophysiological matrix of idiopathic inflammatory demyelinating diseases of the CNS. Optic neuritis or other single demyelinating syndromes can develop over time into MS. Such “conversion” occurs especially in females with relatively early onset. MRI is currently the technique of choice to identify those at risk for conversion to clinical MS. [3] When a clinician detects exacerbation of symptoms, MRI will detect new lesion activity in most cases. However, patients without clinical activity can still manifest new lesion activity on MRI examination. MRI lesion data may strongly predict probability of subsequent attacks and progression to disability.
Onset of MS is usually between the ages of 10-59 years, but can occur as early as age two. [7] Symptoms often begin as sudden but transient motor and sensory disturbances, including blurred vision, dizziness, muscle weakness, and tingling sensations. [8] In about two-thirds of the cases onset is between ages 20 and 40 (onset after age 50 is rare), and women are more frequently affected than men (60% and 40%, respectively). With the passage of time, an almost bewildering array of symptoms can manifest.
MS is difficult to diagnose in its early stages. Initial symptoms begin alone or in combination, and are highly variable in duration and expression. Typically they develop over a few days, remain for a few weeks, then recede. Motor symptoms tend to come first, and include feelings of heaviness, weakness, leg dragging, stiffness, tendency to drop things, and clumsiness. [8] Sensory symptoms include vague numbness and tingling, and “pins and needles” and electrical sensations. Often the sensory organs are also involved. Optic neuritis and other visual symptoms – blurring, fogginess, haziness, eyeball pain, blindness, double vision – can appear early, and afflict more than one-third of the cases. Problems with the ear vestibular apparatus can cause lightheadedness, dizziness, nausea, and vomiting. Later in the disease process, genitourinary nerve tract involvement can create loss of bladder, sexual, and bowel function. Cumulative increase in the number, size, and distribution of lesions correlates well with the patterns of physical and mental disability. [9]
MS usually damages quality of life to a marked degree. Progressive motor degeneration leads to physical disability, while difficulties with mental functioning are measurable in some 43 percent of MS patients and can lead to job loss and social awkwardness. Less than five percent of cases develop severe dementia. Death by suicide occurs seven times more frequently in the MS population than in the general population. [3]
The patterns of MS progression vary. Patients with relapsing-remitting (RR) MS constitute 70-80 percent of all cases, and primary- progressive (PP) MS make up the rest. But 80 percent of the RR MS patients develop secondarily progressive (SP) MS after some period, typically within 7-15 years. [5] For most patients the transition from RR to SP means that extended periods of remission will no longer occur; and on MRI the lesions become more confluent and extensive.
Impairment scales are used to grade clinical signs and symptoms based on objective criteria; disability scales grade limitations on activities of daily living; and handicap scales grade limitations of social interactions. Currently the closest to a “gold standard” for clinical disability is Kurtzke’s EDSS (extended disability status scale). [10] Scoring of disability extent is most useful to the patient, while for the clinician lesion scoring is the more reliable indicator of clinical status and progression.
Some 20-40 percent of all MS cases become classified “benign” since they have less than moderate disability after ten years (meaning the patient can walk more than a half block without a cane). This is a highly misleading term for these patients, since by their fifteenth year with the disease many of them will require some form of walking aid. [3]
Arresting the progression of MS means arresting disability progression. The current conventional drug therapies do not do so. Half of all patients reach DSS 6 (moderate disability, basically unable to walk unaided) within 12-15 years after onset; only about five percent are better than DSS 6 at 40 years. The average life expectancy is decreased only slightly by MS, although for very disabled individuals the probability of death is more than four-fold greater than the general population. Best estimates suggest more than three-quarters of MS patients live longer than 25 years after diagnosis. [3]
Potential Etiological and Triggering Factors
Numerous factors undoubtedly contribute to the causation, exacerbation, or progression of multiple sclerosis. There is a familial component, although no single gene has been identified and the genetic contribution seems relatively minor. [5] The gender component of women having greater susceptibility has been confirmed. [11] Most likely the disease is a product of innate susceptibility spread over several different genes that interact with multiple environmental factors. People who move from a low-risk to a high-risk area before age 15 have a higher risk of developing the disease.
The disease evolves over decades, going through periods of relative stability and increased activity in relation to exogenous triggers. [3]
Agents that can trigger exacerbations includeviral infections,
emotional stress,
pregnancy,
heat exposure,
allergic reactions to foods, and
irritation or provocation by environmental agents.Among the short-term triggering factors, the best documented are
minor respiratory infections (which in one study preceded 27 percent of relapses), [12]
sinusitis, [3] and
heat exposure.Although now obsolete, a hot bath test was formerly used in the diagnosis of MS. [3]
The major etiological factors best supported by the available evidence are inherited susceptibility, microbial infection, and environmental toxin exposure. Diet has been less studied but undoubtedly makes important contributions.
The Genetics of MS
Almost one-quarter of multiple sclerosis patients have an affected relative, and a genetic influence on susceptibility is strongly suggested from population, twin, and family studies. [5] One group of Class II MHC genes of the D class, located on chromosome 6p21, has been consistently implicated. [3] Studies of several ethnic groups confirmed that the human leukocyte antigen (HLA) haplotypeDR2Dw2 is a modest contributor to disease susceptibility. [5] In patients with this haplotype, certain T-cell polymorphisms are also MS risk factors. [13]
Several genome scans conducted on MS populations around the world have failed to indict any individual genes. [5] This probably means that many genes contribute to MS, each with a small effect on disease susceptibility; but separate, “modifier” genes may control the clinical symptoms. A large Canadian study of 15,504 patients did not find support for any transmissible MS factor between spouses, but did suggest that offspring of MS pairings have a substantially greater risk for developing the disease. [14]
Studies with siblings and fraternal twins indicate the genetic component of the disease has relatively low penetrance. The concordance rate in identical twins is around 25-35 percent, suggesting that some 65-75 percent of MS must be attributable to non-genetic factors.
One promising area for further genetic exploration is mitochondrial DNA. Wherever oxidative stress and/or inflammation are involved, cumulative failure of the mitochondria, the cells’ “energy power plants,” has to be examined as a potential factor. [15] Optic neuritis, which is common in RR MS, has similarities with Leber’s hereditary optic neuropathy (LHON), a known mitochondrial failure syndrome which also can feature demyelination. MRI studies of the MS brain suggest energetic abnormalities, even in the normal appearing white matter. [16] Findings from at least a dozen studies seem to prove that MS can occur in individuals lacking pathogenic mitochondrial mutations of any kind. [16] However, a consistent association was found between the mitochondrial ancestral K* and J* haplotypes and MS in caucasians. [13]
Candidates for Infectious Involvement
Epidemiological studies suggest multiple sclerosis is initiated by a primary encounter with an environmental (i.e., non-genetic) agent during childhood or early adulthood. Evidence that this might be an infectious agent is suggested by increased levels of IgG and the presence of alkaline oligoclonal bands in the CSF. These bands occur in greater than 95 percent of MS patients and in 10 percent of other CNS diseases that are infectious. This pattern of elevated IgG and oligoclonal bands is thought to be characteristic of antibody production within the CNS in response to infectious agents. [17]
Viruses are obvious candidates for the infectious villains in MS because several cause demyelination in humans and animals, [8] conditions that can present clinically as relapsing-remitting symptoms. [17] The demyelination of MS may result from direct viral damage to brain cells, or from viral infection leading to formation of antibodies, which then attack the myelin. Over the years many virus candidates for MS have been considered and discarded. Currently, human herpesvirus-6 (HHV-6) is the front-runner candidate for MS causation or triggering.Human Herpesvirus Type 6 (HHV-6) HHV-6 is perhaps the most neuroinvasive member of the herpesvirus family. It has been detected both in normal brain and in the brains of patients with MS. [18] CNS infection with HHV-6 is a major cause of seizures in children, as well as more severe pediatric neurological disorders, including disseminated demyelination and infarction of the basal ganglia. [19] In both children and adults, the virus can produce encephalitis that can be fatal unless successfully treated with antiviral therapy. [20] As with HIV, its genome becomes integrated into the human genome (at chromosome 17); its intervention in AIDS may result in dementia. The recurrent infection pattern of HHV-6 recalls the clinical relapse patterns of MS. [19]
HHV-6 can be lethal even to immunologically intact adults. In this population, focal encephalitis, chronic, demyelinating myelopathy, and other CNS dysfunction can sometimes result in death. [21] Particularly relevant to MS is that demyelination is the most consistent finding in HHV-6 attack on the CNS. The pattern and extent of HHV-6 associated demyelination range from diffuse and extensive loss of myelin to sharply circumscribed foci of demyelination, combined with axon destruction within those zones most severely involved. [20, 22] These parallel the distinctive histopathological features of MS. [23, 24]
Challoner and colleagues showed in MS patients that the nucleic acid of strain HHV-6B was preferentially distributed in and around the zones of demyelination. [18] Sanders and colleagues found HHV-6 (and other herpesviruses) generally more associated with active MS plaques than inactive ones, [25] and Knox and colleagues used immunohistochemical staining to identify cells in the CNS actively infected with HHV-6. They detected such cells in 8 of 11 patients diagnosed with MS (73%), versus 2 of 28 controls (7%), comprising normal persons and patients with non-MS inflammatory demyelinative diseases. In the MS patient group, 17 of 19 tissue slices showing active demyelination (90%) had HHV-infected cells, compared with 3 of 23 tissue sections free of active disease (13%; p<0.0001).
Knox et al found lymphoid tissue samples from MS patients revealed active HHV-6 infection (in six of nine patients examined, or 67%; all controls negative). [19] Blood samples from 22 of 41 MS patients (54%) had active HHV-6 infections, compared to 0 of 61 control patients. Repeated blood analyses indicated the MS patients were more consistently burdened with active HHV-6 infection than were immunosuppressed patients, namely those who had received liver transplants.
The Knox study made a pivotal contribution to the circumstantial case for HHV-6 causal involvement in MS. Four arguments support this position:(1) HHV-6 can cause demyelinative CNS degeneration and active HHV-6 infections are associated with other demyelinating diseases of the CNS;
(2) a strong relationship was found between the presence of cells actively infected with HHV-6 and active demyelination;
(3) the cumulative outcomes of previous studies pointed to an HHV-6 association with MS; and
(4) the high degree of neuroinvasiveness of HHV-6 combined with its capacity to establish latent infections that periodically reactivate is consistent with the periodic exacerbations of clinical MS.Previous to the Knox study, efforts to unequivocally define HHV-6 involvement in MS produced mixed results. DNA of HHV-6 had been detected in the brain tissues and occasionally in the cerebrospinal fluid (CSF) of patients with MS. Abnormally high titers of antibodies to HHV-6 were found in the serum. The Polymerase Chain Reaction (PCR) is a means to tremendously amplify DNA and facilitate detailed matching of gene identities. A negative finding for HHV-6 DNA in the CSF was probably due to interference with the PCR assay by CSF constituents, and perhaps also by the low absolute level of HHV-infected cells ambient in the CNS. When technologies were used that could detect active HHV-6 infections, differences between MS patients and controls were found. Techniques that did not distinguish between active and latent HHV-6 infections failed to show differences. Recent studies demonstrated that at least 50-70 percent of MS patients carry HHV-6 specific IgM antibodies, and at least 30 percent have HHV-6 in their sera. [19]
The mechanisms by which HHV-6 affects demyelination and other degenerative CNS changes in MS remain to be elucidated. Case reports establish beyond doubt that HHV-6 can trigger fulminant demyelinating disease and encephalitis in MS patients. Fulminant demyelinating activity in the MS brain can feature virtually total segregation of HHV-6 from the areas free of demyelinative changes. [19] This virus can infect and destroy oligodendrocytes, the cells which produce myelin. However, the relatively low number of HHV-6 infected cells in brains of MS patients, as compared with encephalitis patients, suggests mechanisms other than direct cell destruction may be involved.
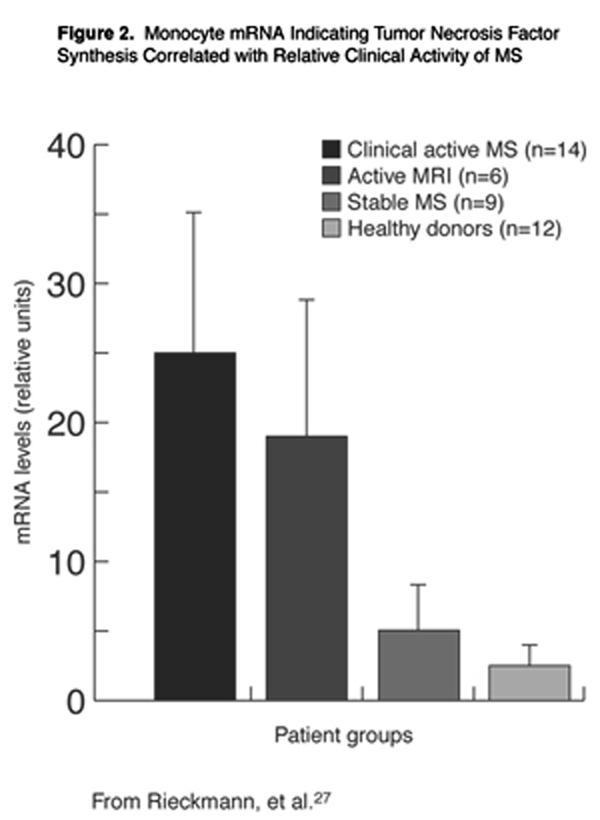
The cytokine tumor necrosis factor-alpha (TNF-±) may be an indirect mediator of HHV-6 damage to the CNS cell populations of MS patients. Cells that produce this cytokine have been identified in MS lesions, [26] and the expression of TNF-± in blood monocytes of MS patients correlates with disease activity. As illustrated graphically in Figure 2, significant differences in cellular expression of TNF-± were observed between clinically active patients and patients with active lesions from MRI, compared to stable patients or healthy control subjects. [27] HHV-6 is unique, even within its own virus family, for its ability to up-regulate TNF-± production in blood monocytes. [28]
A second indirect mechanism of demyelinative damage to the CNS of MS patients by HHV-6 may be the induction of autoimmunity against CNS tissues. A large body of evidence indicates MS is an autoimmune (as well as inflammatory) disease. Antigen mimicry is likely involved, whereby one or more antigenic determinants on an HHV-6 protein may cross-react with a myelin determinant, such as myelin basic protein or myelin oligodendrocyte glycoprotein. [29] Of the majority of MS patients with RR MS, most change to a progressive disease course and at the time of their death many have antibodies specific for myelin proteins bound within their CNS tissues. [30, 31]
The antiviral drug acyclovir, which provides effective prophylaxis against HHV-6 infections in bone marrow transplant patients, has been shown to markedly reduce the frequency of disease exacerbations in patients with MS. [32]
Chlamydia The latest organism implicated as an MS pathogen is Chlamydia pneumoniae, an intracellular bacterium known to infect humans. [17] A great deal of circumstantial evidence links Chlamydia to MS:(1) several chlamydial species produce a relapsing-remitting pattern of disease;
(2) one species – C. trachomata – is more common in women than in men, and may prefer the HLA-DR16 gene allele that is also linked to MS; and
(3) some chlamydial species are linked to immune-mediated, inflammatory neurodegeneration. Still, direct proof of C. pneumoniae involvement in MS has been lacking.In 1999 Sriram, and collaborators [33] reported isolating C. pneumoniae from the CSF of 64 percent of a group of 37 MS patients, compared with 11 percent of non-MS controls (n = 27). Using sophisticated assay technology, they claimed the C. pneumoniae gene was present in the CSF of 97 percent of their MS patients, and that in 86 percent the CSF carried oligoclonal antibodies to the organism. [17]
Unfortunately, other labs have so far been unable to replicate these remarkable findings. [34] The organism has been identified from CSF in only a minority of MS cases; sensitive techniques of immunohistochemistry and in situ DNA hybridization failed to detect it in the CNS tissue; and, in contrast to CSF, it did not culture out of brain tissue. [35] Little is known about how the MS host responds immunologically to this infectious agent. It could be directly causal for MS, or could nonspecifically potentiate the disease. The degree of actual participation, if any, of C. pneumoniae in MS remains to be elucidated.
Mycoplasmas One under-appreciated microbe present in MS is Mycoplasma pneumoniae. This virus-like, primitive bacterium has been proven capable of invading the human CNS [35] where it can cause acute meningoencephalitis, perivascular demyelination, autoimmune sequelae, and a CNS vasculitis. In many cases the organism cannot be detected by sensitive DNA identification techniques, does not culture out from CSF, and leaves no antibody trail. Nicolson and Nicolson reported 50 percent of MS patients they examined had mycoplasmal infections, often as coinfections with HHV-6 (found in 60 percent of their patients), Chlamydia (20%), or other bacteria (25%). [36, 37] They also reported finding mycoplasmas contaminating commercial vaccines. [36]
Other Viruses Under active investigation for MS are Varicella zoster, [38, 39] retroviruses, and nidoviruses. The retroviruses can generate human CNS symptoms that closely resemble MS. [40]Of the nidoviruses, Coronavirus produces an MS-type demyelinating syndrome in experimental animals. [41] A virus called IM and closely resembling the Japanese SMON (subacute- myelo-optic-neuropathy) virus reportedly was isolated from several MS patients. [42] All MS patients are seropositive for Epstein-Barr Virus (EBV), although supposedly it does not reach the brain. [43]
Surveying the considerable bulk of information about the wide variety of viruses or other microbes proven capable of entering the brain, attacking cells therein, and creating neurodegenerative damage, the thoughtful reader might be tempted to suspect that any single one could be pathogenic in MS. The microbe that succeeds may be specified by the genotypic and/or phenotypic characteristics of that individual; or conversely, the MS patient, while in a weakened state, may be subject to colonization by any combination of these infectious agents. Some clinicians favor empirical treatment against putative infectious agents in MS; for example, doxycycline for Chlamydia pneumoniae and acyclovir or valacyclovir for HHV-6 and/or other herpesviruses. [7]
A real-life approach to consideration of MS might suggest that virtually any acute or chronic stressor that weakens the immune system – toxic attack, intensified oxidative stress, illness from a “garden variety” virus or bacterium, malnutrition, even sustained emotional stress – could conceivably create a time window for one of these organisms to enter the brain and establish itself. After that, interaction of the organism with responsive host immunity might set the stage for recovery or for eventual progression to full-blown MS.
Toxins and Other Environmental Factors Linked to MS
Environmental factors that are prime candidates for MS causation include toxins (solvents, pesticides), exposure to X-rays (diagnostic or occupational), and exposure to domesticated animals (dogs, cats, caged birds). [11]
Multiple sclerosis can occur in high concentrations within limited geographic areas. These clusters are reminiscent of other disease clusters stemming from community infectious or toxic exposure. In 1973 Eastman and colleagues reported on a cluster in Mansfield, Massachusetts. [44] There the frequency of MS in 1970 was 141 per 100,000 – two to three times the prevalence rate reported in other portions of the northeastern United States. A historical analysis revealed that eight patients had lived within several blocks of each other in the center of the town in the 1930s. During that period the water supply was heavily contaminated with bacteria and perhaps also with toxins. The time window between this exposure and subsequent development of MS – 23 years – was in line with the estimated incubation period for MS. The authors failed to mention the center of town also housed a large iron foundry.The Mercury Connection Ingalls reviewed and analyzed several MS clusters [45] and suggested Mansfield’s iron foundry probably also produced compounds of lead and/or mercury. He further demonstrated by mapping that the tiny town of Mossyrock, Washington (population 500) had a cluster of six very tightly localized cases. During the Great Depression one enterprising young man sought to improve his lot by salvaging “quicksilver” (mercury) from cinnabar (HgS, mercuric sulfide) leftover from an old mining venture in the area. He melted the cinnabar on the family stove in the presence of six siblings and cousins aged 14 to 18, all of whom subsequently developed MS.
The largest-ever MS cluster observed was the 1983-1985 “outbreak” of 30-40 cases that occurred in Key West, Florida. [45] Of 19 cases confirmed as MS, three-fourths were women and seven were nurses who worked at the same hospital. Across the street from the hospital was a landfill from which red matter oozed continuously, and which would regularly catch fire and fill the air with burning matter. The red matter was strongly suspected, although never officially confirmed, to be anti-fouling ship paint containing mercury. Soon after publishing his analysis of the Key West cluster, Ingalls published a reasoned claim that his own symptoms of MS were triggered by his mercury dental fillings. [46] Mercury is well known to combine with and alter the structure of numerous proteins. Huggins and Levy published a study [47] which involved the photo-labeling of proteins in CSF drawn from MS patients before and after removal of their mercury amalgams. Previous to amalgam removal, the CSF had an abnormal content of multiple extraneous proteins. After amalgam removal (24-48 hours) there was marked absence of protein except for a single band of albumin. These researchers hypothesized that mercury from amalgam in the body could generate multiple abnormal proteins that might help trigger MS.
Siblerud and Kienholz [48] examined two groups each of 24 MS patients, one group who had their mercury amalgams removed 2.75 years previously and the other still with amalgams. Subjects with amalgams had significantly lowered red blood cell counts, hemoglobin, and hematocrit, compared to those without amalgams. The mercury-contaminated group had significantly lowered thyroxine, serum IgG, total T-cells, and CD8 (suppressor) cells. This group also reported significantly more (33.7%) amalgams than during the previous 12 months. Interestingly, the MS subjects combined had significantly higher hair mercury than did the non-MS control subjects.
In a previous study, Siblerud similarly compared MS patients and determined their mental and emotional status. [49] Those still carrying amalgams scored significantly higher for depression, anger, hostility, psychosis, and obsessive-compulsive disorder.
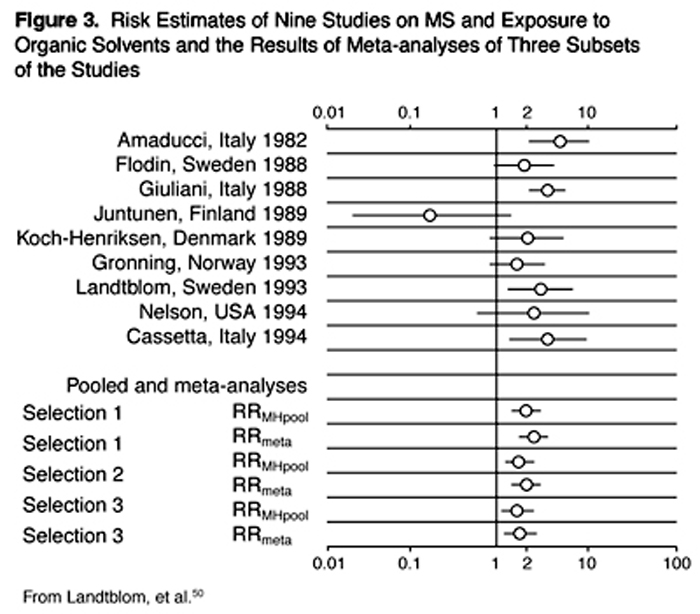
Organic Solvents A number of epidemiological studies have targeted occupational exposures, including exposures to organic solvents. Landtblom and colleagues did a sophisticated meta-analysis that covered all studies from the period 1966-1994 and found 13 published studies. [50] They then did three separate analyses, each of which generated two slightly differing calculations of relative risk (RR). Their results are illustrated in Figure 3. All six of their derived RR estimates were in the range of 1.7-2.6, uniformly consistent with organic solvent exposures increasing the risk of MS. These findings, derived using solid methodology, point to organic solvents as major toxic contenders for MS causation.
While the field of immunity seeks microbial antigens as possible explanations for MS causation, solvents and heavy metals or other toxins can also present novel antigens to the immune system. By chemically reacting with a biomolecule, they can change its topography or other antigenic aspects; this is known as a haptenic reaction. As one example, Thrasher and colleagues demonstrated evidence of formaldehyde antibodies and altered cellular immunity in persons exposed to formaldehyde from contaminated homes. [51]
Dietary/Digestive Contributions to MS Etiology
Much of the epidemiological data in multiple sclerosis is consistent with a role for diet in its initiation, exacerbations, or progression. This type of research is also unable to clearly differentiate between “risk factors” and “triggers.” Some of the dietary factors contributing to the worsening of MS include a high animal-fat diet; food allergies and intolerances; and digestive malfunctions, including malabsorption and dysbiosis.High Animal Fat Diet Swank and his colleagues reported in 1952 on a comprehensive geographical assessment of diet and MS in Norway. [52] The inland farming communities in Norway had a higher MS incidence than the coastal areas, and included much more animal and dairy products than did the coastal diets, which consisted largely of cold-water fish. They reported a strong positive association between butterfat consumption and MS incidence, and a strong negative association with fish consumption.
Subsequent studies have supported a strong association between dietary animal and dairy intake and MS. Agranoff and Goldberg [53] found an extremely high correlation between milk consumption and MS in 1949-1967 mortality rates in the United States. Fish and vegetable intake were negatively correlated. Their protective correlation data for fish and vegetables held across 20 countries, while the dietary categories total fat, animal protein, butter fat, and meat fat all were positively associated with the disease. This pattern has basically maintained through a number of subsequent studies. As reviewed in Lauer, [54] MS rates have repeatedly correlated with consumption of animal fat, animal protein, and meat from non-marine mammals. The negative correlation with vegetable and/or fish intake has also held.
Controlled clinical trials support a beneficial role for certain long-chain fatty acids in MS. [54] A meta-analysis of three trials revealed that intakes of large amounts of linoleic acid, an omega-6 polyunsaturated fatty acid (17.2 or 23 grams per day) lowered severity of relapses and slowed progression in some cases. [55] In another trial, when omega-3 fatty acids were added to this high omega-6 intake the added benefits were few, if any. [56] This was a faulty trial design, as enzymes in the omega-6 fatty acid pathway are known to compete with those in the omega-3 pathway. [57] Nonetheless, the cumulative epidemiological, experimental, and clinical data dictate that MS patients reduce animal fat intake and increase intakes of vegetables and cold-water marine fish. [54]
A modified-fat diet has been empirically proven for MS. Beginning in 1948, Dr. Roy Swank began treating MS patients with his modified diet consisting of 50-90 grams of protein (with one egg per day and several glasses of skim milk for animal protein); a maximum of 15 grams of animal fat, 10-15 grams of fluid vegetable oils, and 5 grams of cod liver oil; and carbohydrates as required for calories. Margarine, shortening, and hydrogenated oils were not allowed; three fish meals per week were encouraged; and most patients increased their fruit and vegetable intakes. This diet is not very different from what would currently be considered a healthy diet.
In 1988 Dr. Swank reported on 150 patients followed long-term, from 1949 to 1984. [58, 59] Those who complied, with daily fat consumption of less than 20 grams of fat (<15 grams of saturated fat), fared much better on morbidity and length of life than did those who consumed larger amounts of fat. The death rate was lower in the former group compared with the latter (31 percent versus approximately 80 percent). Swank also concluded that 95 percent of the patients placed on his diet before the development of disability suffered no significant increased disability from MS during the 35-year period. [60, 61]
Food Allergies Diets high in gluten and milk are generally much more common in areas with high MS prevalence, [62, 63] and a connection between MS and food allergies has been suspected since the 1930s. In addition, patients with generalized allergy may be more readily susceptible to symptom exacerbation. In one study of 15 patients, the avoidance of allergenic foods, tobacco, or house dust led to almost complete control of symptoms. [1]
MS patients can manifest anatomic proof of food allergy. Small-intestinal biopsy indicated 50 percent of a small group (6 of 12) had inflammatory abnormalities of the lining reminiscent of celiac disease and other food allergies. [64] Celiac disease is closely linked to gluten allergy, but negative findings from controlled studies seemed to rule out any central role for gluten allergies in MS. [8] But other foods, or the cumulative effects of food allergy, may be MS triggers. Thus, a 1952 study found 31 percent of 49 MS patients improved when they avoided foods to which they were found allergic. [65] Reintroduction of such foods caused their symptoms to recur. According to Gaby, cow’s milk and sulfite food additives can trigger exacerbations, and in occasional MS cases the degree of improvement from an allergy elimination diet can be highly impressive. [1]
Compelling rationale for an intestinal-immune system axis in MS has been articulated by Embry. [66] Constituents of common foods are known to cause autoimmune diseases. Celiac disease is caused solely by gluten from grains. Clinical trials in rheumatoid arthritis and in Crohn’s disease have shown that avoidance of milk, grains, and legumes results in major symptom improvements. T-lymphocytes reactive with milk proteins are very common in persons with MS; experimentally, such cells cross react with self-antigens in myelin. Peptides (small proteins) from milk have been found to be molecular mimics of self-antigens in myelin. Patterns of CNS degeneration closely resembling MS can be precipitated in mice by injecting milk peptides or including them in the diet.
Digestive Malfunctions and Dysbiosis Aside from food allergy per se, poor GI function seems endemic in MS. According to Wright, a majority of the patients he sees with MS have poor digestion and assimilation of nutrients. [58] Most have poor stomach function, with inadequate acid and pepsin production. Some have inadequate intestinal digestive enzyme secretion. This problem is commonly seen in otherwise healthy individuals as they age.
Gupta and colleagues examined 52 MS patients and found42 percent had fat malabsorption,
41 percent demonstrated undigested meat fibers,
27 percent had abnormal absorption, and
12 percent experienced malabsorption of vitamin B12. [67]Pancreatic enzyme preparations may help correct malabsorption as well as to help disperse circulating immune complexes.
Gut dysbiosis has been implicated in MS. [1] Perlmutter reported finding anti-Candida antibodies or Candida immune complexes in seven of ten MS patients evaluated. [7] Eight of these patients also demonstrated depressed levels of colonic symbiotic bacteria (Lactobacillus species). Aggressive anti-Candida treatment together with the requisite re-colonization of probiotic organisms has been claimed to occasionally resolve MS symptoms. [68]
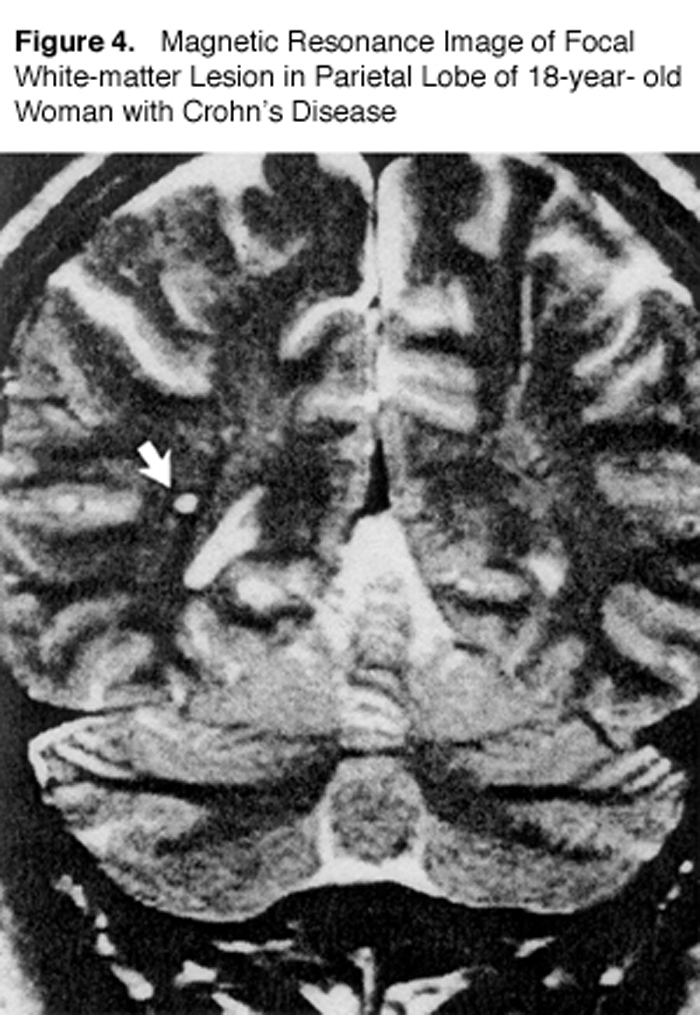
Gut dysbiosis is also commonly recognized in patients with inflammatory bowel disease (IBD). One study used MRI to look at MS-type lesions (“white matter plaques”) in IBD patients compared to normal individuals (Figure 4). [69]
Hyper-intense, focal lesions 2-8 mm in diameter were found in 42 percent of patients with Crohn’s disease and in 46 percent of those with ulcerative colitis. Only 16 percent of the healthy volunteers showed lesions. The authors stated, “The frequency of focal white-matter lesions in the patients with inflammatory bowel disease was almost as high as that in patients with multiple sclerosis.…” [69]
Current Medical Management of MS
Conventional drug development has yielded several new drugs to treat multiple sclerosis, all classed as “partially effective.” Interferon β-1b (Betaseron®), interferon β-1a (Avonex®), and glatiramer acetate (Copaxone®) have all been approved by the U.S. Food and Drug Administration for use in relapsing forms of MS. [70] Each of these reduces clinical evidence of acute, relapse-associated worsening of the disease. In addition, evidence from European trials indicates a role for intravenous immunoglobulin in reducing relapses.
The use of these drugs is not without side effects, however. Investigators from Europe and Canada report that doses of interferon β-1a, exceeding those previously evaluated in the United States, reduced clinical and MRI evidence of disease progression. [70] But this drug (Avonex) produced flu-like symptoms in up to 61 percent of patients who took it at the currently recommended lower dose, as well as nausea in 33 percent and anemia in eight percent. [71] In another study, interferon β-1b was shown to delay clinical and MRI worsening in patients with secondary progressive MS. This drug (Betaseron) produced flu-like symptoms in 76 percent of the patients receiving it and significant injection site reactions in 85 percent. It caused asthenia in 49 percent, myalgia in 44 percent, menstrual disorders in 28 percent of the female patients, sweating in 23 percent, was liver toxic in 19 percent, and caused depressed white cell counts in 16 percent of patients. [4]
These drugs are regarded by some parties as significant therapeutic advances against MS, although they are expensive (at approximately $11,000 per year in U.S. currency), inconvenient since they are not effective by mouth, and have marked adverse effects. Although they reduce relapse rate by 35 percent, to date no effort has been made to collect data that might help assess long-term benefits for quality and length of life.
Copaxone is a synthetic peptide drug designed to interfere with receptors on myelin-sensitized T-cells. It does appear to have a degree of effectiveness against the autoimmune aspect of the disease. Although it may be slightly less toxic than the interferons, Copaxone has a fierce adverse effects profile. Some of the more frequent toxic effects reported from clinical trials include bacterial infection, lymphadenopathy, vaginal moniliasis, edema, weight gain, eye disorders, palpitations, vasodilatation, dyspnea, nausea, and pruritus. [72]
A number of other possible drug therapies are in the pipeline for MS, but the development scenario has become more challenging. To demonstrate benefits that would exceed the existing “partially effective” therapies would require longer and larger trials. The most promising among these are: Valacyclovir, an anti-herpesvirus medication; TNF inhibitors and other inhibitors of pro-inflammatory cytokines; pentoxifylline; transforming growth factor-β, and other agents to enhance the TH2 arm of immunity; dpenicillamine and other matrix metalloproteinase inhibitors; antibodies of many kinds to manipulate MHC and other Tcell antigenic sites; inhibitors of cathepsin B, a proteinase linked to the inflammatory demyelination of MS; oxygen radical scavengers; autologous bone marrow transplantation; gene therapy; implantation of oligodendroglial precursor cells; and the use of mathematical modelling to conduct virtual clinical trials that screen new agents. [70]
Rational Bases for Integrative Management of MS
Multiple sclerosis is a frustrating disease to have, to treat, and to study, particularly since its etiology and triggers are so poorly understood. The seemingly arbitrary waxing and waning of MS symptoms and the omnipresent likelihood of disease exacerbation necessarily dictate strict adherence to a basic plan, with willingness to augment or otherwise modify the plan as circumstances change. As with many other diseases, diet and lifestyle changes, dietary supplementation, and moderate physical exercise all contribute to better quality of life in MS, but additional medical modalities also show promise.
Anti-Inflammatory Diet
Steadily accumulating data indicate the "mainstream" Western diet is pro-inflammatory, imposing oxidative challenge on the body while failing to adequately support antioxidant defenses. One class of pro-inflammatory dietary constituents is the animal-source fats. These have a significant content of saturated fats; a low content of the anti-inflammatory omega-3 fatty acids docosahexanoic acid (DHA) and eicosapentanoic acid (EPA); and a high proportion of their omega-6 fatty acids in the form of long-chain omega-6 arachidonic acid (AA). Unlike the omega-6 linoleic acid, AA is a precursor to pro-inflammatory prostaglandins; and as reviewed by Lauer, coastal populations that consume more fish, therefore less AA and more DHA and EPA, have lower MS rates. [54]
The delicate endothelial linings of the blood vessels are vulnerable to pro-inflammatory attack, [57] and inflammation of blood vessels in the brain is characteristic of MS. [73] Plaques frequently arise around a vein or venule, and active inflammation in these vessels is often accompanied by thrombosis and increased platelet stickiness. [74] Omega-3 fatty acids help maintain anti-inflammatory balance in the circulation, while supporting myelination and nerve cell membrane renewal. Wright monitors systemic fatty acid (FA) balance by testing red cell membrane FA profiles, then prescribes omega-3 FAs as necessary. [68]
Swank's healthy-fat MS diet probably benefits MS in several ways. From its overall composition it would be expected to lower cholesterol and reduce platelet stickiness. Polyunsaturated oils appear to help prevent MS deterioration; [55] cod liver oil inhibits autoimmunity in experimental animals; [1] and keeping the "bad" fatty acids low (saturates, trans-fats) reduces their competition with the "good" fatty acids, including the omega-6 gamma-linolenic acid and the omega-3 alpha-linolenic acid, EPA, and DHA. [57]
Another implication from its anti-inflammatory orientation is that the Swank Diet should down regulate autoimmunity in the MS patient. The potent immune-suppressing steroids have short term symptomatic efficacy, but dietary rebalancing of the omega-6 to omega-3 ratio could favorably reset the body's autoimmune-inflammatory set point.
Rational Dietary Supplementation
As with the other neurodegenerative diseases, supplementation of the diet with vitamins and other nutrients is indicated, both to support well being and to ameliorate deficiencies engendered by the ongoing demyelinating, autoimmune-inflammatory process.Linoleic Acid: Linoleic acid is an essential omega-6 fatty acid, meaning a deficiency state is known and it must be obtained from the diet. Homa and collaborators found abnormally low levels in the red cells of 14 percent of their MS patients. [75] Plant oil sources of linoleic acid were administered to MS patients in three double-blind, placebo-controlled trials. The results were mixed: two of the trials found benefit while one did not. A meta-analysis of the 181 patients concluded that linoleic acid at 20 grams per day reduced disability and the severity and duration of relapses, especially in patients with early disease and minimal disability. [55]
Gamma-Linolenic Acid (GLA): Gamma-linolenic acid contributes to anti-inflammatory balance by competing with the pro-inflammatory arachidonic acid. The use of GLA bypasses the enzymatic conversion of LA to GLA, which is subject to inactivation by a number of factors including viral activity. Initial studies used encapsulated evening primrose oil as a source of GLA and failed to find effectiveness in MS. Horrobin theorized that the capsule dyes (tartrazine, Ponceau R) interfere with GLA utilization. [76] Working with two collaborators, he conducted a preliminary non-controlled trial on 14 patients using primrose oil in dye-free capsules, either by itself or in conjunction with colchicine. They reported that five of eight patients benefited from primrose oil supplementation (2.4 mL/day) and four out of the remaining six benefited from primrose oil plus colchicine (2.4 mL/day plus 1.0 mg/day, respectively. New Zealand researchers noted that MS patients often have cold hands and feet, usually an indication of impaired peripheral blood flow. Their non-controlled study on 16 patients [77] concluded that GLA from primrose oil improved peripheral blood flow characteristics and consequently, hand-grip strength.
Omega-3 Fatty Acids: Omega-3 fatty acids include alpha-linolenic acid from flax, and the longer-chain EPA and DHA from cold-water fish and algae. Immunological experiments showed omega-3 fatty acids suppressed inflammatory reactivity in the mouse EAE model of MS, and increased omega-3 FA intake in humans has been shown beneficial for the autoimmune diseases rheumatoid arthritis and Crohn's disease. From red cell analysis, it was suggested MS patients might have low systemic DHA and EPA content. [78] A small clinical trial (12 patients) with no control patient group suggested that omega-3 fatty acid supplements from fish oil might reduce MS exacerbations. [79]
Antioxidants: Due to their high propensity for oxidation, long-chain fatty acid preparations should always be administered in conjunction with high intakes of antioxidants. These nutrients offer benefit in virtually any clinical scenario that involves inflammation and oxidative stress. There is little doubt that oxidative stress is increased in MS. [80] Vitamin E was reported low in MS patients' serum, and lipid peroxidation markers were increased in the CSF, especially during periods of disease exacerbation. The enzyme glutathione peroxidase (GP), which detoxifies peroxides, is markedly decreased in the red cells, [81,82] the white cells, [82] and the CSF [80] of MS patients. A group in Denmark [82] gave a high-dose mixed antioxidant supplement (sodium selenite 6 mg per day, providing 2,740 micrograms of elemental selenium; vitamin C 2000 mg; vitamin E 480 mg) to 18 MS patients daily for five weeks. GP activity, which was abnormally low at baseline, increased five-fold to the normal range without significant side effects.
The GP enzyme has an absolute requirement for selenium, a dietarily essential trace mineral, at its active sites. It also has an absolute requirement for the thiol (SH) tri-peptide glutathione (reduced glutathione; GSH) as its substrate cofactor. Perlmutter has reported cases of successful oral application of GSH precursors in Parkinson's, Alzheimer's, stroke, and multiple sclerosis. [7]
GSH replacement, whether by mouth or combined with intravenous supplementation, is safe and well tolerated. But when taken orally, GSH may have poor systemic bioavailability. One orally effective GSH precursor is N-acetylcysteine (NAC), a potent antioxidant. Following its intestinal absorption, NAC is first metabolized to cysteine, then cysteine is incorporated into GSH in depleted patients.
The antioxidant alpha-lipoic acid (ALA) is another orally effective GSH repleter. ALA is a broad-spectrum, fat- and water-phase antioxidant with potent electron-donating capacity (more so even than GSH), and has added biochemical versatility as a Krebs cycle cofactor. ALA has shown consistently impressive efficacy for the treatment of neuropathies in a number of controlled trials. [83]
Flavonoids: Derived from plant sources, these potent free-radical scavengers support overall antioxidant defense and particularly protect capillary integrity. In an effort to decrease intestinal epithelial and blood-brain barrier permeability, both of which are reported abnormal in MS, standardized preparations of flavonoids may offer benefit.
The popular Ginkgo biloba leaf contains, in addition to flavonoids, terpene substances which are also anti-inflammatories. One such terpene, ginkgolide B, is a potent inhibitor of platelet-activating factor, a well-characterized inflammatory mediator. In a double-blind, placebo-controlled trial, ginkgolide B failed to acutely reduce MS exacerbations. The extremely short period of study only seven days limits the meaning of this trial. [84]
Vitamin D, Possible Key to MS Geographical Distribution: One factor that correlates with MS is latitude. The higher latitudes, in both the northern and southern hemispheres, have up to 10 times higher rates of MS (50 to 100 cases per 100,000 population, versus 5 to 10 cases per 100,000 in the tropics). [8] One hypothesis most likely to explain this phenomenon is the influence of latitude over production of vitamin D in the skin.
Availability of vitamin D, the "sunshine vitamin," decreases with increasing latitude in patterns closely correlated with increasing MS rates. [85] Individuals with a high exposure to sunlight have a significantly lower risk of MS, independent of country of origin, age, sex, race, and socioeconomic status. Conversely, most MS patients have vitamin D deficiency, which leads to low bone mass and high risk of fracture, compounded by the osteopenic effects of the glucocorticoids widely used in MS therapy. [86]
Fish oil is an excellent vitamin D source. Within specific nations, fishing communities located on the coast consistently have lower MS incidence compared with farming communities living inland.52,54 In addition to lower rates of MS in coastal communities, rates are generally lower in countries in which a lot of fish is eaten. Perhaps the vitamin D component of fish oil complements the benefits afforded by the omega-3 fatty acids. The case for this vitamin being the determinant of MS geography is circumstantial, but in any case it is an important dietary supplement for MS patients.
Minerals: Calcium and magnesium complement and balance each other, and are essential minerals involved in human metabolism. Goldberg and collaborators [87] administered dietary supplements with calcium (about 1,100 mg daily), magnesium (about 680 mg), and 20 grams of cod liver oil to 16 young MS patients for periods of one to two years. They found the number of exacerbations observed during the program was less than one-half the predicted number.
Injectable NutrientsVitamin B12 Vitamin B12 is a key nutrient factor supporting myelin formation. Acquired B12 deficiency and inborn errors in its metabolism are recognized causes of CNS demyelination, so its deficiency in MS would be expected to contribute to progression. Early studies of B12 status in MS produced conflicting results, but improved testing techniques confirmed B12 levels were lower in the CSF of MS patients, if not always lower in the serum. [88] For more than 30 years, clinicians have been reporting consistent clinical improvement of MS from B12 injections. Many integrative physicians routinely prescribe intramuscular injections of B complex with B12 and folic acid to their MS patients, reportedly with improvement.
Reynolds and colleagues at Kings College, London, reported on a significant association between MS and disturbed B12 metabolism. They discovered some of their MS patients were significantly macrocytic, a condition reminiscent of classic B12 deficiency. Their patient group also had significantly lower serum B12. [88] Kira and collaborators confirmed this observation in Japan, administering methylcobalamin by mouth at a large dose of 60 mg per day to patients for six months. [89] They observed improvement in sensory nerve potentials, but no motor nerve improvement. Immune-suppressive steroids or cyclophosphamide can produce comparable results, but with severely adverse side effects. [8]
Adenosine Monophosphate (AMP) Fatigue can be a significant factor in MS. Wright reported success from patients self-administering intramuscular injections of AMP, which is a precursor of the “energy currency” substance ATP (adenosine triphosphate). AMP has been used to treat viral infections and other conditions. In one uncontrolled study, 16 patients with severe disability from established MS received a series of AMP injections over 6-10 months. Marked improvements were noted in endurance and in bladder function. [90] Intravenous AMP administration is not advised. [68]
Phytotherapies Some of the herbs used in traditional folk medicine may make a difference in MS. In a double-blind study, 100 MS patients received a traditional Tibetan herbal formula known as Adaptrin (formerly Padma 28). [91] Half of the patients who received this formula experienced overall improvement in addition to increased muscle strength and improved bladder function.
Natural Interferon Treatment Whereas the beta-interferons currently approved for MS drug therapy are synthetic, a natural alpha-interferon derived from leukocytes could prove just as advantageous and perhaps with a better benefit-risk profile. In three preliminary studies conducted between 1987 and 1991, 45 patients were treated with 5-30 million units per week for 3-12 months, and were observed for two years. [92] Side effects included flu-like symptoms, myalgias, and fatigue which caused some patient dropout. In the first year of treatment, 58 percent of the patients improved and 22 percent stabilized, and after the second year 53 percent remained improved and 22 percent stable. Additional controlled studies are needed.
Histamine Replacement Rediscovered Histamine is a small orthomolecule which exists naturally in the body. In humans, histamine is synthesized from histidine, a conditionally- essential amino acid. Histamine is a proven mediator of allergy, and one of the most persistent dogmas in medicine is that anti-histamines are the best way to block allergy. Yet in MS patients, raising histamine may help block autoimmunity.
According to Drs. Gillson and Wright and their collaborators, [93] in the 1940s while anti-histamines were being discovered, Horton and others at the Mayo Clinic were achieving remarkable results for allergies and other illnesses by raising the body’s histamine (through injection). Later, Horton’s disciple Dr. Hinton Jonez set up a clinic in Tacoma, Washington, exclusively for MS management. Treatments included elimination of food- and other allergies, injections of histamine, B12 and AMP, and physical therapy. The clinic was closed after Dr. Jonez died in 1952.
Physicians at the Tahoma Clinic, in Kent, Washington, recently collaborated in a revival of histamine application for MS. A proprietary transdermal cream (Procarin) was developed by Elaine Delack, RN, herself afflicted with MS, and was administered through the clinic to 55 MS patients. It was judged effective in 67 percent of the cases. [93] Patients improved in one or more areas, including extremity strength, balance, bladder control, fatigue, activities of daily living, and cognitive functioning, sustained for periods of up to three months. This promising work continues.
Oral Antigen Therapy Oral tolerance is the phenomenon whereby oral dosing of an antigen can neutralize the body’s immune sensitization against the molecule. Oral tolerance was first observed when guinea pigs given hen’s egg protein orally were found to become resistant to the anaphylactic reactions normally induced by the ingested protein. In 1986 the oral administration of human collagen was employed to suppress collagen-induced arthritis. Subsequently, attention turned to MS.
In experimental allergic encephalomyelitis (EAE), rats develop an MS-like disease that features autoimmune reactivity against myelin basic protein (MBP). When such rats were orally fed MBP, their EAE was suppressed, demyelination was reduced, clinical neurological signs improved, and antibodies against MBP decreased. [94] A preliminary study with human subjects indicated they did produce the class of T-cells required (in theory, at least) to suppress autoimmunity in the brain, but with appreciable subject-to-subject variation. [95] However, a later controlled human trial failed to find significant clinical improvement. [96] Recent reports from animal research have sparked debate about possible adverse effects. [97]
Hyperbaric Oxygen Therapy (HBOT) Hyperbaric oxygen therapy involves exposure to oxygen at pressures higher than normal air, in multiple sessions repeated almost on a daily basis. Most HBOT protocols involve 20 sessions of 90 minutes each for four weeks, in a chamber with oxygen supplied at 1.75-2.0 times normal atmospheric pressure. Side effects are generally minor. Earlier, uncontrolled trials and anecdotal reports indicated benefit from HBOT for MS. The first double-blind trial reported initial marked improvement in 12 of 17 patients, with more favorable and lasting response in those with less severe disease. [98] Seven controlled trials showed virtually no improvement. A 1995 meta-analysis of these eight available studies concluded HBOT therapy had little to offer for MS; [99] however, all these trials were short term, lasting less than two months.
The Federation of Multiple Sclerosis Therapy Centres in the United Kingdom has very different data on HBOT outcomes in MS. Their HBOT protocol involves indefinite long-term treatment, preferably on a weekly basis, following the initial intense treatment period of four weeks. Their network of 56 treatment centers has treated more than 10,000 patients over a period of 14 years. In 1996, the Federation’s Perrins and James reported on the progress of 703 patients followed for up to 13 years; [100] the results suggested HBOT significantly benefits MS.
The degree of benefit obtained from HBOT was clearly related to the number and frequency of treatments the patients obtained over the years. Patients with all the MS subtypes were interviewed immediately following the initial course and assigned a Kurtzke Disability Scale (KDS) score. They were later assessed at three years (2-4), seven years (6- 8), and 10-13 years after beginning treatment. After the initial course there was little average change in the KDS, although 25 percent of the RR patients had improved scores. Of all the patients, 59-77 percent reported symptomatic improvement. Visual defects were reported by 51 percent of the patients, but many resolved during the initial course. Other initial adverse effects occurred in a minority of the patients, and featured mostly fatigue, leg weakness, and limb numbness, none of which persisted. Minor problems with eardrum pressure did not necessitate stopping treatment.
Following the initial treatment course, 66 percent of the patient cohort continued for three years, 34 percent for seven years, and 24 percent for a minimum of 10 years. At three years there was overall average deterioration on the KDS, but those patients with more treatments had deteriorated less. At seven years, none of the 10 RR patients who had received at least eight treatments in every quarter of each year had deteriorated and four of them had actually improved.
At 10-13 years, 447 patients of the Federation’s initial cohort of 703 remained eligible for assessment. Of these, 23 percent were no worse after regular treatments for 10-13 years; seven percent had actually improved; and the eight percent who had received less than one follow-up treatment per year had seriously deteriorated. Further analysis revealed that a minimum of one treatment every two weeks was correlated with appreciable arrest of progression, and that one per week was probably most effective. Consistent with the known natural history of MS, the relapsing-remitting patients and those who began HBOT at earlier stages experienced greater benefit. The Federation’s study concluded that the degree of KDS maintenance or improvement attainable after 10 years could make the difference between remaining mobile without assistance and needing canes or a wheelchair.
HBOT in the UK has proven beneficial, practical, and cost-effective with minimal adverse effects. The Federation also advises its patients to take advantage of physiotherapy and to adopt healthy dietary habits consistent with the Swank diet and other natural principles . [101] Perlmutter [7] and other practitioners make HBOT available to MS patients in an integrative medical setting.
Emerging Options for Modulating MS Autoimmunity
The immune system is normally able to distinguish between foreign invaders and “self” body tissues in healthy individuals. [102] Autoimmunity results when the immune system inappropriately attacks self tissues. In MS the targets of autoimmunity are mainly the myelin-producing cells in the CNS. [103] T-cells reactive to the major constituents of myelin such as myelin basic protein and proteolipid protein (PLP), migrate from the peripheral circulation, across the blood-brain barrier, and into the brain. There they proceed to attack myelin-ensheathed axons and myelin-producing cells, initiating an inflammatory cascade that eventuates in the white matter lesion or plaque of multiple sclerosis.
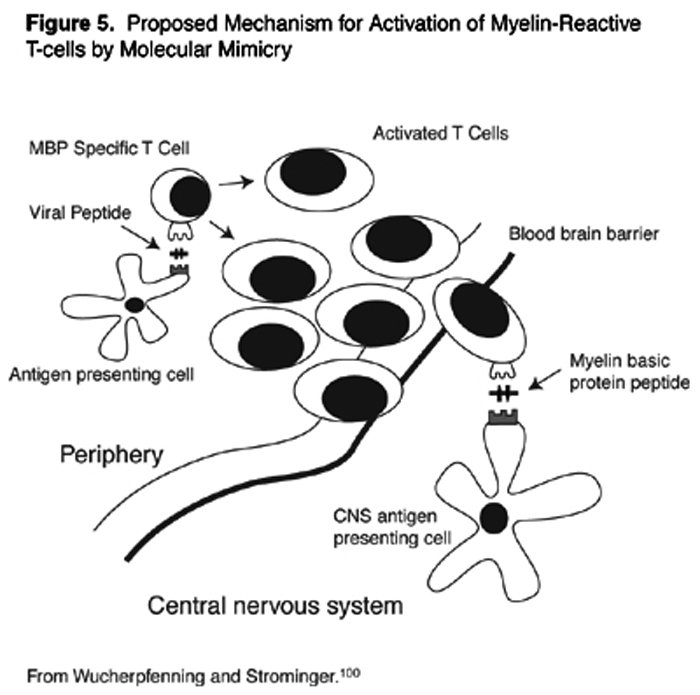
Myelin-reactive T-cells in the peripheral circulation may become activated by microbes. Structural similarities between the foreign antigens on the microbe, and the self-antigens of myelin may lead to inadvertent auto-sensitization of the T-cell against self. This is the concept of molecular mimicry (Figure 5). [104] MBP, for example, shares extensive homologies in amino acid sequences with measles, influenza virus, adenovirus, herpesviruses, papilloma virus, and bacteria, including pseudomonas.
Cytokines are small peptide substances that act as cell-to-cell messengers to regulate immune cell activity. Patterns of cytokine production can set the immune system into one of two dominant modes: T-helper types 1 (Th1) or 2 (Th2). [102] Th1 activation features predominantly cell-mediated cytotoxicity, and inflammatory and hypersensitivity reactions. Th2 activation features predominantly antibody-mediated, allergic responses. Various chronic diseases show differing cytokine patterns; in MS the Th1 pattern clearly dominates. Of the known Th1 cytokines, interferon-gamma worsens disease when given systemically to MS patients, and IL-12 has been implicated in a number of experimental autoimmune diseases; both these cytokines were found to be up-regulated in MS patients. [105] IL-12 was even found abnormally elevated in the serum of secondary progressive MS patients. Therapeutics are being pursued that down-regulate Th1 cytokines, with the goal of systemically turning off the autoimmunity of MS.
Vitamin D3 is a potent immune-modulating substance; [106] it acts via specific receptors carried on antigen-presenting cells and activated T-cells. Exogenous 1,alpha,25-dihydroxyvitamin D3 (DHD3), the biologically active metabolite of vitamin D3, can inhibit different experimental models of autoimmune disease and can significantly ameliorate EAE, the “mouse multiple sclerosis.” [105, 106] Experiments conducted in vitro and in vivo with both DHD3 and its synthetic analogue Ro 63-2023 showed specific inhibition of the Th1 cytokines IL-12 and IFN-gamma. As a consequence of this down-regulation of Th1, inflammation, demyelination, and axonal loss were markedly lowered in the EAE mice. [105] Interestingly, this effect did not up-regulate Th2 pathways; but appeared to calm an overactivated Th1 immune mode. Th1 may not be typically pro-inflammatory, but in the case of EAE/MS, this pathway has seemingly become locked into an exaggerated sensitivity to myelin-related antigens.
An Integrative Management Strategy for MS
A few physicians have been practicing for decades what is effectively integrative medical management of MS. They concur that the implementation of a personalized regimen early in the course of MS can facilitate symptom-free, long-term stabilization of the disease with good quality of life, all without the use of pharmaceuticals. [17, 58, 91] Dietary supplementation combined with dietary reform can halt the deterioration and bring about improvement, as achieved by 94 percent of the patients with newly diagnosed RR-MS over a period of two years. [107] Conscious adherence to an anti-inflammatory diet that incorporates the Swank principles, and aggressive dietary and injectable supplementation with nutrients, are the foundation of the patients’ responsibility for managing their disease.
In addition to the obvious aspects of dietary and lifestyle modification required of the integrative, personalized approach to health maintenance, other measures of personal attention to good health are important, including moderate exercise that reinforces balance and muscle tone, [108] and the conscious avoidance of toxic situations, excessive fatigue, emotional stress, or marked hot and cold temperature oscillations.
An integrative protocol for MS management is submitted (Table 1). For the most effective management of MS, it is suggested the practitioner work closely with the patient to eliminate toxic metals (e.g., lead, mercury) and chemicals from the daily environment and to deplete the total body load. All solvents, insecticides and other pesticides, and for some patients every synthetic in their environment, must be considered suspect. Mere exposure to cigarette smoke can be an exacerbating factor. [109] Allergies, food or inhalant, should be tested for and eliminated. Patients at an early stage of symptoms are likely to benefit from intestinal detoxification and normalization, including food allergy testing. Correction of nutrient deficiencies, GI malabsorption, and probiotic balance often results in multisystem improvement. The viral-microbial load should be identified and treated.
For those few unfortunate patients who sincerely implement an integrative program but still progress to significant disability, a desire may arise to try a pharmaceutical intervention. At this point it becomes crucial to retain the integrated program, as a means to protect against the toxicity attendant with any of the approved MS pharmaceuticals. Hyperbaric oxygen might first be tried, if it is available. Although a fully controlled study on HBOT is still lacking, the use of this modality seems to offer an additional dimension for healing with little risk. And the histamine strategy may provide breakthrough benefit for some patients.
Making a commitment to explore “natural” treatment modalities does not have to mean abandoning opportunities for benefit from pharmaceutical medications, and vice versa. Nutrients and other modalities support homeostasis and help protect the system from the shock of synthetic monotherapies such as Avonex, Betaseron, and Copaxone.
Prospects for Eventual Effective Management of MS
Its unpredictable behavior and apparent complex etiopathogenesis render multiple sclerosis a particularly challenging disease. Whatever the perspectives brought to bear on further studies of MS, it is clear these will require large sample sizes and longer periods of study in order to adequately define benefit. Experts in the MS field have much to disagree about, but there is consensus that MS has significant genetic and environmental components. The genetic contributions are progressively being defined, but discrete environmental factors (viruses, chemical toxins) are still poorly defined.
Most workers in the field agree that infectious agents play a key role in MS, but no one of the several current candidate agents has been conclusively proven. Exogenous toxins are linked with MS clusters but do not account for the north-south gradient of disease prevalence. A role exists for diet, but (with notable exceptions) hardly any organized effort has been made to investigate dietary factors. The overlapping areas of nutrition, lifestyle, and the overall potential for an authentic integrative strategy to stabilize or reverse the disease have generally been neglected in favor of the pharmacological intervention model. Authentic research into the integrative management of MS is sorely needed.
Multiple sclerosis, once established, is likely to lead to serious disability, and in this scenario the best prognosis is partial improvement. But if management is begun early, an authentic integrative strategy will in many cases achieve near-complete relief of symptoms. Strict adherence to diet and lifestyle changes; elimination of toxic and microbial body load; aggressive use of nutrients; and exceptional commitment to minimizing stress, are all mandatory requisites for a quality coexistence with this disease.
A number of ambitious new strategies, mainly high-tech, are being brought to bear on MS. These include the predictable trio of gene therapy, stem cell transplantation, and pharmacological neuroprotection. But the unraveling of its autoimmune component may well be the final frontier for conquering MS. Once this challenging task can be achieved, multiple sclerosis should yield to integrative medical management.
References:
Gaby AR.
Commentary: Multiple sclerosis.
Nutrition & Healing 1997;4:1-11.Waxman SG.
Multiple sclerosis as a neuronal disease.
Archs Neurol 2000;57:22-24.Weinshenker BG.
The natural history of multiple sclerosis.
Neurologic Clinics 1995;13:119-146.Berlex Laboratories.
Betaseron®. Richmond, CA 94804Willer CJ, Ebers GC.
Susceptibility to multiple sclerosis: interplay between genes and environment.
Curr Opin Neurol 2000;13:241-247.Anderson DW, Ellenberg JH, Leventhal CM, et al.
Revised estimate of the prevalence of multiple sclerosis in the United States.
Ann Neurol 1992;31:333-336.Perlmutter D.
BrainRecovery.com: Powerful Therapy for Challenging Brain Disorders.
Naples, FL: The Perlmutter Health Center
(www.brainrecovery.com); 2000.Murray MT, Pizzorno JE.
Multiple Sclerosis. In: Murray MT, Pizzorno JE, eds.
Encyclopedia of Natural Medicine.
Rocklin, CA: Prima Health; 1998:666-676,900-901.Weiner HL, Guttman CR, Khoury SJ, et al.
Serial magnetic resonance imaging in multiple sclerosis: correlation with attacks, disability and disease stage.
J Neuroimmunol 2000;104:164-173.Kurtzke JF.
The Disability Status Scale for multiple sclerosis: apologia pro DSS sua.
Neurology 1989;39:291-302.Landtblom AM, Flodin U, Karlsson M, et al.
Multiple sclerosis and exposure to solvents, ionizing radiation, and animals.
Scand J Work Environ Health 1993;19:399-404.Sibley WA, Bamford CR, Clark K.
Clinical virus infections and multiple sclerosis.
Lancet 1985; i:1313-1315.Kalman B, Li S, Chatterjee D, et al.
Large scale screening of the mitochondrial DNA reveals no pathogenic mutations but a haplotype associated with multiple sclerosis in caucasians.
Acta Neurol Scand 1999;99:16-25.Ebers GC, Yee IML, Sadovnick AD, et al.
Conjugal multiple sclerosis: population-based prevalence and recurrence risks in offspring.
Ann Neurol 2000;48:927-931.Kidd PM.
Parkinson’s disease as multifactorial oxidative neurodegeneration: implications for integrative management.
Altern Med Rev 2000;5:502-529.Kalman B, Alder H.
Is the mitochondrial DNA involved in determining susceptibility to multiple sclerosis?
Acta Neurol Scand 1998;98:232-237.Stratton CW, Mitchell WM, Sriram S.
Does chlamydia pneumoniae play a role in the pathogenesis of multiple sclerosis?
J Med Microbiol 2000;49:1-3.Challoner PB, Smith KT, Parker JD, et al.
Plaque-associated expression of human herpesvirus 6 in multiple sclerosis.
Proc Natl Acad Sci US 1995;92:7440-7444.Knox KK, Brewer JH, Henry JM, et al.
Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease.
Clin Infect Dis 2000;31:894-903.Drobyski WR, Knox KK, Majewski D, et al.
Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient.
New Engl J Med 1994;330:1356-1360.Mackenzie IRA, Carrigan DR, Wiley CA.
Chronic myelopathy associated with human herpesvirus-6.
Neurology 1995;45:2015-2017.Novoa LJ, Nagra RM, Nakawatase T, et al.
Fulminant demyelinating encephalomyelitis associated with productive HHV-6 infection in an immunocompetent adult.
J Med Virology 1997;52:301-308.Raine CS.
The Norton lecture: a review of the oligodendrocyte in the multiple sclerosis lesion.
J Neuroimmunol 1997;77:135-152.Trapp BD, Peterson J, Ransohoff RM, et al.
Axonal transection in the lesions of multiple sclerosis.
New Engl J Med 1998;338:278-285.Sanders VJ, Felisan S, Waddell A, et al.
Detection of herpesviridae in postmortem multiple sclerosis brain tissue.
J Neurovirol 1996;2:249-258.Hofman FM, Hinton DR, Johnson K, et al.
Tumor necrosis factor identified in multiple sclerosis brain.
J Exp Med 1989;170:607-612.Rieckmann P, Albrecht M, Kitze B.
Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity.
Ann Neurol 1995;37:82-88.Gosselin J, Flamand L, D’Addario M, et al.
Modulatory effects of Epstein-Barr, herpes simplex, and human herpes-6 viral infections and coinfections on cytokine synthesis.
J Immunol 1992;149:181-187.Hafler DA.
The distinction blurs between an autoimmune versus microbial hypothesis in multiple sclerosis.
J Clin Invest 1999;104:527-610.Wucherpfennig KW, Catz I, Hausmann S, et al.
Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients.
J Clin Invest 1997;100:1114-1122.Warren KG, Catz I.
Increased synthetic peptide specificity of tissue-CSF bound anti-MBP in multiple sclerosis.
J Neuroimmunol 1993;43:87-96.Lycke J, Svennerholm B, Hjelmquist E, et al.
Acyclovir treatment of relapsing-remitting multiple sclerosis.
J Neurol 1996;243:214-224.Sriram S, Stratton CW, Yao S, et al.
Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis.
Ann Neurol 1999;46:6-14.Boman J, Roblin PM, Sundstrom P, et al.
Failure to detect Chlamydia pneumoniae in the central nervous system of patients with MS.
Neurology 2000;54:265.Greenlee JE, Rose JW.
Controversies in neurological infectious diseases.
Seminars Neurology 2000;20:375-386.Nicolson GL, Nicolson NL.
Autoimmune neurological and rheumatic diseases: role of chronic infections in morbidity and progression. In: American Biologics’ 13th Annual Symposium on Integrative Medicine, Malta.
Chula Vista, CA: American Biologics; 2001.Nicolson GL.
Suggested therapy of chronic systemic chlamydial and chlamydial/mycoplasmal co-infections.
Huntington Beach, CA: Institute for Molecular Medicine; 2001;
www.immed.org.Ross RT, Cheang M, Landry G, et al.
Herpes zoster and multiple sclerosis.
Can J Neurol Sci 1999;26:29-32.Bergstrom T.
Herpesviruses – a rationale for antiviral treatment in multiple sclerosis.
Antiviral Res 1999;41:1-19.Greenberg SJ.
Human retroviruses and demyelinating diseases.
Neurol Clin 1995;13:75-97.Lavi E, Schwartz T, Jin YP, et al.
Nidovirus infections: experimental model systems of human neurologic diseases.
J Neuropathol Exp Neurol 1999;58:1197-1206.Melnick JL, Wang SS, Seidel E, et al.
Characterization of IM virus, which is frequently isolated from cerebrospinal fluid of patients with multiple sclerosis and other chronic diseases of the central nervous system.
J Virology 1984;52:739-744.Munch M, Hvas J, Christensen T, et al.
The implications of Epstein-Barr virus in multiple sclerosis – a review.
Acta Neurol Scand 1997;169:59-64.Eastman R, Sheridan J, Poskanzer DC.
Multiple sclerosis clustering in a small Massachusetts community, with possible common exposure 23 years before onset.
New Engl J Med 1973;289:793-795.Ingalls TH.
Endemic clustering of multiple sclerosis in time and place, 1934-1984.
Am J Forensic Med Pathol 1986;7:3-8.Ingalls TH.
Triggers for multiple sclerosis.
Lancet 1986;ii:160.Huggins HA, Levy TE.
Cerebrospinal fluid protein changes in multiple sclerosis after mercury amalgam removal.
Altern Med Rev 1998;3:295-300.Siblerud RL, Kienholz E.
Evidence that mercury from silver dental fillings may be an etiological factor in multiple sclerosis.
Sci Total Environ 1994;142:191-205.Siblerud RL.
A comparison of mental health of multiple sclerosis patients with silver/mercury dental fillings and those with fillings removed.
Psychological Rep 1992;70:1139-1151.Landtblom AM, Flodin U, Soderfeldt B.
Organic solvents and multiple sclerosis: a synthesis of the current evidence.
Epidemiol 1996;7:429-433.Thrasher JD, Wojdani A, Heuser G, et al.
Evidence for formaldehyde antibodies and altered cellular immunity in subjects exposed to formaldehyde in mobile homes.
Arch Env Hlth 1987;42:347-350.Swank RL, Lerstad O, Strom A, et al.
Multiple sclerosis in rural Norway.
New Engl J Med 1952;246:721-728.Agranoff BWA, Goldberg D.
Diet and the geographical distribution of multiple sclerosis.
Lancet 1974;ii:1061-1066.Lauer K.
Diet and multiple sclerosis.
Neurology 1997;49:S55-S61.Dworkin RH, Bates D, Millar JHD, et al.
Linoleic acid and multiple sclerosis: a reanalysis of three double-blind trials.
Neurology 1984;34:1441-1445.Bates D, Cartlidge NEF, French JM, et al.
A double-blind controlled trial of long-chain N-3 polyunsaturated fatty acids in the treatment of multiple sclerosis.
J Neurol Neurosurg Psychiatry 1989;52:18-22.Kidd PM.
Cell membranes, endothelia, and atherosclerosis – the importance of dietary fatty acid balance.
Altern Med Rev 1996;1:148-167.Swank RL.
Multiple sclerosis: twenty years on a low fat diet.
Arch Neurol 1970;23:460-474.Swank RL, Grimsgaard A.
Multiple sclerosis: the lipid relationship.
Am J Clin Nutr 1988;48:1387-1393.Swank RL, Dugan BB.
Effect of low saturated fat diet in early and late cases of multiple sclerosis.
Lancet 1990;336:37-39.Swank RL.
A prospective discussion of past international nutrition catastrophes – indications for the future.
Nutrition 1997;13:344-348.Hewson DC.
Is there a role for gluten-free diets in multiple sclerosis?
Human Appl Nutr 1984;38A:417-420.Butcher PJ.
Milk consumption and multiple sclerosis – an etiological hypothesis.
Med Hypotheses 1986;19:169-178.Lange LS, Shiner M.
Small-bowel abnormalities in multiple sclerosis.
Lancet 1976;ii:1319-1322.Ehrentheil OF.
Role of food allergy in multiple sclerosis.
Neurology 1952;2:412-426.Embry AF.
The critical need for dietary research into the cause and progression of multiple sclerosis.
In: Embry AF, ed. 2000;
www.direct-ms.org.Gupta JK, Ingegno AP, Cook AW, et al.
Multiple sclerosis and malabsorption.
Am J Gastroenterol 1977;68:560-565.Wright JV.
Multiple sclerosis.
Nutrition & Healing 1997;4:1-12.Geissler A, Andus T, Roth M, et al.
Focal white-matter lesions in brain of patients with inflammatory bowel disease.
Lancet 1995;345:897-898.Noseworthy JH.
Progress in determining the causes and treatments of multiple sclerosis.
Nature 1999;399:A40-A47.Biogen, Inc.
Avonex® Interferon Beta-1a.
Cambridge, MA 02142: Biogen, Inc.; 2000.Teva Marion Partners.
Copaxone® glatiramer acetate.
Kansas City, MO 64134: Teva Marion Partners; 2001.Adams CWM, Poston RN, Buk SJ, et al.
Inflammatory vasculitis in multiple sclerosis.
J Neurol Sci 1985;69:269-283.Wright HP, Thompson RHS, Zilkha KJ.
Platelet adhesiveness in multiple sclerosis.
Lancet 1965;ii:1109-1110.Homa ST, Belin J, Smith AD, et al.
Levels of linoleate and arachidonate in red blood cells of healthy individuals and patients with multiple sclerosis.
J Neurol Neurosurg Psychiatr 1980;43:106-110.Horrobin DF, Botez T, Botez MI.
Polyunsaturated fatty acids and colchicine in multiple sclerosis.
Br Med J 1979;i:199-200.Simpson LO, Shand BI, Olds RJ.
Dietary supplementation with Efamol and multiple sclerosis.
New Zealand Med J 1985;98:1053-1054.Cunnane SC, Ho SY.
Essential fatty acid and lipid profiles in plasma and erythrocytes in patients with multiple sclerosis.
Am J Clin Nutr 1989;50:801-806.Cendrowski W.
Multiple sclerosis and MaxEPA.
Br J Clin Practice 1986;40:365-367.Jimenez-Jimenez FJ, de Bustos F, Molina JA, et al.
Cerebrospinal fluid levels of alphatocopherol in patients with multiple sclerosis.
Neurosci Letts 1998;249:65-67.Shukla VKS, Jensen GE, Clausen J.
Erythrocyte glutathione peroxidase deficiency in multiple sclerosis.
Acta Neurol Scand 1977;56:542-550.Mai J, Sorensen PS, Hansen JC.
High dose antioxidant supplementation to MS patients.
Biol Trace Elem Res 1990;24:109-117.Ziegler D, Reljanovic M, Mehnert H, et al.
Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials.
Exp Clin Endocrinol Diabetes 1999;107:421-430.Brochet B, Guinot P, Orgogozo JM, et al.
Double blind placebo controlled multicentre study of ginkgolide B in treatment of acute exacerbations of multiple sclerosis.
J Neurol Neurosur Psychiatr 1995;58:360-362.Hayes CE, Cantorna MT, DeLuca HF.
Vitamin D and multiple sclerosis.
Proc Soc Exptl Biol Med 1997;216:21-27.Nieves J, Cosman F, Herbert J, et al.
High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis.
Neurology 1994;44:1687-1692.Goldberg P, Fleming MC, Picard EH.
Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium, and vitamin D.
Med Hypotheses 1986;21:193-200.Reynolds EH.
Multiple sclerosis and vitamin B12 metabolism.
J Neuroimmunol 1992;40:225-230.Kira J, Tobimatsu S, Goto I.
Vitamin B12 metabolism and massive-dose methyl vitamin B12 therapy in Japanese patients with multiple sclerosis.
Intern Med (Tokyo) 1994;33:82-86.Lowry ML.
Adenosine-5-monophosphate in the treatment of multiple sclerosis.
Am J Med Sci 1953;226:73-83.Korwin-Piotrowska T, Nocon D, Stankowska-Chomicz A, et al.
Experience of PADMA 28 in multiple sclerosis.
Phytother Res 1992;6:133-136.Squillacote D, Martinez M, Sheremata W.
Natural alpha interferon in multiple sclerosis: results of three preliminary series.
J Int Med Res 1996;24:246-257.Gillson G, Wright JV, DeLack E, Ballasiotes G.
Transdermal histamine in multiple sclerosis: Part one – clinical experience.
Altern Med Rev 1999;4:424-428.Whitacre CC, Gienapp IE, Meyer A, et al.
Treatment of autoimmune disease by oral tolerance to autoantigens.
Clin Immunol Immunopathol 1996;80:S31-S39.Fukaura H, Kent SC, Pietrusewicz J, et al.
Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-B1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients.
J Clin Invest 1996;98:70-77.Brod SA. Gut response.
Therapy with ingested immunomodulatory proteins.
Arch Neurol 1997;54:1300-1302.Trentham DE.
Oral tolerization as a treatment of rheumatoid arthritis.
Rheum Dis Clinics North Am 1998;24:525-536.Fischer BH, Marks M, Reich T.
Hyperbaricoxygen treatment of multiple sclerosis.
New Engl J Med 1983;308:181-186.Kleijnen J, Knipschild P.
Hyperbaric oxygen for multiple sclerosis.
Acta Neurol Scand 1995;91:330-334.Perrins DJD, James PB.
Long-term study on hyperbaric oxygenation for multiple sclerosis.
Bedford, United Kingdom: Federation of Multiple Sclerosis Therapy Centers;
1996: www.ms-selfhelp.org.Federation of Multiple Sclerosis Therapy Centers.
Nutritional and physical advice to patients.
Bedford, United Kingdom: Federation of Multiple Sclerosis Therapy Centers;
2001: www.ms-selfhelp.org.Roitt I, Brostoff J, Male D.
Immunology.
Philadelphia, PA: Mosby; 1998 (Fifth Edition).Steinman L.
Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system.
Cell 1996;85:299-302.Wucherpfennig KW, Strominger JL.
Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein.
Cell 1995;80:695-705.Mattner F, Smiroldo S, Galbiati F, et al.
Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis.
Eur J Immunol 2000;30:498-508.Hayes CE.
Vitamin D: a natural inhibitor of multiple sclerosis.
Proc Nutr Soc 2000;59:531-535.Nordvik I, Myhr KM, Nyland H, Bjerve KS.
Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients.
Acta Neurol Scand 2000;102:143-149.Schwarz J.
Tai Chi Chuan.
Berkeley, CA: Judith Schwartz; 2001:
www.judithschwartz.com.Imre M, de Becker C.
Effects of cigarette smoking on motor functions in patients with multiple sclerosis.
Arch Neurol 1992;49:1243-1247.

Return to ANTIOXIDANTS
Return to MULTIPLE SCLEROSIS
Return to OMEGA-3 FATTY ACIDS
Return to NEURODEGENERATIVE DISEASES & EFAs
Since 12-04-2001


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |