

Impact of Nutrition on Telomere Health: Systematic Review
of Observational Cohort Studies and Randomized Clinical TrialsThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Advances in Nutrition 2020 (May 1); 11 (3): 576–601 ~ FULL TEXT
Serena Galiè, Silvia Canudas, Jananee Muralidharan, Jesús García-Gavilán, Mònica Bulló, Jordi Salas-Salvadó
Human Nutrition Unit,
Department of Biochemistry and Biotechnology,
IISPV, Sant Joan de Reus University Hospital,
Rovira i Virgili University, Reus, Spain.
Diet, physical activity, and other lifestyle factors have been implicated in the pathophysiology of several chronic diseases, but also in a lower total mortality and longer life expectancy. One of the mechanisms in which diet can reduce the risk of disease is with regard to its impact on telomeres. Telomere length (TL) is highly correlated to chronological age and metabolic status. Individuals with shorter telomeres are at higher risk of chronic diseases and mortality. Diet may influence TL by several mechanisms such as regulating oxidative stress and inflammation or modulating epigenetic reactions. The present systematic review aims to examine the results from epidemiologic and clinical trials conducted in humans evaluating the role of nutrients, food groups, and dietary patterns on TL. We also discuss the possible mechanisms of action that influence this process, with the perspective that TL could be a novel biomarker indicating the risk of metabolic disturbances and age-related diseases. The available evidence suggests that some antioxidant nutrients, the consumption of fruits and vegetables, and Mediterranean diet are mainly associated with longer telomeres. However, most of the evidence is based on high heterogenic observational studies and very few randomized clinical trials (RCTs). Therefore, the associations summarized in the present review need to be confirmed with larger prospective cohort studies and better-designed RCTs.
Keywords dietary pattern; food; macronutrients; micronutrients; telomerase; telomere length.
From the FULL TEXT Article:
Introduction
Telomeres are special structures at the end of chromosomes that protect the integrity of DNA information throughout the cell cycle, preventing loss of DNA during cell division. [1] After consecutive cellular divisions, telomere length (TL) naturally shortens until a critical size is reached, which causes cellular senescence or apoptosis, also known as the end replication problem. [2] In people, telomeres shorten with age in all replicating somatic cells that have been examined, including fibroblasts and leukocytes. [3] Thus, TL can serve as a biomarker of a cell's biological (versus chronological) ‘‘age’’ as potential for further cell division. Telomerase, the enzyme with catalytic activity, answers the end replication problem by promoting telomere lengthening. [4]
Telomere attrition is a natural phenomenon widely recognized as one of the hallmarks of aging. [5] A large number of population-based studies have observed a decrease in leukocyte telomere length (LTL) in parallel with increasing age. [6] In this regard, positive relations were established between clinically different pathological conditions, modulated by lifestyle variables through oxidative stress and inflammation, and the accelerated shortening of telomeres. [7] Therefore, nutrition, oxidative damage, telomere shortening, and cell senescence represent a sequence of processes, which may play an important role in in vivo aging and longevity.
As stated previously, telomere shortening has been shown to be accelerated by inflammation [7] and oxidative stress [8, 9], but also by metabolic conditions that increase inflammation and oxidative stress such us abdominal obesity, hyperglycemia, and hypertension. [10] Prenatal conditions and early adversity contribute to adult TL in addition to current stress and other lifestyle factors. In fact, different studies suggest that telomere attrition is modifiable, as substantial variability exists in the rate of telomere shortening that is independent of chronological age. [11] Telomere attrition has also been linked with other potentially modifiable lifestyle factors, such as poor nutrition and physical inactivity [12, 13] indicating the plasticity of TL. In fact, findings from different studies show that a healthy diet, low stress, exercise, and a good sleep pattern are related to longer telomeres. [14, 15] Therefore, TL variability may be partially explained by lifestyle practices, including dietary patterns.
Unfortunately, most of the studies relating lifestyle and telomere health are observational and very few randomized clinical trials (RCTs) generating high-quality evidence have been conducted. Indeed, some studies have not found such associations, which leads to contradictory results regarding the effect of lifestyle on TL. As far as we know, only 2 systematic reviews have been conducted analyzing the effect of diet on TL. [16, 17] However, no one specifically analyzed the association between nutrients, food groups, and dietary patterns using RCTs and observational studies.
Therefore, the aim of this systematic review is to examine the results from epidemiologic and clinical trials conducted in humans evaluating the role of nutrients, food groups, and dietary patterns in TL and telomerase activity. We also discuss the possible mechanisms of action that influence this process, with the perspective that TL could be a novel biomarker, measured from blood samples, that could indicate the risk of suffering age-related diseases.
Although a positive effect of dietary restriction on TL and telomerase activity in rodents has been well documented [18], no clear evidence exists in humans that dietary restriction delays aging related to TL. [19] It is important to mention that in the present review, the effect of reduced calorie intake on telomere health is not addressed.
Methods
Protocol registration
The protocol for the systematic review is available in PROSPERO (http://www.crd.york.ac.uk/PROSPERO; identifier: CRD42019139580).
Search strategy
The studies included in this systematic review were identified by a literature search conducted in both MEDLINE-PubMed and Cochrane Library databases as well as a manual search. Published literature from the earliest available online indexing year up to 1 February, 2019 for PubMed and up to 4 February, 2019 for the Cochrane Library was included.
Both searches were conducted by combining 6 separate search subsets of dietary variables with a unique search subset for telomere health. In each database, a list of Medical Subject Headings and keywords was used, in order to obtain a comprehensive vision of all the published literature available for the purpose of this review (Supplemental Tables 1and2).
Eligibility criteria and study selection was used The search included case-control studies, cross-sectional studies, prospective cohort studies, and RCTs. We considered studies involving males or females, healthy adults or patients affected by some major disease, such as cancer, diabetes, hypertension, or overweight and obesity. We excluded studies conducted in pregnant women, children, or infants.
After a primary screening, the full texts of the selected articles were obtained. Using a standardized model, 2 independent researchers analyzed the full text of the articles that passed the first screening. Finally, a third author evaluated eventual discrepancies in the selected articles. This systematic review includes 59 observational studies and 11 RCTs (Supplemental Figure 1).
Study Quality Assessment
The quality of all included studies in the present systematic review was assessed by 2 independent researchers. The NIH National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-sectional Studies was used in order to assess the risk of bias in observational studies. The Cochrane Collaboration Tool was used to assess the risk of bias in the included RCTs. Depending on the score assigned, the studies were categorized as either good, fair, or poor quality, according to the Agency for Healthcare Research and Quality standards. Discrepancies between researchers were solved by consensus. After analyzing the quality of all studies, 81.3% of the observational and cross-sectional studies were categorized as good quality, whereas 81.8% of the RCTs were categorized to have low risk of bias.
Results and Discussion
Macronutrients and TL

Table 1 Total protein and carbohydrates have not been clearly associated with TL [20, 12] (Table 1). However, results from 2 cross-sectional studies have shown positive associations between dietary fiber intake and TL [12, 21] suggesting that the quality of carbohydrates, and specially dietary fiber, may have a potential beneficial effect on telomere health in parallel to reducing the risk of chronic diseases. [22, 23]
By contrast, the effects of dietary fats with respect to telomeres have been studied in more detail than the other macronutrients. Fat is a key diet component that has been shown to play an important role in inflammation. To date, 2 case-control studies have investigated the association of dietary fat with TL. One cross-sectional study involving prostate cancer participants indicated no associations (P = 0.77) [24], whereas Song et al. [25] showed a positive cross-sectional association with certain classes of fatty acids (FAs). In this case-control study among postmenopausal women, MUFAs and PUFAs showed no associations (P = 0.33 and P = 0.55, respectively), whereas short-to-medium-chain fatty acids (SMSFAs) were inversely associated with LTL. Replacing 1% of energy intake from SMSFAs with other energy sources was associated with longer telomeres. In the same cross-sectional analysis, no association was observed for total fat (P = 0.09). By contrast, in a cross-sectional analysis of the Helsinki Birth cohort, high total fat intake was associated with shorter LTL in men, but not in women (P = 0.26). [26] Similar inverse correlations between dietary total fat, short-chain fatty acids (SFAs), and LTL were seen in a prospective study conducted amongst 609 participants from the Jerusalem Lipid Research Clinic (LRC) cohort. [27] In that study, along with SFAs, MUFAs and PUFAs (expressed as percentage of total energy) showed significant inverse associations in the bivariate models. Interestingly, these associations were seen only in men, but the significance was maintained only in the case of MUFA intake after multivariable backward stepwise regression. [27] PUFA intake, specifically linoleic acid (LA), was also inversely associated with LTL in another cross-sectional study. [12]
An RCT investigating the effect of ω-3 (n–3) supplementation on telomerase activity or LTL showed no significant effects of supplementation. The participants in this placebo-controlled RCT took ω-3 supplements at different concentrations (2.5 g/d or 1.25 g/d) or placebo (FAs in the typical American diet) for a period of 4 mo. Even though no significant differences in TL between the 3 groups were noted (P = 0.39), they found a significant inverse association between changes in the ω-6:ω-3 PUFA ratio in plasma and LTL. [28] It is worth mentioning that the ratio of ω-6:ω-3 has been an interesting topic of study as these 2 FAs regulate several inflammatory pathways by competing for enzymes and receptors. [29] Consequently, ω-6 compared with ω-3 supplementation was investigated in another 6–mo RCT in adults with mild cognitive impairment. Dietary supplementation with both ω-3 FAs, DHA and EPA, or ω-6 LA FA showed a trend towards telomere shortening in the LA-supplemented group and a protective effect on TL in the DHA group. [30] In another ω-3 supplementation study with concentrated fish oil (enriched in EPA + DHA) or placebo (olive oil, rich in MUFA), amongst schizophrenia patients, a significant increase in the peripheral blood mononuclear cell (PBMC) telomerase concentration was reported in the fish oil–supplemented group. [31] Unfortunately, most of the RCTs are of short duration, preventing determination of the effects on telomere shortening.
Taking into account all the aforementioned studies we can conclude that even though most studies have demonstrated a positive relation between ω-3 FA and LTL, the overall relation of MUFAs and PUFAs with LTL is inconsistent, as these studies demonstrated either no significant association (P > 0.05) [25, 26] or an inverse association [27] between MUFAs or PUFAs and LTL. Hence, this requires further investigation in the future by means of longer RCTs conducted in large samples of individuals.
Alcohol has been related to all-cause mortality in a J-shaped curve, although debated for different disease associations. [32, 33] However, its relation with TL is controversial. A case-control study and a cross-sectional study have investigated the association between alcohol intake and TL. The case-control study conducted by Pavanello et al. [34] showed that among 457 participants (200 alcohol abusers, 257 controls), subjects drinking more than 40 g of alcohol per day (drink units) had significantly shorter TL compared with those that drink less. Similar results were seen in a cross-sectional analysis of the Asklepios study, where alcohol consumption was negatively associated with TL in both men and women. [35] In the following section, alcohol beverage intake as a food group will be further discussed in order to give a wider perspective.
Dietary micronutrients and TL

Table 2 TL has also been associated with the intake of micronutrients (Table 2). Various classes of micronutrients such as vitamins, minerals, and other bioactive compounds have been shown to play a protective role against oxidative stress and DNA damage. [36–38] Folate and vitamin B-12, in particular, have been of interest in many studies due to their essential role in purine and pyrimidine synthesis. [39] Vitamin D, a fat soluble vitamin, was inversely associated with low all-cause mortality, type 2 diabetes, and inflammation in some studies. [40, 41]
A retrospective case-control study analyzing the effect of vitamin D supplementation in hemodialysis participants showed that patients not receiving vitamin D treatment had shorter telomeres. [42] This association between vitamin D and TL, however, was not seen in another case-control study with prostate cancer participants (P = 0.77). [24]
The intake of vitamins C and E have also been shown to play important biological roles. Amongst the 3 cross-sectional studies reported in the present review, 1 of the studies showed a positive association between vitamins C or E and TLs. [43] The cross-sectional study by Marcon et al. showed significant correlations for vitamins C and E, which vanished after adjustments. In fact, among all the micronutrients analyzed, β-carotenes showed significant correlations after adjustment for potential confounders. [44] The third study reported a similar positive trend for vitamin E and TL. [12] In the cross-sectional Sisters Study, women taking daily multivitamin supplements had 5.1% longer telomeres compared with the nonusers. [43] With respect to the consumption of vitamin C, similar positive associations to the cross-sectional studies mentioned above were seen in a prospective cohort with 10 y of follow-up among older and middle-aged Korean men, where vitamin C was positively associated with changes in LTL. [50] This study also reported a positive association between folate and potassium intake with changes in LTL. Age stratification in this study showed that all these associations remained significant only among men that were aged <50 y. The authors suggested that a higher fruit and vegetable intake at earlier ages would help delay biological aging. [50] Amongst the cross-sectional studies evaluating the effects of micronutrients on TL, the epidemiological data from the NHANES study indicated a significant positive correlation between TL and dietary copper intake [46], whereas a case-control study found a positive correlation between phosphorous and TL. [42]
Three RCTs exploring the effects of different vitamins and minerals have been published. A double-blind RCT conducted amongst 37 African Americans showed that oral vitamin D supplementation for 16 wk significantly increased PBMC telomerase activity in the intervention group. Even though age and sex are important factors altering TL and telomerase activity, this effect persisted after adjusting for these variables. [47]
In another RCT, supplementation with a commercial compound containing different antioxidants and ω-3 FA for 12 wk was tested in 66 healthy women. In this study, a significant increase in telomerase activity was noted, even if no changes were observed in TL, after supplementation. In the same study, significant changes in the expression of sirtuin family membersSIRT1and 2 in PBMCs were noted. [48] Finally, zinc supplementation in an elderly population in Southern Australia increased from baseline to week 12 of supplementation, but no differences in TL changes between the placebo and the supplemented group were observed (P > 0.05). [49] In summary, because of the heterogeneity between the studies (in terms of design, type of subjects studied, and length), it is extremely difficult to establish beneficial effects of micronutrients and vitamins on telomere health. Therefore, larger and longer studies are required in the future to understand the effect of these nutrients and the beneficial amounts on telomere biology.
Food groups and TL

Table 3 Several epidemiological studies and clinical trials have analyzed the relation between food groups and telomere health under the hypothesis they may influence the aging process via antioxidative and anti-inflammatory effects (Table 3). Vegetables, fruits, legumes, and nuts are sources of polyphenols, unsaturated FAs (in the case of nuts), and fiber. Their consumption has been associated with positive effects on markers of inflammation and oxidative stress, but also with insulin resistance or other cardiovascular risk factors [51, 52] in parallel with longer telomeres. By contrast, processed meats, alcoholic beverages, or sweetened carbonated beverages, and other foods rich in saturated FAs, alcohol, and sugar, have frequently been related to inflammation and oxidative stress in parallel with shorter telomeres.
Nuts, oils, and other dietary fats
Eight observation studies and 1 RCT analyzed the effect of nut consumption [20, 45, 56–58, 60, 64, 67, 71] on telomere health with contradictory conclusions. The effect of walnut consumption on TL has been evaluated only in the context of a clinical trial (the Walnuts and Healthy Aging Study). [71] The authors evaluated the effect of a supplement of 30–60 g/d of walnuts in the context of a typical diet (equivalent to 15% estimated energy requirements) in comparison to the usual diet without walnuts. [71] After 2 y, the participants in the control group had significantly shorter telomeres expressed as percentage of telomeres with a length <3 kb.
Eight cross-sectional studies evaluated the association between nut consumption and TL, and only 3 of them reported positive associations after adjusting for multiple confounding factors [20, 45, 64]. In cohorts such as the NHANES [64] or the Korean Genome Epidemiology Study (KGES) [45] carried out in US and Korean populations, respectively, nut consumption was cross-sectionally associated with longer telomeres, whereas no association was found in the Nurses' Health Study (NHS) (P = 0.91) [58] or the Washington Heights-Inwood Community Aging Project (WHICAP) (P = 0.13). [60] Only Karimi et al. [67] found a negative association between nut and seed consumption with the T/S ratio [telomere (T) to single-copy gene (S) sequence] in a waste recyclers healthy Iranian group.
Six cross-sectional studies and only 1 RCT have analyzed the association between butter or vegetable oil consumption and TL. In 4 cross-sectional studies an inverse association between butter and telomeres [25, 26, 57, 67] was reported, whereas no association was reported in the remaining 2 food groups (P > 0.05). [44, 66] In elderly Chinese women a negative association was found between the intake of fats and oils for cooking and TL but was not found in men (P = 0.61). [57] Song et al. [25] obtained similar cross-sectional results in the case of the Women's Health Initiative (WHI) study that included a subsample of 4029 postmenopausal women; whereas in the Helsinki Birth Cohort Study (HBCS) the association was only found in men. [26] The consumption of solid fats was also negatively associated with TL in 300 Iranian men. [67] However, no associations were reported between fat used for cooking or oil consumption in 2 European studies conducted in Italian (P = 0.12) [44] and Belgian participants (P = 0.91). [66] Finally, in a double-blind RCT, an increased level of telomerase activity was observed in 35 schizophrenia Polish patients after 26 wk of supplementation with 2.2 g/d of olive oil. [31]
Fruits and vegetables
Current evidence is scarce and inconsistent regarding fruit and vegetable intake and their relation with telomeres. Three case-control [53–55] and 4 cross-sectional studies [26, 44, 60, 45] found a positive association between fruit and vegetable consumption and longer telomeres, whereas another 10 cross-sectional studies did not find any association (all, P > 0.05). [12, 20, 24, 35, 56–58, 66–68]
In a gastric cancer case-control study, fruit but not vegetable consumption was associated with longer TL only among controls. [53] In another case-control study with 271 hypertensive and 455 normotensive Chinese participants, vegetable but not fruit intake was associated with longer telomeres. [54] By contrast, in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCOCST; n = 1,661, US participants) no significant associations between fruit ( P = 0.44) or vegetables (P = 0.28) and TL were reported. [24] Kahl et al. [55] only found beneficial associations for fruit and vegetable consumption in those participants who were not exposed to pesticides in a sample of 124 healthy Brazilian farmers, but not in those exposed.
In 4 cross-sectional studies [44, 45, 51, 60] a positive association was observed between fruit or vegetable consumption and TL. In the first 3 studies conducted in occidental populations, only positive associations between the consumption of vegetables and longer telomeres were observed, whereas in the Lee et al. study, a positive association with fruits, but not vegetables was reported in full-adjusted models. Several cross-sectional studies did not find a significant association between fruit and vegetable consumption and TL (all, P > 0.05) [12, 20, 24, 35, 56–58, 66–68]. For example, in a large sample of 2,509 Belgian adults from the Asklepios study, neither fruit nor vegetable intake was associated with TL (P = 0.53) [35] and the same results were observed both in a subsample (P = 0.75) [12] and in the total population [58] of NHS.
Cereals and legumes
A total of 5 cross-sectional studies have found positive associations either with cereals [12, 66, 67] or legumes [20, 45] intake. In 2,284 US women [12], 2,509 Belgian subjects [66], and 100 Iranian males [67], whole grains or whole-grain products were positively associated with longer TL. However, these studies did not take into consideration the type of legumes in their analyses. In 2 different Asian populations [20, 45], legumes but not cereal consumption (P > 0.05) was positively related to TL. Legumes contain folic acid, antioxidants, and other phytochemicals that may be involved in DNA integrity. In the WHICAP study, a negative association between total cereal consumption and TL [60] was reported, even though a secondary analysis with only whole-grain cereals reported no significant association (P = 0.85). Some studies failed to show the significant benefits of consuming cereals [44, 56–58, 62, 68], legumes [26, 57, 58, 60], or both aforementioned food groups [58] on TL, including the single clinical trial that compared both food groups (70) (all, P > 0.05).
Meat, poultry, and derivatives
Thirteen publications have analyzed meat consumption and telomere health [20, 44, 45, 54, 56–58, 60–62, 66–68]. Processed meat contains high concentrations of advanced glycation end products and nitrosamines that may promote inflammation and oxidative stress. Results from 5 cross-sectional studies have shown negative associations between red meat or processed meat and TL [45, 58, 61, 62, 66]. In addition, De Meyer et al. [66] found a negative association between poultry consumption and TL in 1,218 Belgian men. In a subsample of participants from the Multi-Ethnic Study of Atherosclerosis (MESA), those in the highest quartile of processed meat consumption showed shorter TL compared with those in the first quartile. [56] In the Strong Heart Family Study (n = 2,846) a lineal inverse association between processed meat consumption and TL was also observed. [61] A similar inverse association was seen in 2 other studies including an Asiatic population. [45, 68] Only 2 studies included in this review have reported a positive association between red meat consumption and TL. One of these studies showed a significant positive correlation in a sample of 28 healthy people aged 18–65 y. [62] The second study (60) reported a comparable association in a non-Hispanic white population (n = 506) but not in the African-American (n = 536; P = 0.88) and Hispanic groups (n = 679; P = 0.32). In a prospective evaluation of 1,459 Philippine adults, an association between processed or grilled/fried meat consumption and TL was shown after 12 y of follow-up. [58] Similarly, no associations between any meat subgroup and TL [20, 44, 54, 57,58] (all, P > 0.05) were reported in 5 other cross-sectional studies.
Fish
Zhou et al. (20) and Karimi et al. [67] reported a positive correlation between fish intake and TL in normoglycemic or altered-glycemic Chinese and healthy Iranian individuals, respectively. However, 1 case-control study [54], 1 prospective cohort [68], and 7 cross-sectional studies [44, 45, 57, 58, 60, 62, 66] that investigated the relation between fish intake and TL showed no associations (all, P > 0.05). Similar to the above-mentioned prospective cohort [68], no associations [44, 58] have been reported between fish consumption and TL in Italian (n = 56) and US healthy participants ( n = 4,676; P > 0.05). In the MESA [56] nonfried fish consumption was associated with shorter TL in an adjusted model including age and energy intake, but this association was lost after additional adjustments.
Milk and other dairy products
In the KGES [45]) a significant positive association between total dairy product consumption and TL was reported, whereas in the WHI observational study [25] including 4,029 healthy postmenopausal US women, the association was inverse and significantly stronger only when skimmed milk and low-/no-fat cheese were not considered. De Meyer et al. [66] observed a positive association only between cheese intake and TL in men, but not in women (P = 0.51) or the total population (P = 0.33) with other dairy products. Similar results were reported in 8 other cross-sectional studies. [20, 26, 44, 54, 56, 57, 60, 62]
Beverages
Numerous epidemiological studies have analyzed the effect of beverage consumption on TL [20, 45, 54–57, 59, 62, 63, 65, 66]. In 2 cross-sectional studies with a total of 2,741 US men and 7,865 US women from NHANES and NHS, respectively, those participants who drank coffee had longer telomeres than noncoffee drinkers. [63, 65] In another study evaluating this association in an elderly Chinese population, only tea consumption was related to TL in men, but not in women (P = 0.50). [57] In line with the above 2 studies, the results of a crossover intervention study with 40 chronic hepatitis C patients showed that the consumption of coffee for 30 d lowered oxidative damage and increased TL by 40% in 89% of the participants. [69] Caffeine, the main component of tea and coffee, was also associated with longer telomeres.
Leung et al. [59] analyzed the effect of several beverages on TL in 5,309 US adults from the NHANES and reported a marginal association between natural fruit juice consumption and longer telomeres, whereas sugar-sweetened beverage consumption was associated with shorter telomeres. Two studies conducted in Asiatic populations also showed inverse associations between the intake of sweetened carbonated beverages and TL [20, 45]. Although soy has a high content of polyphenols and antioxidants, Lian et al. [54] found that individuals who regularly consumed soymilk had shorter telomeres. Four studies [55, 56, 62, 66] did not find any association between any of the beverage groups considered and TL, including sweetened beverages, alcoholic beverages, nonsweetened beverages, coffee, and juices (P > 0.05).
Taking into account all the studies analyzed in this section, we can conclude that, in general, associations between food groups and TL are inconsistent. Even if several studies have shown significant positive associations in the case of the consumption of nuts, vegetables, coffee, and legumes, and negative associations for butter, processed meat, and sweetened carbonated beverages, other studies were not able to confirm them. However, it is important to mention that most of these studies were cross-sectional, thus more prospective observations and especially RCTs are needed in order to elucidate any possible causal associations, as only this type of study enables establishing cause–effect relations.
Dietary patterns and TL
Dietary pattern analysis has received considerable attention in nutritional epidemiologic studies during recent years, in relation to their emerging influence on the prevention of chronic diseases. Conceptually, dietary patterns describe the overall diet and eating routines of a population, and may thus be more predictive of disease risk than individual foods or nutrients. Increasing evidence suggests that the health benefits of foods are attributed to the additive and synergistic interactions of nutrients and other phytochemicals on different biological mechanisms which are also involved in telomere maintenance, as we will see in the last section of this review. Special dietary quality indices have been proposed to evaluate the adherence to a certain dietary pattern (e.g., Mediterranean diet, Western diet, vegetarian diet, vegan diet, and others). Dietary patterns also reflect adherence to formal dietary guidelines recommended for disease prevention. [72]

Table 4 Several epidemiological studies showed that adherence to specific dietary patterns may be related to changes in telomere attrition. Of the 70 articles selected in this systematic review, 16 evaluated the effect of dietary patterns on TL [45, 56, 58, 60, 66, 67, 73–82] (Table 4).
A priori food patterns
Related to food a priori patterns, 11 cross-sectional [58, 60, 66, 73–76, 78–81] 3 prospective cohort [75, 76, 81] studies, one of them RCT [76], were included in this systematic review (Table 4).
The Mediterranean diet (MedDiet) is one of the best dietary patterns analyzed in relation to cardiovascular disease (CVD) risk and other health outcomes [83] including reduction of overall mortality [84–87] and increased likelihood of healthy aging. [88] Some key foods of the Mediterranean diet, such as, vegetables, seeds, fruits, olive oil, nuts, and wine, are especially rich in antioxidant and anti-inflammatory components and their consumption has been broadly associated with an improvement of several inflammatory and oxidant biomarkers (see the food and nutrient sections), which have been implicated in the maintenance of TL. However, it is unclear whether the protective effects on TL provided by the MedDiet result from its individual constituents or the combination of these.
A total of 8 studies [58, 60, 74, 76, 78–81] focused on analyzing the relation between adherence of MedDiet and TL and their conclusions are controversial. Most of the studies are cross-sectional. Crous-Bou et al. [58], in a cohort of 4,676 healthy American women, showed that greater adherence to the MedDiet was significantly associated with longer TL. However, in that study, no association between any of the individual Mediterranean food items analyzed and TL was demonstrated, which supports the concept of the dietary pattern as a powerful tool to detect health benefits. In a similar way [74], adherence to the MedDiet was positively associated with telomerase activity, and accordingly with longer TL. These findings were confirmed by another study conducted in 1,743 individuals from the WHICAP study [60], where a significant association between the MedDiet and TL among a non-Hispanic white population was reported, even though no association was found in the total population studied (P = 0.15). This positive association between baseline adherence to the Mediterranean diet and TL was also reported in a population of women at high cardiovascular risk [Prevention with Mediterranean Diet study (PREDIMED)] [76] and in the NHANES study. [79]
Four cross-sectional studies [66, 75, 78, 81] have analyzed the relation between the Dietary Inflammatory Index (DII) and TL. In 2 of them, respectively, conducted in healthy US adults and Spanish people at high CVD risk, a high DII score was associated with shorter TL after adjusting by potential confounders [75, 78], whereas no association was reported in the HBCS study (P = 0.24 in women and P = 0.36 in men) [81] and in 2509 Belgian males and females from the Asklepios population (P = 0.95). [66]
Few other studies have been conducted on other a priori dietary patterns that have also demonstrated beneficial effects on health. [73, 79–81] Leung et al. [79] showed in the NHANES cohort that not only MedDiet, but also the Healthy Eating Index (HEI-2010), the Alternate Healthy Eating Index (AHEI-2010), and the Dietary Approaches to Stop Hypertension (DASH) scores were each positively associated with TL at baseline. On the contrary, no association between TL and the Baltic Sea Diet Score (BSDS; P = 0.41) [81] or other a priori healthy dietary index have been shown in 2 other studies [73, 80] (P > 0.05 in both).
Only 2 studies have prospectively examined the association between a priori dietary patterns and changes in TL. [75, 81] In a population of 1,046 females and males from the HBCS aged 56–70 y, no association was reported between the Mediterranean diet score (P > 0.05), BSDS, and anti-inflammatory dietary index with changes in TL after 10 y of follow-up (P > 0.05). [81] In that study a positive association between MedDiet adherence and telomere shortening was reported only in women. In contrast, in the context of the PREDIMED study, a greater anti-inflammatory potential of the diet (i.e., a decrease in the DII) was associated with increased TL. After 5 y of follow-up, participants with a high proinflammatory diet index had a 2-fold higher risk of accelerated telomere attrition compared with participants with an anti-inflammatory diet. [75]
To the best of our knowledge, the effect of a dietary pattern has only been tested in 1 RCT. In the context of the PREDIMED trial, no beneficial effects on changes in TL were reported in those participants allocated to the 2 Mediterranean intervention arms compared with those allocated to the control group after 5 y of intervention (P = 0.58). [76]
A posteriori food patterns
Two main approaches to characterize dietary patterns are those that are determined a priori (i.e., HEI-2010, MedDiet, DII) and those that are derived a posteriori (e.g., principal component or cluster or factor analyses). The key advantage of a posteriori–derived food patterns is that they take into account many aspects of the diet rather than focusing on a few hypothesized key food or nutrient groups. On the other hand, a posteriori dietary patterns do not build on previous research and thus do not appraise current diet–disease paradigms.
Four cross-sectional [56, 67, 77, 82] and 1 prospective a posteriori food-derived dietary patterns [45] have been related to TL. In the context of the MESA study including 840 white, African-American and Hispanic adults, neither a dietary pattern characterized by the high consumption of fats and processed meat, nor a dietary pattern composed of whole grains and fruit were associated with TL after adjustment for potential confounders. These results were unexpected in light of the observed association between the intake of processed meat and TL in the same study. [56] On the other hand, 3 food patterns (FAs, minerals and vitamins, and PUFAs), together explaining 56.8% of the variance of the dietary nutrient consumption, were identified in the NHANES study. [77] A food pattern, which was representative of minerals and vitamins, was positively associated with TL in adjusted models. Moreover, the intake of dietary fiber, total folate, vitamin B-6, magnesium, iron, copper, and vitamin C also increased across the quartiles of TL whereas total fat and caffeine decreased across TL quarters.
Karimi et al. [67] found a positive relation between adherence to a healthy lifestyle and TL in a cross-sectional study of healthy male residents of Tehran and a negative association with the Western pattern. Adherence to a healthy lifestyle and a dietary pattern with an increased consumption of fruit, vegetables, whole grains, fish, and dairy products appears to be necessary to prevent TL attrition.
A similar dietary pattern, also including nuts, eggs, and tea, was cross-sectionally related to TL in women but not in men from a Chinese cohort. [82] In a Korean population followed for 10 y, a posteriori–derived food pattern so-called “prudent dietary pattern” characterized by a high intake of whole grains, seafood, legumes, vegetables, and seaweed was prospectively associated with leukocyte TL in contrast to a “Western dietary pattern” characterized by the high intake of refined grain, red or processed meat, and sweetened carbonated beverages. In the analysis of particular food items, the higher consumption of legumes, nuts, seaweed, fruit, and dairy products and lower consumption of red meat or processed meat and sweetened carbonated beverages were associated with longer TL. These results suggest that diet in the remote past, that is, 10 y earlier, may affect the degree of biological aging in middle-aged and older adults. [45]
However, current evidence regarding dietary intake and TL is still scarce and there are also inconsistencies. More studies are needed to better understand the relation between diet and TL. Further large prospective and RCTs are warranted in the future to establish a causal relation between food or dietary patterns and telomere attrition.
Blood concentrations of selected nutrients and TL

Table 5 Despite an increasing interest in studying the relation between diet and telomere health, it is sometimes difficult to directly correlate dietary intake of certain macro- and micronutrients with a specific response at the molecular level. Therefore, we will present a separate section, where we have included all those studies in which plasma or serum concentrations of macro- and micronutrients (in some cases reflecting the status of this nutrient) have been measured and analyzed in association with telomere attrition and maintenance (Table 5).
FAs
Multiple epidemiologic studies have demonstrated greater survival rates among individuals with a higher dietary intake of marine ω-3 FA. [86, 87] Therefore, considering TL as a potential biomarker for biological aging, the association between ω-3 FA intake and TL is gaining more and more attention in the scientific community. Farzaneh-Far et al. [14] found a significant inverse association of baseline concentrations of ω-3 FA in whole blood with telomere shortening after 5 y of follow-up in a prospective cohort study of 608 outpatients with stable coronary artery disease from California. Previous findings suggested that deceleration of telomere attrition could be a potentially novel pathway for ω-3 FAs to exert their known antiaging properties. [103, 104] Nonetheless, a specific mechanism is still missing; for instance, the oxidative stress could be a powerful driver for telomere shortening, such as ω-3 FA reshaping the FA composition of cell membranes in order to reduce oxidative damage. Furthermore, a higher telomerase activity had been previously correlated with ω-3 FA supplementation in PBMCs. [105]
At the same time, trans-fatty acids (TFAs) have also been differently correlated with TL in NHANES, the periodic cross-sectional surveys conducted in US adults. Palmitelaidic and linolelaidic acids detected in plasma were positively associated with an increasing telomere shortening over time. [102] In fact, a diet rich in TFAs had already been correlated with a lower antioxidant effect on DNA damage in animal models, in a way that these findings confirm the hypothesized relation between telomere attrition and oxidative DNA damage.
Vitamins
Telomere attrition is accelerated in oxidative stress and chronic inflammatory conditions. In this regard, it will be relevant to investigate the potential associations between dietary intake of anti-inflammatory agents and TL, which is detailed in the following paragraphs.
Vitamin D is a known potent inhibitor of the proinflammatory response and thereby diminishes the turnover of leukocytes, with a consequent decrease in LTL. Nonetheless, dietary intake of vitamin D is not a good indicator of its authentic metabolically active form. Rather, the hydrolyzed form, 1,25-dihydroxyvitamin D [1,25(OH)D3] is the biologically active form. [106]
Correlations between this biologically active 1,25(OH)D3 with TL were evaluated in the Health Professionals Follow-Up Study (HPFS), among 2,843 men. The cross-sectional analysis showed no associations between vitamin D and LTL. [107] Similarly, no associations were found in the Northern Finland Birth Cohort, with 5,096 participants (P = 0.97). [98]
Instead, another cross-sectional study conducted with 2,160 adult women from a large population-based cohort of twins in the UK, observed a positive association between the serum concentration of 1,25(OH)D3 and TL. In addition, a similar trend of association has been observed in this study between serum concentrations of vitamin D and C-reactive protein (CRP) concentrations. [105] Pusceddu et al. [92] evaluated the effects of folate treatment against vitamin D supplementation in an elderly population. The results obtained from a cross-sectional analysis of baseline values revealed that subjects with TL above the median had higher concentrations of total folate.
When examining TL correlations in a representative population of US Radiologic Technologists Study (USRT) the results are contradictory. In fact, no association was found in either of the different race subsets of the analyzed population (P = 0.87) except for vitamin D deficiency, which was significantly correlated with shorter telomeres, at least in the white population of the cohort. [96] Vitamin B-12, folate, α- and γ-tocopherols all exert beneficial effects on brain aging other than contributing to telomere maintenance, at least in in vitro models. [108] Nonetheless, results obtained from different studies about their relations with TL are inconsistent, as can be observed from the studies included in this review.
For instance, no direct association has been found between folate, vitamin B-12, and telomere shortening in adults aged 55 y involved in a cross-sectional study embedded within the population-based KGES cohort. [97] Furthermore, no significant association has been found in the Framingham Offspring Study between plasma folate concentrations and TL, after 4 y of folic acid supplementation even if a positive trend has been observed in participants with a higher plasma concentration of folic acid. [98]
Two cross-sectional studies from the NHANES population showed contrasting results for analysis with bioactive compounds, micronutrients in serum, and TL. One study showed no association between TL and any of the analyzed micronutrients. Nomura et al. [99] did not find any significant association with TL for any of the nutrients analyzed; whereas another study reported an inverse association between γ-tocopherol and TL. [100]
Carotenoids
Various epidemiological studies have explored the antioxidative effect of both dietary and serum concentrations of carotenoids with different health and aging-related diseases in which oxidative stress plays a major role. In a representative population of 3,660 US adults from the NHANES study, TL was positively associated with serum concentrations of different carotenoids, like α-carotene, β-carotene (trans + cis), β-cryptoxanthin, combined lutein/zeaxanthin, and trans-lycopene. [91] The same population was also examined by Nomura et al. [99] in order to evaluate the potential association between serum concentrations of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene and TL in 7458 participants of the NHANES study. However, no association between carotenoids and leukocytes TL were reported (P > 0.05). [109] Nonetheless, the opposite results were obtained in an older population of Italians, in which telomerase activity has been significantly and positively correlated with β-carotene plasma concentrations. [89]
Minerals
The plasma or serum concentrations of certain minerals have been correlated with diseases in which telomere shortening also plays an important role, such as CVD and cancer. Therefore, new emerging studies have explored these associations in cohort studies. [49]
O'Callaghan and colleagues [110] reported several relations between plasma minerals (calcium, magnesium, potassium, selenium, and zinc) and TL, which was strictly dependent on age and sex. In particular, a significant negative association between TL and plasma concentrations of magnesium and calcium was observed only in older females.
Body iron status has also been inversely associated with TL in US adults aged ≥65 y belonging to a NHANES cohort population. The association between high body iron status and shorter TL is biologically plausible, as it is a powerful pro-oxidant and hydroxyl radical promoting agent. Even so, the detailed molecular mechanisms, which explain this relation, remain unclear (101).
In conclusion, although in many cases blood concentrations of some nutrients better reflect the nutrient status of the individual, no clear associations have been systematically reported between blood concentrations of FAs, vitamins, or minerals and telomere length.
Potential mechanisms implicated in nutrition regulation of telomere maintenance
Telomere maintenance has been shown to be positively associated with nutrition status. As has been shown, various nutrients influence TL probably through mechanisms involved in cellular functions embracing inflammation, oxidative stress, DNA methylation, DNA integrity, and telomerase activity.
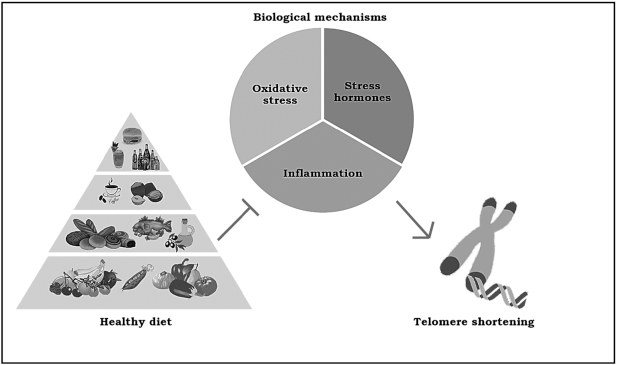
Figure 1 Even if the biochemical pathways that explain the relation between environmental impact and telomere maintenance remain unclear, there are 3 main potential mechanisms involved in this association: stress hormones, inflammation, and oxidative stress (Figure 1). First, it is important to state that the production of reactive oxygen species could potentially increment telomere erosion. In fact, telomere sequences are highly prone to oxidation into 8-oxoG because of the high content of guanine residues, as has been observed in different in vitro studies. [111] When present in telomeres, 8-oxoG residues are likely to decrease both the affinity of shelterin proteins for telomeric DNA and disrupt G-quadruplex structures of telomeres that play an important protective role in avoiding telomere shortening. [112] In addition, oxidative damage of telomeres inhibits telomerase, leading to telomere shortening, giving rise to premature cell senescence. [113]

Table 6

Table 7 Therefore, whatever molecular mechanism is involved in increasing oxidative stress, it could potentially be related to dysregulation of telomere maintenance, such as during chronic inflammatory status. In fact, IL-6 and TNF seem to influence and directly modulate telomerase activity. [114] Insulin resistance is also a chronic state related to both oxidative stress and inflammation that has a great role in promoting telomere shortening, as has been observed in type 2 diabetes populations. [114] At the same time, chronic stress seems to exert a critical function in telomere maintenance, both by direct exposure or mother transmission in early life. [115] Even if biological mechanisms are still unclear, the dysregulation of the hypothalamic-pituitary-adrenal axis and anomalous secretion of cortisol during a chronic stress condition may increase telomere shortening [113] and reduce telomerase activity [117] in humans.
Obviously, different environmental factors, such as diet and lifestyle, could modulate these biological mechanisms of telomere maintenance by promoting or preventing cellular oxidative stress and inflammation.
Other than promoting an antioxidative environment, diet could also play a great epigenetic role in telomere maintenance, by means of the acetylation and/or methylation of histones, which are directly related to telomere recombination and regulation of TL. [118]
Conclusions
In this systematic review, an overall understanding of the relation between diet and telomere maintenance has been discussed. Generally, it could be observed that adherence to healthy dietary patterns, such as MedDiet, and more specifically the introduction of certain micronutrients in the diet, could have a protective effect on telomere shortening. The same positive tendency has also been found for certain food groups like fruit and vegetables or coffee, even if results remain inconsistent (Tables 6 and 7). In addition, potential common risk factors have been identified in these studies. Among them, there are some known proinflammatory food groups such as processed meat and sweetened beverages. Confirming their negative contribution on telomere health, the same negative association has been observed with a high inflammatory dietary pattern.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SC, MB, JS-S: initiated the idea of this review and designed it; SG, SC: performed the screening procedure in order to collect the selected articles. SG, SC, JM, JG-G: wrote the manuscript; SG, SC, JM, JG-G: critically reviewed the article for important intellectual content; JS-S: assessed the articles and helped to draft and critically review the article for important intellectual content; and all authors: read and approved the final manuscript.
Notes
The Physiopathology of Obesity and Nutrition Networking Biomedical Research Center (CIBEROBN) is an initiative of the Carlos III Health Institute(ISCIII) of Spain, which is financed by the European Regional Development Fund (ERDF) (CB06/03). SG is a doctoral fellow from AGAUR no. 2018FI_B_00444, Generalitat de Catalunya. SC is supported by an RYC-2013-12598 grant by the Spanish Ministry of Science, Innovation and Universities; JM is a doctoral fellow funded by the European Union's Horizon 2020 research and innovation program under the Marie Skodowska-Curie grant agreement no. 713679 and from the Rovira i Virgili University (URV); JG-G received a PFIS grant no. FI17/00255 into the AES program from the Carlos III Health Institute (ISCIII), Spanish Ministry of Health.
Author disclosures:
The authors report no conflicts of interest.
Abbreviations used:
BSDS = Baltic Sea Diet Score;
CVD = cardiovascular disease;
DII = Dietary Inflammatory Index;
FA = fatty acid;
HBCS = Helsinki Birth Cohort Study;
HEI-2010 = Healthy Eating Index;
KGES = Korean Genome Epidemiology Study;
LA = linoleic acid;
LTL = leukocyte telomere length;
MedDiet = Mediterranean diet;
MESA = Multi-Ethnic Study of Atherosclerosis;
NHS = Nurses' Health Study;
PBMC = peripheral blood mononuclear cell;
PREDIMED = Prevention with Mediterranean Diet Study;
RCT = randomized clinical trial;
SFA = short-chain fatty acid;
SMSFA = short-to-medium-chain fatty acid;
TFA = trans-fatty acid;
TL = telomere length;
WHI = Women's Health Initiative;
WHICAP = Washington Heights-Inwood Community Aging Project;
1,25(OH)D3 = 1,25 dihydroxyvitamin D.
REFERENCES
Return to ALL ABOUT TELOMERES
Since 1-20-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |