

Inhibition of Protein Glycation by Skins and Seeds
of the Muscadine GrapeThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Biofactors 2007; 30 (3): 193–200 ~ FULL TEXT
Farrar JL, Hartle DK, Hargrove JL, Greenspan P
Department of Pharmaceutical and Biomedical Sciences,
Nutraceutical Research Laboratories,
University of Georgia, Athens, GA, USAThe formation of advanced glycation end products (AGEs) leading to protein glycation and cross-linking is associated with the pathogenesis of diabetic complications. The inhibition of protein glycation by phenolic and flavonoid antioxidants demonstrates that the process is mediated, in part, by oxidative processes. In this study, the effects of seed and skin extracts of the muscadine grape on AGEs formation were examined. Seeds and skins were extracted (10% w/v) with 50% ethanol and incubated at 37 degrees C with a solution containing 250 mM fructose and 10 mg/ml albumin. After 72 h, fluorescence was measured at the wavelength pair of 370 and 440 nm as an index of the formation of AGEs. Both seed and skin extracts were found to be efficacious inhibitors of AGE formation. A 1:300 dilution of the seed extract decreased fluorescence by approximately 65%, whereas muscadine grape skin extract produced a 40% lowering. This difference correlates with the greater antioxidant activity found in muscadine seeds in comparison to skins, however, on a mass basis, the inhibitory activities of the seeds and skins were found to be nearly equivalent. Gallic acid, catechin and epicatechin, the three major polyphenols in the seeds, all significantly decreased the AGE product related fluorescence at a concentration of 50 microM. Neither muscadine seed extract nor skin extract inhibited the methylglyoxal-mediated glycation of albumin. These results suggest that consumption of the muscadine grape may have some benefit in altering the progression of diabetic complications.
From the FULL TEXT Article:
Introduction
Chronic circulatory diseases are a major complication of diabetes mellitus. [9, 13] Apossible biological process responsible for these pathological states is protein glycation or the formation of advanced glycation end products (AGEs). [1, 20] In this reaction, free amino groups of protein react slowly with the carbonyl groups of reducing sugars to produce Schiff base intermediates, which undergo spontaneous Amadori rearrangement to stable ketoamine derivatives. [17] The Amadori products then degrade into α-dicarbonyl compounds that are more reactive than the parent sugars with respect to their ability to react with amino groups of proteins. Thus, reactive carbonyl species such as 3-deoxyglucosone and methylglyoxal are mainly responsible for forming inter- and intramolecular cross-links of proteins. [1] These processes produce structural and functional alterations in plasma and extracellular matrix proteins.
Oxidative processes participate in the formation of AGEs under experimental conditions. Hydrogen peroxide [31] and superoxide anion [22] have been shown to be generated during these reactions, while binding of AGEs to its receptor (RAGE) induces the formation of reactive oxygen species. [26] Inhibition of the protein glycation, both in vitro and in vivo, has been observed with antioxidants such as alpha-lipoic acid [23], plant extracts [16] and isolated phenolic compounds. [5]
In this communication, the effect of ethanolic extracts of the muscadine grape on the glycation of albumin is examined. The muscadine grape (Vitis rotundifolia) is native to the southeastern United States. [10] Having been cultivated since the 16th century, the grapes are very well adapted to the heat of the southeast and are more prevalent than any other grape variety in this climate. The grape possesses one of the highest antioxidant capacities of any fruit. [24] The seeds of the muscadine have a greater antioxidant capacity than the skins. The results presented demonstrate that both muscadine skins and seeds can significantly inhibit the non-enzymatic glycation of albumin.
Materials and methods
Chemicals
Bovine serum albumin (BSA) (Fraction V, Essentially Fatty Acid Free, D- (-) fructose, Chelex 100 (sodium form), Folin-Ciocalteu reagent, methylglyoxal solution, gallic acid, epicatechin and catechin, and TPTZ (2,4,6-tri[2-pyridyl]-s-triazine) were purchased from Sigma Chemical Company (St Louis, MO). Powdered muscadine grape seeds and skins were a gift of Muscadine Products Corporation, LLC (Wray, GA).
Preparation of muscadine extracts
Muscadine grapes, from the Ison variety, were pressed for juice production and deseeded. The grape skins were dried at 50°C for 12 h in a forced-air pan dryer manufactured by Powell Manufacturing Company (Bennettsville, SC). The dried skins were ground in a Fitz Mill Comminutor Hammermill manufactured by the Fitzpatrick Company (Elmhurst, IL). To prepare the muscadine seed extract, dried muscadine seed was made into a powder using a commercial coffee grinder. The seeds and skins were extracted 1:10 (w/v) with 50% ethanol for 2 h at room temperature with periodic vortexing. The extract was then centrifuged to remove the precipitate and filter sterilized to obtain the final extract.
Total phenolic content
Phenolic content of sampleswas determined with the Folin-Ciocalteu method as described by Singleton and Rossi. [28] Gallic acid was employed as the standard. Absorbance was measured at 765 nm in a Beckman DU 600 series spectrophotometer. Results are expressed as mg gallic acid equivalents per g of pulverized extract.
Ferric reducing antioxidant power (FRAP)
FRAP values were determined by a modified version of the Benzie and Strain method [4], with ferrous sulfate as the reference standard. Absorption was measured at 593 nm in a Beckman DU 600 series spectrophotometer. The FRAP assay is based on the reduction of a ferric 2,4,6-tripyridyl-s-triazine complex to the ferrous form. The results are expressed as mmoles of ferrous sulfate formed per 100 g of dry weight of grape skin or seed.
Albumin glycation
The fluorescence assay to determine the glycation of albumin was performed by a modification [16] of the method described by McPherson et al. [20] Bovine serum albumin (10 mg/ml) was incubated with D- (-) fructose (250 mM) in potassium phosphate buffer (200 mM; pH 7.4; 0.02% sodium azide) in a 5% carbon dioxide incubator at 37oC for 72 h. The buffer was treated with Chelex 100 prior to use. Various concentrations of the extracts were added to the 3-ml incubation mixture. To control for the ethanol present in the extract, control incubations were incubated in the presence of the appropriate concentration of ethanol. The fluorescence intensity was measured at an excitation/emission wavelength pair of 370/440 nm using a Perkin-Elmer LS 55 Luminescence Spectrometer. Slit widths were set at 3 nm. Values were corrected for the intrinsic fluorescence of muscadine seed and skin extracts.
Modification of albumin by methylglyoxal
Bovine serum albumin (100 µM) was incubated with 1 mMmethylglyoxal in 0.1Msodium phosphate, pH 7.0 according to the method of Lee et al. [17] The buffer was treated with Chelex prior to use. After 96 h, the fluorescence was measured using the wavelength pair of 350 and 409 nm. Values were corrected for the intrinsic fluorescence of muscadine seed and skins extracts.
Statistical analysis
Experiments were performed in triplicate. Values are expressed as mean ± SEM. Data within skin and seed groups were analyzed using one-way analysis of variance (ANOVA) and multiple comparisons were performed employing the Duncan’s Multiple Range test. P < 0.05 was considered statistically significant.
Results
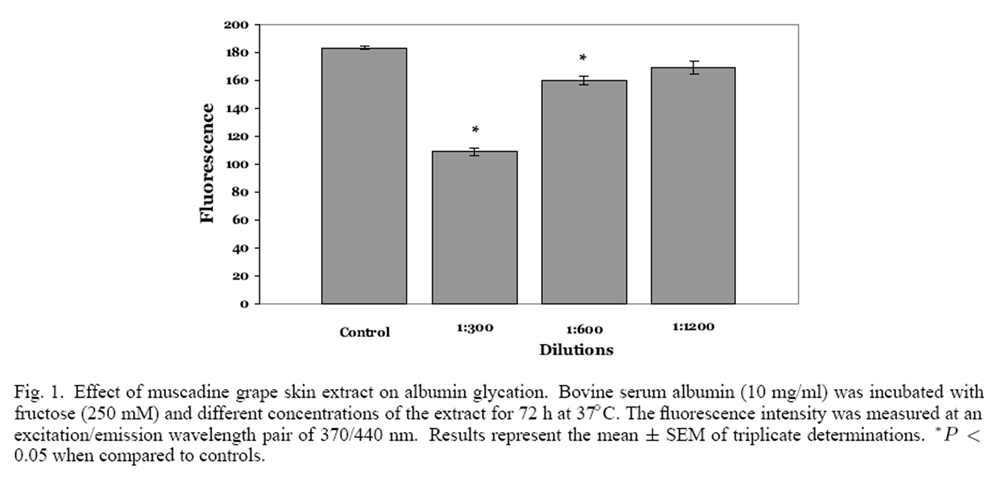
Figure 1
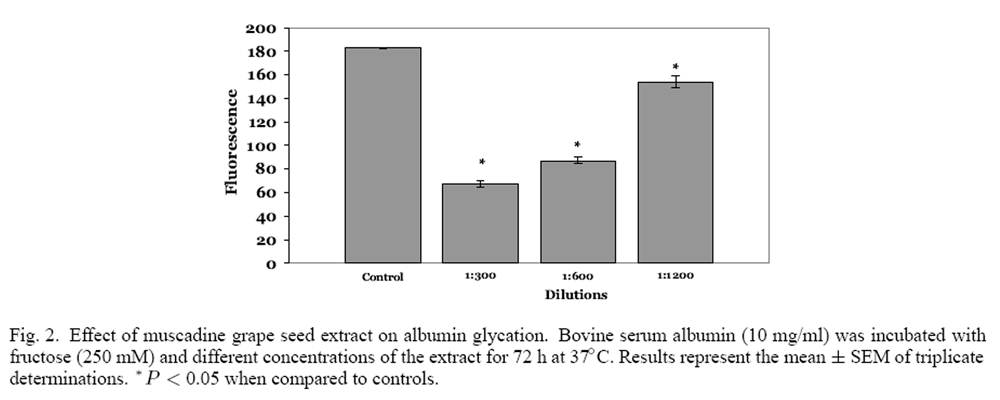
Figure 2 Both muscadine seeds and skins were extracted with 50% ethanol (10% w/v) and the phenolic content of the extracts was ascertained. Muscadine seeds had a phenolic content of 38.7 mg/g, which was significantly greater than muscadine skins (20.0 mg/g), in agreement with the results of Pastrana- Bonilla and colleagues. [24] The effect of muscadine seeds and skin extracts on the glycation of albumin was examined at three different dilutions of the extract. Control incubations of fructose and albumin resulted in significant albumin glycation; the relative fluorescence intensity was found to be approximately 180 units (Figure 1). When fructose and albumin were incubated with 1:300 and 1:600 dilutions (6.6 and 3.3 µg phenolics/ml) of muscadine skin extract, a significant concentration dependent decrease in fluorescence intensity was observed. Conversely, the 1:1200 dilution (1.6 µg phenolics/ml) was not significantly different from the fluorescence intensity observed from the control incubation. A significant concentration-dependent decrease in fluorescence intensity was also observed with muscadine seed extracts (Figure 2). Muscadine grape seed extract at a dilution of 1:1200, representing 3.2 µg phenolics/ml, produced a significant inhibition in albumin glycation; inhibition was not observed with lower concentrations of the extract.
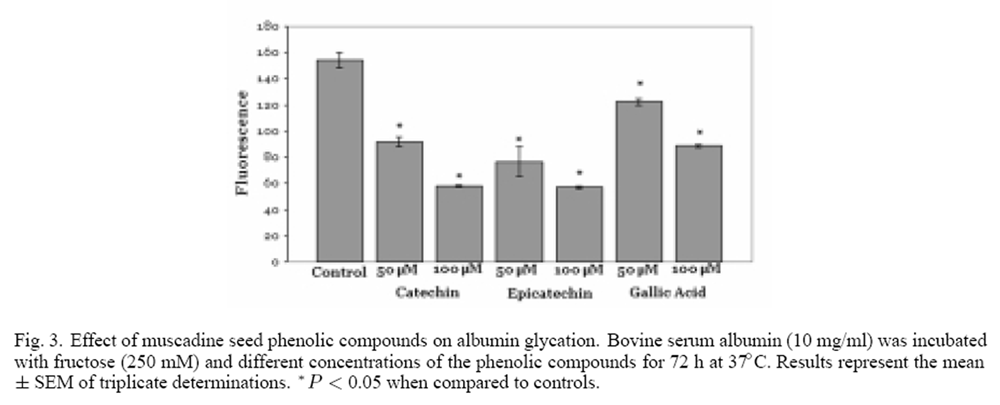
Figure 3 Three of the major phenolics present in the muscadine seed fractions are catechin, epicatechin and gallic acid. [33] The effect of these three compounds on the glycation of albumin was examined. As seen in Figure 3, all three phenolic compounds inhibited the glycation of albumin. Catechin and epicatechin produced the greatest effect, an approximate 65% inhibition of glycation was observed at a concentration of 100 µM. In contrast, gallic acid was the weakest inhibitor of glycation; 100 µM gallic acid produced an approximate 40% decrease in the extent of albumin glycation.

Figure 4 Muscadine grape seeds were extracted with 50% ethanol or distilled water to determine whether the inhibition of glycation was dependent on the type of solvent used for extraction. After the 2 h incubation with periodic vortexing, the phenolic content of the extracts was determined. The ethanolic extract had a phenolic content of 2.4 mg/ml while the content of the aqueous extract was 1.6 mg/ml. The antioxidant property of the extracts was determined employing the FRAP assay. In agreement with phenolic content, the ethanolic extract has a greater FRAP value (27.8 mmoles/100 g vs. 23.5 mmoles/100 g, respectively). The glycation of albumin was measured in the presence of two dilutions (1:300 and 1:600) of the muscadine grape seed extracts. As seen in Figure 4, both ethanolic and aqueous extracts at both dilutions inhibited the glycation of albumin as evidenced by a significant decrease in fluorescence. The ethanolic extract inhibited glycation to a greater extent than the corresponding aqueous extract, in agreement with the greater phenolic concentration found in the ethanolic extracts.
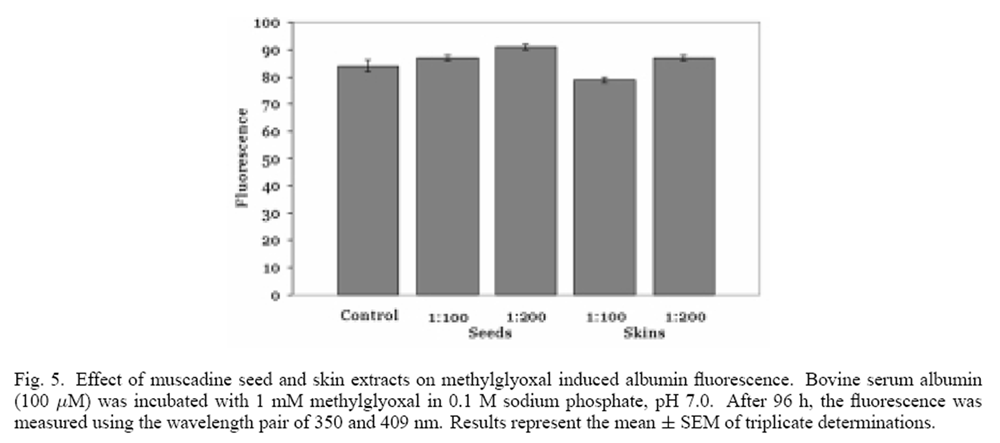
Figure 5 Methylglyoxal, an important intermediate in the auto-oxidation of reducing sugars, can readily glycate proteins. [21] When methylglyoxalwas incubated with albumin, a significant fluorescencewas produced at the wavelength pair of 350 and 409 nm, indicative of albumin glycation (Figure 5). When the 1:100 dilutions of muscadine seed and skin ethanolic extracts (representing 38 and 20 µg phenolics/ml) were incubated with methylglyoxal and albumin, a significant decrease in fluorescence intensity was not observed. These results indicate that muscadine seed and skin extracts do not inhibit all pathways of protein glycation.
Discussion
The formation of advanced glycation end products is quite complex; both oxidative and non-oxidative processes can lead to modification of proteins. [3] Oxidative reactions have been associated with several processes in the formation of AGEs. Glycation of proteins can proceed in vitro via the auto-oxidation of reducing sugars mediated by the presence of metal ions [30] resulting in the production of reactive carbonyl intermediates. The modified proteins, both glycated and oxidized, can promote an enhancement of oxidative, pro-inflammatory cascades. [12] Antioxidants can inhibit metal-mediated auto-oxidation of glucose leading to the formation of AGEs [5, 32],however there are several mechanisms that can contribute to this activity. While flavonoids and other phenolic compounds possess free radical-scavenging activity, certain flavonoids, such as quercetin, are capable of complexing metal ions directly. [19]
The phenolics present in seeds, catechin, epicatechin and gallic acid, all inhibited fructose mediated albumin glycation (Fig. 2). The results agree with those reported by Wu and Yen [32] on the effects of catechin and epicatechin on glucose mediated protein glycation. However, the phenolics present in the skin are different than those in the seed. The skins have high levels of ellagic acid, myricetin, quercetin, and kaempferol. [24] Although not performed in this study, the previous report of Wu and Yen showed that quercetin and kaempferol decreased glucose mediated albumin glycation. Individual phenolics have distinct inhibitory properties on protein glycation, a result that has been correlated with antioxidant properties. [16] It is interesting to note that the lowest concentration for both muscadine skin and seed inhibition of albumin glycation was approximately 3 µg phenolics/ml; this demonstrates the importance of the total phenolic content, rather than individual, specific phenolics in inhibiting this non-enzymatic process.
Protein glycation by methylglyoxal is a non-enzymatic modification whereby arginine and lysine side chains of proteins participate in forming a heterogeneous group of advanced glycation end-products. [8] It is interesting to note that while muscadine grape skin and seed extracts strongly inhibited fructose mediated albumin glycation, the extracts did not alter methylglyoxal mediated albumin glycation. In control experiments, 5 mMN-acetylcysteine, an aldehyde binding thiol compound [29], inhibited methylglyoxal modification of albumin by approximately 60% (data not shown). In a manner similar to that observed with the muscadine extracts,Wu and Yen [32] demonstrated that catechin significantly inhibited glucose, but not methylglyoxal, mediated, albumin glycation. The modification of albumin by malondialdehyde was also examined during the course of these studies. [14]
The modification of albumin by malondialdehyde was inhibited by only 9% in the presence of a 1:100 dilution of muscadine skin extract and by 20% in the presence of a 1:100 muscadine seed extract. The results of the methylglyoxal and malondialdehyde experiments suggest that the phenolic compounds in the muscadine extracts were not inhibiting the glycation process by binding carbonyl compounds in a manner similar to N-acetylcysteine. Price et al. [25] demonstrated the importance of chelation and antioxidant activity, rather than carbonyl trapping, of several glycation inhibitors.
Previous studies reported that green tea [2] and spice extracts (garlic, Cassia tora) [18] inhibit protein glycation. Our laboratory has recently demonstrated that twenty-four herbs and spices inhibit fructose-mediated glycation. The degree of inhibition was positively correlated to phenolic and antioxidant capacity [6] The current study demonstrates that an extract from a commonly edible grape or berry can significantly decrease glycation. Food products such as a water-soluble extract of tomato paste [15], a methanolic extract of Finger and Kodo millet [11] and ethanolic extracts of specific varieties of sorghum bran [7] have been previously shown to decrease glycation.
The possibility that muscadine products and other foods may have a pharmacological effect is dependent on the absorption of phenolic compounds in the gastrointestinal tract. As recently reviewed, bioavailability of some phenolic compounds is significant; approximately 40% of certain phenolic compounds have been found to be excreted in the urine. [27] Since both muscadine seed and skin fractions come from a generally recognized as safe (GRAS) fruit, there is no known toxicity associated with these products. Muscadine seeds and skins, as well as other inhibitors of glycation, may eventually be incorporated into nutraceutical products and functional food and beverages for the diabetes market.
Acknowledgement
This work was supported by a grant from the USDA SBIR Phase II program awarded to Paulk Vineyards, Wray, GA.
References:
N. Ahmed, Advanced glycation end products–role in pathology of diabetic complications, Diabetes Res Clin Pract 67 (2005), 3–21.
P.V. Babu, K E. Sabitha and C.S. Shyamaladevi, Green tea impedes dyslipidemia, lipid peroxidation, protein glycation and ameliorates calcium-ATPase and Na/K ATPase activity in the heart of streptozotocin-diabetic rats, Chem Biol Interact 162 (2006), 157–164.
J.W. Baynes and S.R. Thorpe, Role of oxidative stress in diabetic complications. A new perspective on an old paradigm, Diabetes 48 (1999), 1–9.
I.F. Benzie and J.J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay, Anal Biochem 239 (1996), 70–76.
I. Bousova, J. Martin, L. Jahodar, J. Dusek, V Palicka and J. Drsata, Evaluation of in vitro effects of natural substances of plant origin using a model of protein glycoxidation, J Pharm Biomed Anal 37 (2005), 957–962.
R.P. Dearlove, P. Greenspan, D.K. Hartle, R.B. Swanson and J.L. Hargrove, Inhibition of protein glycation by extracts of culinary herbs and spices, J Med Food (2007), (in press).
J.L. Farrar, D.K. Hartle, J.L. Hargrove and P. Greenspan, A novel nutraceutical property of select sorghum (Sorghum bicolor) brans: Inhibition of protein glycation, Phytotherapy Res (2007), (in press).
R.A. Gomes, H.V. Miranda, M.S. Silva, G. Graca, A.V. Coelho, A.E. Ferreira, C. Cordeiro and A.P. Freire, Yeast protein glycation in vivo by methylglyoxal, FEBS J 273 (2006), 5273–5287.
M.A. Haidara, H.Z. Yassin, M.Rateb, H. Ammar and M.A. Zorkani, Role of oxidative stress in development of cardiovascular complications in diabetes mellitus, Curr Vasc Pharmacol 4 (2006), 215–227.
D.K. Hartle, P. Greenspan and J.L. Hargrove, Muscadine Medicine, Lulu Press, St. George Island, FL, 2005.
P.G. Hegde, G. Chandrakasan and T. Chandra, Inhibition of collagen glycation and crosslinking in vitro by methanolic extracts of Finger millet (Eleusine coracana) and Kodo millet (Paspalum scrobiculatum), J Nutr Biochem 13 (2002), 517.
Y. Iwashima, M. Eto, S. Horiuchi and H. Sano, Advanced glycation end product-induced peroxisome proliferatoractivated receptor gamma gene expression in the cultured mesangial cells, Biochem Biophys Res Commun 264 (1999), 441–448.
A. Jawa and V. Fonseca, Cardiovascular effects of insulin sensitizers in diabetes, Curr Opin Investig Drugs 7 (2006), 806–814.
Z. Kang, H. Li, G. Li and D. Yin, Reactive of pyridoxamine with malondialdehyde: Mechanism of inhibition of formation of advanced lipoxidation end-products, Amino Acids 30 (2006), 55–61.
T. Kiho, S. Usui, K. Hirano, K. Aizawa and T. Inakuma, Tomato paste fraction inhibiting the formation of advanced glycation end-products, Biosci Biotechnol Biochem 68 (2004), 200–205.
H.Y. Kim and K. Kim, Protein glycation inhibitory and antioxidative activities of some plant extracts in vitro, J Agric Food Chem 51 (2003), 1586–1591.
C. Lee, M.B. Yim, P.B. Chock, H.S. Yim and S.O. Kang, Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation, J Biol Chem 273 (1998), 25272–25278.
G.Y. Lee, D.S. Jang, Y.M. Lee, J.M. Kim and J.S. Kim, Naphthopyrone glucosides from the seeds of Cassia tora with inhibitory activity on advanced glycation end products (AGEs) formation, Arch Pharm Res 29 (2006), 587–590.
M. Leopoldini, N. Russo, S. Chiodo and M. Toscano, Iron chelation by the powerful antioxidant flavonoid quercetin, J Agric Food Chem 54 (2006), 6343–6351.
J.D. McPherson, B.H. Shilton and D.J.Walton, Role of fructose in glycation and cross-linking of proteins, Biochemistry 27 (1988), 1901–1907.
R.H. Nagaraj, P. Sarkar, A. Mally, K.M. Biemel, M.O. Lederer and P.S. Padayatti, Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal, Arch Biochem Biophys 402 (2002), 110–119.
Y. Nohara, T. Usui, T. Kinoshita and M. Watanabe, Generation of superoxide anions during the reaction of guanidine compounds with methylglyoxal, Chem Pharm Bull (Tokyo) 50 (2002), 179–184.
L. Packer, E.H. Witt and H.J. Tritschler, alpha-Lipoic acid as a biological antioxidant, Free Radic Biol Med 19 (1995), 227–250.
E. Pastrana-Bonilla, C.C. Akoh, S. Sellappan and G. Krewer, Phenolic content and antioxidant capacity of muscadine grapes, J Agric Food Chem 51 (2003), 5497–5503.
D.L. Price, P.M. Rhett, S.R. Thorpe and J.W. Baynes, Chelating activity of advanced glycation end-product inhibitors, J Biol Chem 276 (2001), 48967–48972.
T. Sato, M. Iwaki, N. Shimogaito, X. Wu, S. Yamagishi and M. Takeuchi, TAGE (toxic AGEs) theory in diabetic complications, Curr Mol Med 6 (2006), 351–358.
A. Scalbert, C. Morand, C. Manach and C. Remesy, Absorption and metabolism of polyphenols in the gut and impact on health, Biomed Pharmacother 56 (2002), 276–282.
V.L.R. Singleton and J.A.Rossi Jr., Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid agents, Am J Enol Vitic 16 (1965), 144–158.
S. Vasdev, C.A. Ford, L. Longerich, S. Parai, V. Gadag and S. Wadhawan, Aldehyde induced hypertension in rats: prevention by N-acetyl cysteine, Artery 23 (1998), 10–36.
S.P.Wolff and R.T. Dean, Glucose autoxidation and protein modification, Biochem J 245 (1987), 243–250.
S.P. Wolff, Z.Y. Jiang and J.V. Hunt, Protein glycation and oxidative stress in diabetes mellitus and ageing, Free Radic Biol Med 10 (1991), 339–352.
C.H. Wu and G.C. Yen, Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation end products, J Agric Food Chem 53 (2005), 3167–3173.
Y. Yilmaz and R.T. Toledo, Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid, J Agric Food Chem 52 (2004), 255–260.

Return to RESVERATROL
Since 9-20-2009


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |