

Polyphenols as Therapeutic Molecules in Alzheimer's Disease
Through Modulating Amyloid PathwaysThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Molecular Neurobiology 2015 (Apr); 51 (2): 466–479 ~ FULL TEXT
Johant Lakey-Beitia, Ruben Berrocal, K. S. Rao & Armando A. Durant
Centre for Biodiversity and Drug Discovery,
Institute for Scientific Research and High Technology Services (INDICASAT-AIP),
City of Knowledge,
Panama City, Republic of Panama.
Alzheimer's disease (AD) is a complex and multifactorial neurodegenerative condition. The complex pathology of this disease includes oxidative stress, metal deposition, formation of aggregates of amyloid and tau, enhanced immune responses, and disturbances in cholinesterase. Drugs targeted toward reduction of amyloidal load have been discovered, but there is no effective pharmacological treatment for combating the disease so far. Natural products have become an important avenue for drug discovery research. Polyphenols are natural products that have been shown to be effective in the modulation of the type of neurodegenerative changes seen in AD, suggesting a possible therapeutic role. The present review focuses on the chemistry of polyphenols and their role in modulating amyloid precursor protein (APP) processing. We also provide new hypotheses on how these therapeutic molecules may modulate APP processing, prevent Aβ aggregation, and favor disruption of preformed fibrils. Finally, the role of polyphenols in modulating Alzheimer's pathology is discussed.
KEYWORDS: Alzheimer’s disease . Polyphenols . α-Secretase activator . β-Secretase inhibitor . γ-Secretase inhibitor . Aβ aggregation . Amyloid fibril disaggregation . Molecular mechanisms . Structure–activity relationships
From the FULL TEXT Article:
Introduction
Neurodegenerative disease occurs as a result of changes in the native conformation of proteins, followed by accumulation of these misfolded amyloidogenic proteins in the central nervous system, which in turn causes progressive neurological impairment and neuronal dysfunction [1, 2]. This is the molecular basis of the most devastating neurodegenerative diseases known to date such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) [3–5]. AD is the most prevalent neurodegenerative condition with approximately 29 million aging people suffering from this disease — a figure that is expected to triple by 2050 [6, 7].
AD is a process in which, due to uncontrolled cleavage of amyloid precursor protein (APP) by unknown inducing factors, toxic amyloid beta fragments are generated [8–10]. AD is also characterized by amyloid fibril and phosphorylated tau aggregates and tangles [11–16]. At present, there are two major challenges in AD drug discovery: first, the nonavailability of an animal model that reflects all the pathological events seen in AD human brain and, second, the lack of reliable biomarkers to detect and understand the progression of AD [17–20]. Scientists are trying to develop drugs that can simultaneously perform multiple tasks such as reducing inflammation, inhibiting β-secretase, activating α-secretase, preventing of Aβ and tau aggregation, and driving the disintegration of preformed fibrils [21, 22]. However, no perfect drug or perfect treatment of AD has been discovered so far, and many drugs have failed in recent clinical trials [17].
Consequently, research has begun to focus on natural products as alternatives in the treatment of AD [23]. For example, the water extract of the leaves of Caesalpinia crista has been shown to prevent Aβ aggregation from monomers and disintegrated preformed Aβ fibril; Centella asiatica prevents synuclein aggregation [24, 25]; Ginkgo biloba extract has shown to inhibit the formation of oligomers [23, 26]; extracts prepared from the medicinal herb Paeonia suffruticosa inhibited Aβ fibril formation and also de-stabilized the preformed amyloid fibril [26, 27]. The anti-amyloidogenic properties observed are attributed to the polyphenolic compounds present in the extracts. The present review focuses on the chemistry of polyphenols and the mechanisms by which polyphenols may induce changes in APP processing, reduction of Aβ load, prevention of Aβ aggregation, and disintegration of preformed fibrils. New mechanisms that explain the binding pattern of polyphenols to Aβ and modulation of APP processing by polyphenols are also proposed.
Chemistry of Polyphenol Compounds
Polyphenols (PPs) are natural compounds that are widespread in fruits, vegetables, seeds, cereals, oils, etc. [28]. More than 8,000 polyphenolic compounds have been identified in foods. These secondary metabolites provide protection to plants from ultraviolet light, defense against herbivory, and also attract pollinating insects [29, 30]. In the past, polyphenols have not been considered to have any substantial nutritional value; however, there is now an increased interest in exploring their potential as antioxidants [31–33]. Moreover, it has been proposed that polyphenolic compounds may play a role in the prevention of multiple diseases, such as atherosclerosis, cancer, type II diabetes, and cardiovascular and neurodegenerative diseases [34–37].
Polyphenols’ chemical structure includes two or more phenol rings with hydroxyl groups in ortho or para positions, which are necessary for redox reactions [38]. There is a direct positive correlation between the antioxidant capacity and the number of hydroxyl groups present in the polyphenols’ structure; i.e., an increase in the amount of hydroxyl groups in the polyphenol chemical structure is associated with increases in redox potential and antioxidant activity [39, 40].
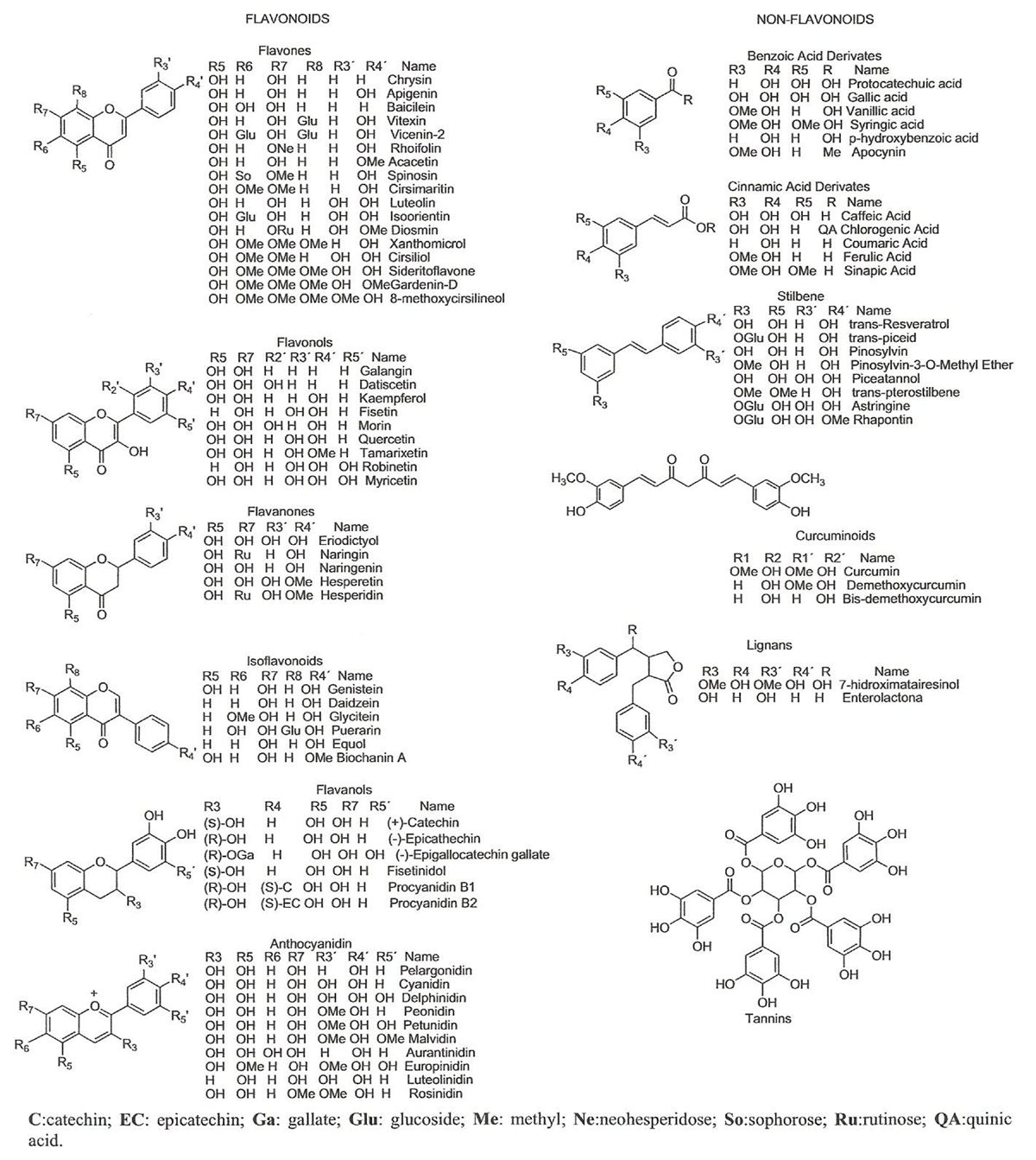
Figure 1
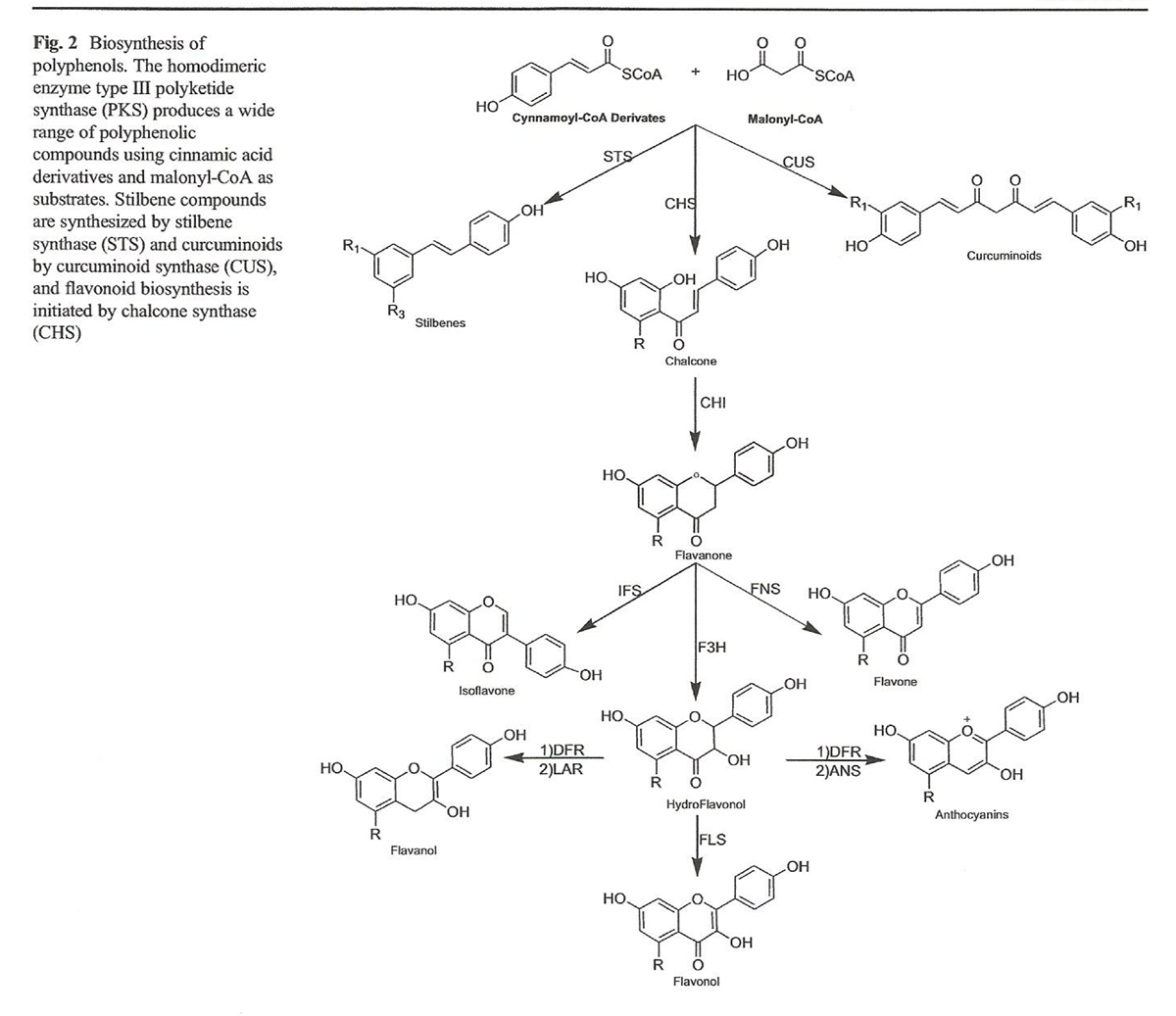
Figure 2
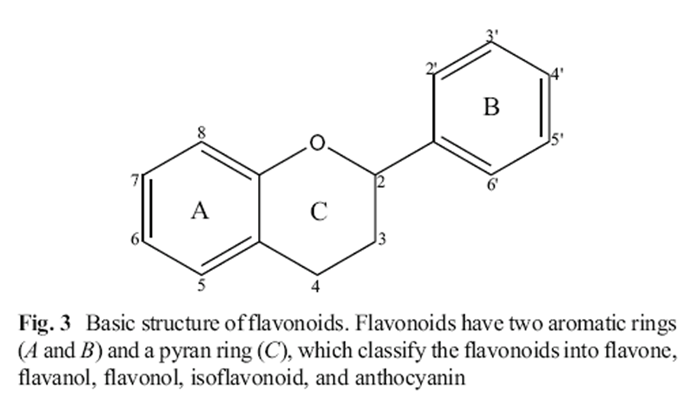
Figure 3 Polyphenols are grouped into two main categories: flavonoids and non-flavonoid compounds (Figure 1). Flavonoid compounds are classified into two groups: anthoxanthins (flavonol, flavanol, isoflavonoid, flavone, and flavanone) and anthocyanins, while non-flavonoid compounds include phenolic acids, stilbenes, curcuminoids, lignans, and tannins [41–43]. Polyphenols are secondary metabolites produced by enzymatic and non-enzymatic reactions. These reactions produce multiple secondary metabolites of biological importance (Figure 2). The homodimeric enzyme type III polyketide synthase (PKS) produces a wide range of natural compounds by acetyltransferring, aromatization, cyclization, condensation, and decarboxylation [44]. PKS is involved in the biosynthesis of polyphenolic compounds in plants by decarboxylative condensation of acetyl units deriving from malonyl-CoA and thioester groups of cinnamoyl-CoA or p-coumaroyl-CoA [45–47]. For example, curcuminoids are formed by biotransformation catalyzed by curcuminoid synthase (CUS), while stilbenes are formed by biotransformation by stilbene synthase (STS). Chalcone synthase (CHS) catalyzes the formation of chalcone using p-coumaroyl-CoA and malonyl-CoA [45–47].
Flavonoid Compounds Flavonoids are the largest group of polyphenols, with more than 5,000 flavonoid compounds widely distributed in plants [48, 49]. Their basic structure consists of two aromatic rings linked through a pyran ring (Figure 3). Depending on the oxidation state of the pyran ring, flavonoids can be classified as flavones, flavonols, flavanols, isoflavonoids, flavanones, and anthocyanins [50, 51]. Flavonoid hydroxylation occurs mainly at C5, C7, and C4'. These compounds are commonly found glycosylated in plants, frequently as O-rhamnosyl and Oglucoside flavonoids, and acylation and methoxylation are less frequent [50, 52].
Flavones are characterized by the presence of a keto-pyrene group. Hydroxylation is common at C5 [41]. Chrysin, acacetin, and baicilein are examples of common flavones found in citrus fruits, celery, and parsley [53, 54]. Flavonols possess a keto-hydroxypyrene group, which is predominantly hydroxylated at C3, while C5 and C7 are frequently hydroxylated [41]. Myricetin, quercetin, and fisetin are examples of flavonols present in apples, beans, and onions [53, 54].
Flavanols contain a pyran ring hydroxylated at C3. Flavanols can be hydroxylated at the A-ring (C5, C7) and Bring (C3', C4', C5') [41, 50]. Fisetinidol, catechin, and epicatechin are flavanols commonly found in berries, cocoa, tea, and onions. Proanthocyanidins or condensed tannins are oligomers of flavanols that are classified as A- and B-type proanthocyanidins [52]. B-type proanthocyanidins can be classified into two groups: procyanidins and prodelphinidins (e.g., epicatechin, catechin, and gallic ester derivate) [55].
Isoflavonoids have a basic structure containing a substituted keto group on the pyran ring. Commonly, they are substituted at C5, while C6 and C4' are methoxylated or hydroxylated. Isoflavonoids may contain a glucosyl or hydroxyl group at C7 or C8. Soybean is a rich source of these polyphenolic compounds [41, 50]. Flavanones also usually have a keto-pyran group usually substituted by hydroxyl groups on ring A at C3 and C5. Occasionally, C7 is glycosylated or hydroxylated, while C4' has a methoxy or hydroxy group. Eriodictyol, naringin, and hesperetin are flavanones present in citrus fruits [53].
Anthocyanins are ubiquitous water-soluble compounds that are responsible for red or blue colors in flowers and fruits and whose color changes according to the pH value [56, 57]. The molecular structure of anthocyanins is based on the 2-phenylbenzopyrylium cation (also named flavylium) [58]. Glucose, rhamnose, arabinose, and galactose are the most common sugars found forming glycosides in anthocyanins [59]. The aglycones of anthocyanins are known as anthocyanidins, which do not exist in nature and are unstable water compounds [60]. These structures perform unique biological functions like antioxidant, antiinflammatory, and anti-aggregation activities [61].
Non-Flavonoids Among the non-flavonoid polyphenols are phenolic acids, stilbenes, curcuminoids, lignans and tannins, which are proven neuroprotectors. Phenolic acids are the simplest polyphenols found in nature. There are classified into two categories, namely, hydroxybenzoic and hydroxycinnamic acid derivates [41]. Hydroxybenzoic acid derivates (C6–C1) bear one aromatic ring attached to a carboxylic group. Gallic acid and protocatechuic acid are hydroxybenzoic acid derivates that are found in red fruits, black radish, and onions [50]. Hydroxycinnamic acid derivates (C6–C3), also known as phenylpropanoids, are more common than hydroxybenzoic acids. Caffeic, p-coumaric, and ferulic acids are hydroxycinnamic acid derivates present in berries, cherries, kiwis, and apples. These polyphenolic compounds can be glycosylated or can be found forming esters with quinic acid, shikimic acid, or tartaric acid. Stilbenes are formed in nature through the phenylpropanoid pathway. These compounds have two aromatic rings connected through a double bond (C6–C2–C6). The widely known polyphenolic compound resveratrol is a stilbene found in red grapes [50].
Curcuminoids [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5- dione] are phenolic compounds isolated from Curcuma longa (curcumin) [50]. Lignans have two phenylpropane units (C6– C3–C3–C6) bound together through β–β' bonds. Examples include secoisolariciresinol, which is found in sesame and pumpkin seeds [50]. Tannins are complex polymers of high molecular weight (>30,000 Da) classified as hydrolizable and condensed tannins. Hydrolyzable tannins are heterogeneous polymers of phenolic acids, which are classified as gallotannins, composed of gallic acid and glycosides and ellagiotannins, constituted by ellagic acid [41].
The role of different polyphenols in modulating APP processing, as well as their anti-aggregation properties, is discussed below.
New Hypothesis on Polyphenols Modulating APP Pathway
APP is a large type-1 transmembrane multidomain protein that performs multiple cellular activities. APP695, APP751, and APP770 are the most frequently expressed isoforms in the brain [8, 62]. APP is catabolized by secretases (α, β, and γ), forming non-amyloidal and/or amyloid-derived products [61, 63]. In the non-amyloidal pathway, α-secretase cleaves APP at Lys 687, producing sAPPα and a C-terminal fragment of 83 aa residues (CTFα), which are further cleaved by γ-secretase leading to the formation of Aβ17–42 (also known as protein 3 (p3)), and APP intracellular domain (AICD) [64]. In the amyloidal pathway, β- secretase cleaves APP at Met 671, releasing a fragment of the secreted amyloid precursor protein beta (sAPPβ), and a Cterminal fragment of 99 aa residues (CTFβ). The latter is then cleaved by the enzyme γ-secretase at Val 711 and Ala 713, leading to the formation of AICD, Aβ40, and Aβ42 peptides [65–67].
Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), also known as β-secretase, is involved in the production of amyloid-β peptide [68]. β-Secretase has 501 aa residues, including a signal peptide of 21 aa residues, a proprotein domain (22–45 aa), a luminal domain (46–460 aa), a transmembrane domain (17 aa), and a cytosolic carboxyl domain of 24 aa [68].A BACE1 homolog, named BACE2, cleaves APP into a short peptide. However, BACE2 is present in small quantities in the brain, and so, this enzyme is probably not crucial in the formation of Aβ peptide. γ-Secretase is a heterotetrameric membraneembedded aspartyl protease consisting of four subunits: nicastrin, presenilin, anterior pharynx, and presenilin enhancer 2 [69–71]. When α- or β-secretase cannot cleave APP, γ- secretase cleaves it, forming the soluble amyloid precursor protein γ (sAPPγ) and AICD [72]. Finding drugs that target APP processing is complex and challenging due to the multiple functional enzymes and substrates involved [17].
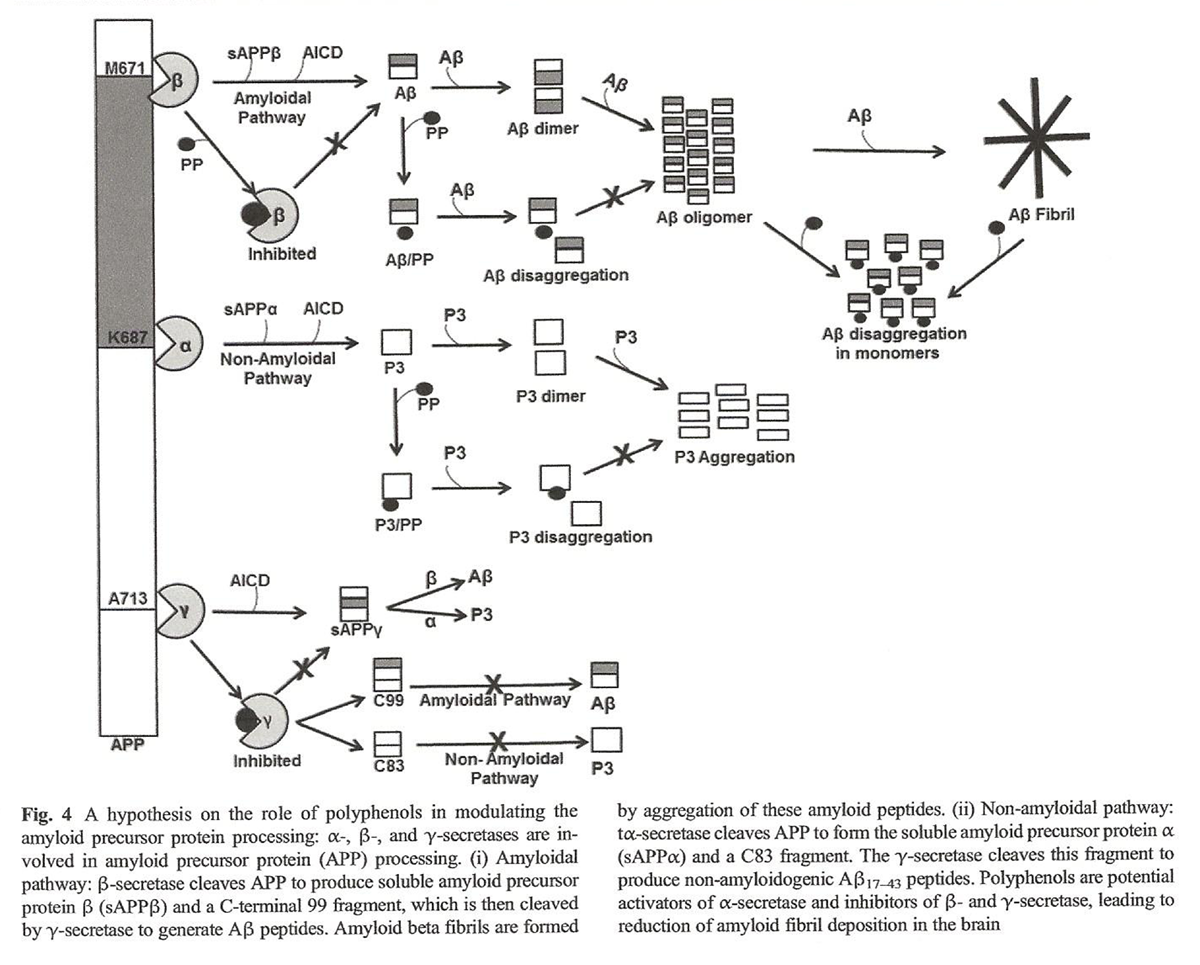
Figure 4
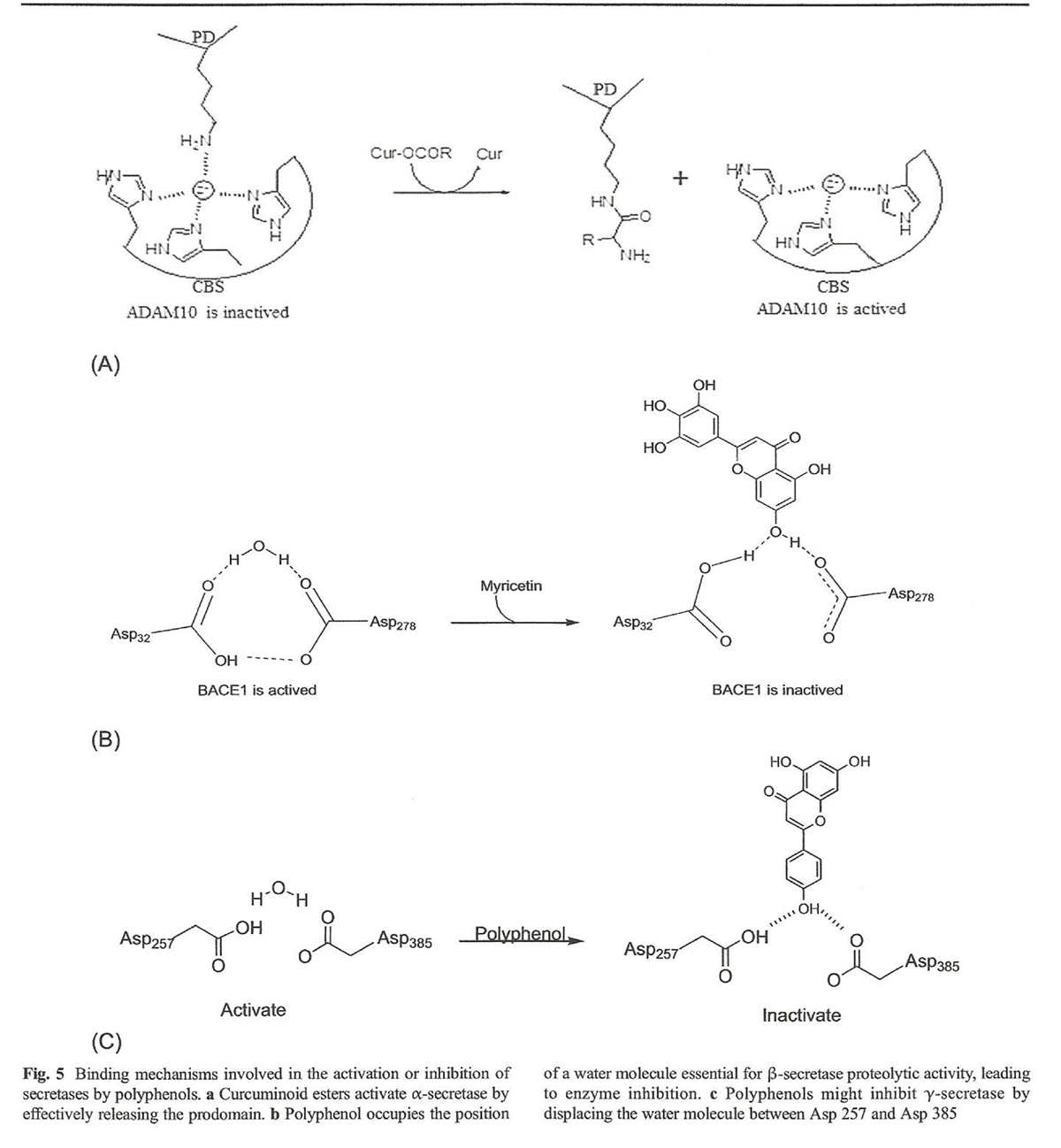
Figure 5 Polyphenols are powerful anti-amyloidogenic compounds due to physicochemical features such as the presence of aromatic rings, molecular planarity, capacity to formhydrogen bonds, the presence of an internal double bond, and molecular weights below 500 g/mol, which allow for potential inhibition of APP pathways (Figure 4) that, in turn, reduces amyloid load [73–76].
Polyphenols as Activators of α-Secretase Several members of the a disintegrin and metalloproteinase (ADAM) family have been proposed as physiologically active α-secretases, namely: ADAM9, ADAM10, and ADAM17. It has been demonstrated that ADAM10 has the highest α- secretase activity “in vivo” [77–79]. Moreover, it has been suggested that the upregulation of ADAM10 could be a potential therapeutic target for the treatment of Alzheimer’s disease. ADAM10 has a potential neuroprotective role because it promotes the non-amyloidogenic pathway [80].
This enzyme is activated by removal of the prodomain, which is probably promoted by the action of proprotein convertases [81–84]. Phlorotannins and epigallocatechin-3-gallate (EGCG) have been shown to increase the overexpression of sAPPα through activation of α-secretase favoring neuroprotection [85–87]. Other polyphenols such as curcumin induce ADAM10 activation, whereas curcumin–amino acid conjugates favor the overexpression of sAPPα. Other esters found in nature such as phorbol 12,13-dibutyrate (PDBU) and phorbol 12-myristate 13-acetate (PMA) also increase the overexpression of sAPPα by activation of α-secretase [88]. Based on this similarity, we propose a hypothetical structure–activity relationship by which a covalent interaction between the ester group of EGCG and curcumin–amino acid conjugates and the enzyme prodomain promote the release of the active site, allowing the cleavage of APP to form sAPPα fragments (Figure 5a) [89, 90].
Polyphenols as Inhibitors of β-Secretase The amino acids Asp32, Asp228, and two water molecules, all located in the catalytic binding site of BACE1, are essential for catalytic activity [91]. One of these watermolecules participates in enzymatic proteolysis, while the second water molecule is key for stabilizing a tetrahedral intermediate that is essential for protein cleavage [92]. Computational modeling studies indicate that Asp32 is protonated, whereas Asp228 is not [91]. Proteolytic BACE 1 activity is initiated through a nucleophilic attack by a water molecule on the carbonyl group of the peptide bond [93]. Inhibition of BACE1 represents a potential therapeutic target in AD treatment as it decreases Aβ load. Several peptides are known to inhibit beta secretase, yet smallmolecules, namely isophthalamides, have shown higher inhibitory effect upon BACE1 [94]. Myricetin is a potential BACE1 inhibitor [95]. We propose a mechanism that explains the role of polyphenolinduced inhibition through the displacement of a water molecule, which then participates in a hydrogen bonding network with Asp32 and Asp228 that is essential for BACE1 proteolytic activity. It is very probable that flavonols having a myricetinlike chemical structure cause inhibition of BACE1 following this same mechanism (Figure 5b) [96].
Polyphenols as Inhibitors of γ-Secretase Presenilin I (PS1) protein is a member of the aspartic protease family implicated in the regulation of intramembrane proteolysis [66, 70]. PS1 has been identified as the catalytic subunit of the γ-secretase complex. This protein is composed of nine transmembrane domains, where domains 6 and 7 form the catalytic site. Mutations in PS1 have been linked to familial Alzheimer’s disease (FAD). Therefore, PS1 is a potential target in the design of drugs against AD [71, 97]. Two aspartyl groups (Asp257 and Asp385) opposed to each other in the active site and are required for the catalytic activity of PS1. One of these aspartates is deprotonated and acts as a base, activating a water molecule present in the catalytic site. The other aspartate donates a proton to the carbonyl group of the substrate, following an acid–base mechanism [97]. We propose that polyphenols that can occupy the active site of γ- secretase (thus displacing the water molecule required by the enzyme for catalysis) would disable the enzyme and reduce Aβ formation [97] (Figure 5c).
Structure–Activity Relationship of Polyphenols on Their Aβ Anti-Aggregation Activity
Aβ42 aggregation is a hallmark of AD pathology; therefore, inhibition of Aβ42 aggregation is a key factor in drug discovery. Aβ42 aggregation biology is a multifold step, where monomers form oligomers, protofibrils, and matured fibrils. Drug discovery efforts are focused on preventing the formation of either oligomers or fibrils. There are also studies focusing on disintegration of preformed fibrils [23–27]. However, there are limited studies exploring how drugs bind to Aβ42 and prevent fibril formation [23–27, 98–102]. The primary sequence of the Aβ42 peptide is H 2 N - D A E F R H D S G Y E V H H Q K L - VFFAEDVGSNKGAIIGLMVGGVVIA-CO2H [103–105].
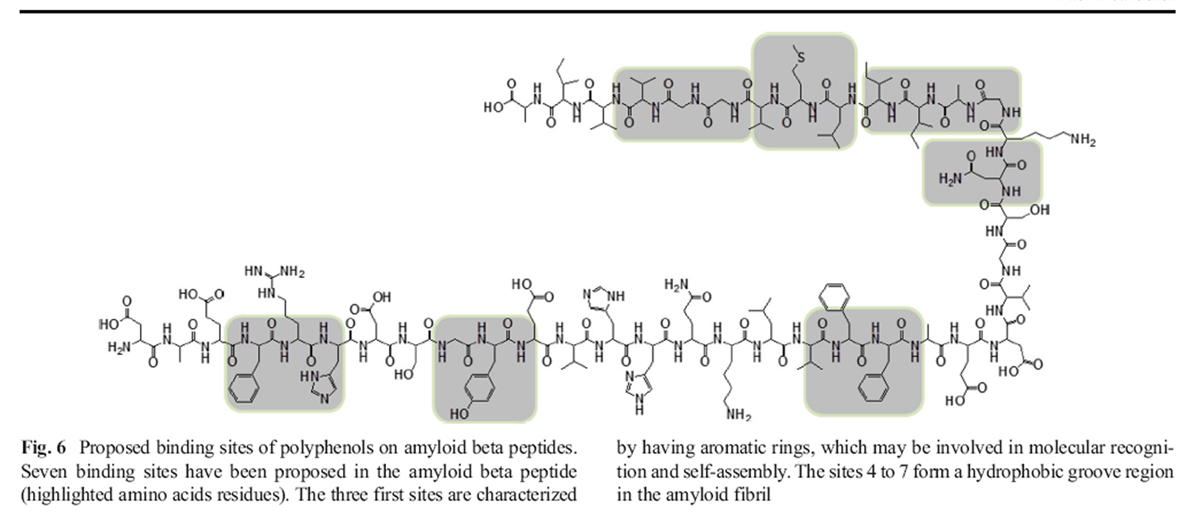
Figure 6 More than 50 % of the amino acids in this peptide are hydrophobic residues [106]. It has been suggested that the hydrophobic core KLVFF is essential for fibrillogenesis [107–109]. Aβ peptide may be considered as a molecule with two faces (upper and lower), which allow it to self-assemble and to form oligomers and matured fibrils [98–101]. Wang et al. have identified significant binding sites on the Aβ42 peptide structure: F4-H6, Y10, F20, N27, I31-M35, M35, and M35 to V39 for molecules like tanshinones [102]. Based on these data, we propose a model that identifies proposed binding sites for polyphenols on amyloid beta peptides (Figure 6) [102].
Aβ fibrillization is a multistep process, which begins with the formation of Aβ oligomers constituted by 24 monomers [110–113]. The toxic spherical oligomers are considered an intermediate into fibril formation and are 3–10 nm in size. Aβ fibrils are characterized by highly stable crossed β-sheet structures at 4.75 and 9.8–10.6 Å [114–117]. Amyloid fibril formation depends on the increase in the concentration of Aβ42 peptide, low pH, the time of incubation, and the length of the carboxyl chain [118]. Studies have indicated that hydrophobic forces, aromatic stacking, and electrostatic interactions stabilize the Aβ structure [119, 120]. The main physicochemical properties of molecules with the potential to inhibit amyloidal fibril formation might be due to the presence of aromatic rings in their chemical structure and the ability to form non-covalent interactions with amino acids residues of the Aβ peptide sequence [73, 74]. Moreover, the planarity of the inhibitor is essential for increasing surface contact with Aβ peptides [75]. Most polyphenols have more than two aromatic rings essential for π–π stacking interactions with hydrophobic amino acid residues of Aβ (Tyr, Phe) and at least three hydroxyl groups that form hydrogen bonds with hydrophilic amino acid residues of Aβ (His6, Ser8, Tyr10, His14, Lys16) [121–123]. The resonance structure of polyphenols provides enough planarity to penetrate the Aβ fibril hydrophobic grove, thus disturbing the fibril structure [124].
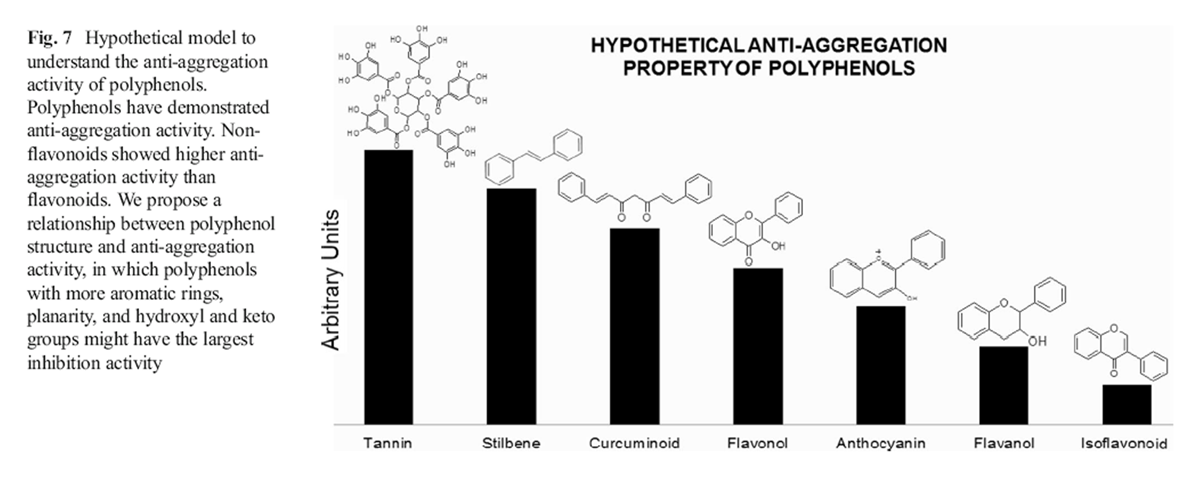
Figure 7 Polyphenolic compounds such as resveratrol, curcumin, and myricetin have demonstrated anti-Aβ aggregation properties [111, 125–130]. The differences observed in the antiaggregation activity among polyphenols are related to their chemical structure. Generally, non-flavonoids (tannins>stilbenes> curcuminoids) show higher anti-amylodogenic activity than flavonoids (flavonols>anthocyanins>flavanol> isoflavonoid) [131, 132]. Nevertheless, no clear mechanisms have been proposed so far to explain how polyphenols prevent Aβ aggregation. Therefore, we suggest hypothetical structure– activity relationships (Figure 7) based on structural comparisons of polyphenols to explain how polyphenolic compounds prevent Aβ aggregation [133, 134].
Isoflavonoids generally show lower anti-aggregation activity than other flavonoids. Structure–activity relationships of isoflavonoids and other flavonoids suggest that the aromatic B ring at C2 is essential for decreasing amyloid fibril formation due to favorable non-covalent interactions between polyphenols and amino acids of the Aβ peptide sequence [135].
Flavanols possess more anti-amyloidogenic activity than isoflavonoids because they contain more hydroxyl groups able to form hydrogen bonds with Aβ peptides. Nevertheless, flavanols have two chiral centers (C2, C3) that may diminish molecular planarity [136]. Furthermore, these compounds lack the presence of a keto group at C4 in the C ring, leading to less non-covalent interactions with Aβ peptides. Both physicochemical features have negative effects on the inhibition of fibril formation [132, 137].
Anthocyanins are characterized by having a pseudo aromatic ring C that increases their structural planarity and promotes amyloid fibril disruption due to effective incorporation of anthocyanins inside the amyloid beta fibril groove. Curcuminoids are more hydrophobic than flavonoids. This physicochemical property might enhance their affinity for binding with the hydrophobic core of Aβ fibril, resulting in an increased anti-amyloidal activity [132]. Stilbenes have more hydroxyl groups in their chemical structure than curcuminoids, which may explain the strong anti-aggregation activity observed for these polyphenols [137, 138]. Tannins are complex polyphenols having the highest number of hydroxyl groups among polyphenolic compounds and therefore the strongest anti-aggregation activity [139–142]. Nevertheless, their large molecular weight reduces their suitability as a therapeutic drug [76].
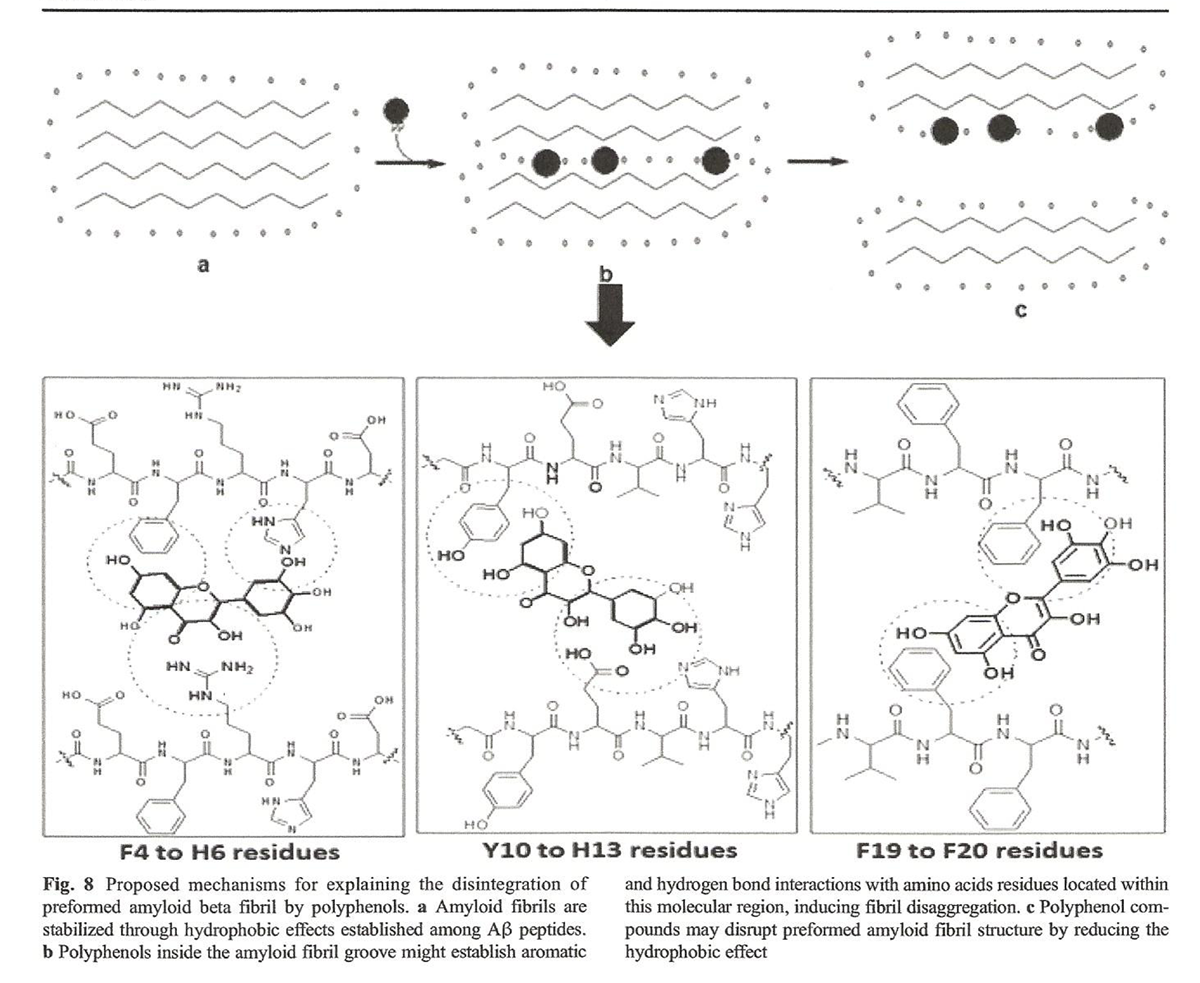
Figure 8 The possible mechanisms used by polyphenols to destabilize preformed fibrils remain unclear [143]. When polyphenols get inside the hydrophobic groove of Aβ fibril, their aromatic rings disrupt the organization of the fibril due to attraction and repulsion between the polyphenol and the Aβ peptide [144, 145]. We suggest that these interactions may lead to conformational changes that might favor widening of the amyloid fibril groove through reduction of the hydrophobic effect (a major driving force that stabilizes the fibril structure), leading to a disaggregation of amyloid fibril (Figure 8) [124].
Conclusion
The understanding of polyphenol bioavailability and health benefits is still not so clear. However, population studies on polyphenols and memory have shown that polyphenols contribute to a healthy brain. There have been studies showing that some polyphenols can cross the blood–brain barrier and confer neuroprotection. A lot of information is available on the influence of polyphenols on the differential expressions of genes involved in inflammation, apoptosis, and tumor necrosis. The current challenge in polyphenol research is related to their bioavailability at pharmacological concentrations. Some polyphenols appear to have pharmacological capabilities against cancers, metabolic disorders, and memory, but we still need to understand the delivery mechanisms of these compounds. The major challenge is to bring blood polyphenol concentrations up to the levels required for pharmacological action [146].
It is a challenge to cover all of the possible molecular mechanisms utilized by any drug in the treatment of AD, as this disease has multiple pathological events. Polyphenols have attracted research interest recently due to their multiple effects such as inhibition of Aβ, metal chelation, and prevention of mitochondrial dysfunction and apoptosis, as well as their antioxidant and anti-inflammatory properties. Although there are no clear mechanisms described so far that fully explain the role of polyphenols in the treatment of AD, we have presented some well-founded hypotheses that associate the physicochemical properties of polyphenols with their possible role in α-secretase activation, β- and γ-secretase inhibition, disaggregation of Aβ fibrils, and anti-Aβ aggregation. To the best of our knowledge, this review thus provides some novel avenues for future research.
Acknowledgments
Johant Lakey is supported by a doctoral scholarship granted by the Institute for Training and Development of Human Resources of Panama (IFARHU) and National Secretariat for Science, Technology, and Innovation of Panama (SENACYT). K.S Rao is grateful to the National Science System (SNI) of SENACYT for the financial support.
References:
Hashimoto M, Rockenstein E, Crews L, Masliah E (2003) Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med 4:21–36. doi:10.1385/NMM:4:1-2:21
Wang SS, Hung YT,WenWS, Lin KC, Chen GY (2011) Exploring the inhibitory activity of short-chain phospholipids against amyloid fibrillogenesis of hen egg-white lysozyme. Biochim Biophys Acta 1811:301–313. doi:10.1016/j.bbalip.2011.02.003
Gadad BS, Britton GB, Rao KS (2011) Targeting oligomers in neurodegenerative disorders: lessons from α-synuclein, tau, and amyloid-β peptide. J Alzheimers Dis 24:223–232. doi:10.3233/ JAD-2011-110182
HegdeML, Hegde PM, Rao KS, Mitra S (2011) Oxidative genome damage and its repair in neurodegenerative diseases: function of transition metals as a double-edged sword. J Alzheimers Dis 24: 183–198. doi:10.3233/JAD-2011-110281
Guerrero E, Padmaraju V, Hegde ML, Britton GB, Rao KS (2013) Recent advances in α-synuclein functions, advanced glycation, and toxicity: implications for Parkinson’s disease. Mol Neurobiol 47:525– 536
Prado-Prado F, García I (2012) Review of theoretical studies for prediction of neurodegenerative inhibitors. Mini Rev Med Chem 12:452–466. doi:10.2174/138955712800493780
Cho JK, Ryu YB, Curtis-Long MJ, Ryu HW, Yuk HJ, Kim DW, Kim HJ, Lee WS, Park KH (2012) Cholinestrase inhibitory effects of geranylated flavonoids from Paulownia tomentosa fruits. Bioorg Med Chem 20:2595–2602. doi:10.1016/j.bmc.2012.02.044
Sambamurti K, Greig NH, Utsuki T, Barnwell EL, Sharma E, Mazell C, Bhat NR, Kindy MS, Lahiri DK, Pappolla MA (2011) Targets for AD treatment: conflicting messages from γ-secretase inhibitors. J Neurochem 117:359–374. doi:10.1111/j.1471-4159. 2011.07213.x
Tweedie D, Brossi A, Chen D, GeYW, Bailey J, Yu QS, KamalMA, Sambamurti K, Lahiri DK, Greig NH (2006) Neurine, an acetylcholine autolysis product, elevates secreted amyloid-β protein precursor and amyloid-β peptide levels, and lowers neuronal cell viability in culture: a role in Alzheimer’s disease? J Alzheimers Dis 10:9–16
Ramesh BN, Rao TSS, Prakasam A, Sambamurti K, Rao KS (2010) Neuronutrition andAlzheimer’s disease. J Alzheimers Dis 19:1123– 1139. doi:10.3233/JAD-2010-1312
PrakasamA,Muthuswamy A, Ablonczy Z, Greig NH, Fauq A, Rao KS, Pappolla MA, Sambamurti K (2010) Differential accumulation of secreted APP metabolites in ocular fluids. J Alzheimers Dis 20: 1243–1253. doi:10.3233/JAD-2010-100210
Padmaraju V, Indi SS, Rao KS (2010) New evidences on Tau–DNA interactions and relevance to neurodegeneration. Neurochem Int 57: 51–57. doi:10.1016/j.neuint.2010.04.013
Barrio JR, Kepe V, Satyamurthy N, Huang SC, Small G (2008) Amyloid and tau imaging, neuronal losses and function in mild cognitive impairment. J Nutr Health Aging 12:61S–65S
SambamurtiK, PappollaMA, Rao KS (2008) Value in development of a TAPIR-like mouse monoclonal antibody to Aβ. J Alzheimers Dis 14:175–177
Utsuki T, Yu QS, Davidson D, Chen D, Holloway HW, Brossi A, Sambamurti K, Lahiri DK, Greig NH, Giordano T (2006) Identification of novel small molecule inhibitors of amyloid precursor protein synthesis as a route to lower Alzheimer’s disease amyloid-β peptide. J Pharmacol Exp Ther 318:855–862. doi:10. 1124/jpet.106.103309
Heredia L, Lin R, Vigo FS, Kedikian G, Busciglio J, Lorenzo A (2004) Deposition of amyloid fibrils promotes cell-surface accumulation of amyloid β precursor protein. Neurobiol Dis 16:617–629. doi:10.1016/j.nbd.2004.04.015
Chiang K, Koo E (2014) Emerging therapeutics for Alzheimer’s disease. Annu Rev Pharmacol Toxicol 54:381–405
Pillai JA, Cummings JL (2013) Clinical trials in predementia stages of Alzheimer disease. Med Clin North Am 97:439–457. doi:10. 1016/j.mcna.2013.01.002
Schenk D, Basi GS, Pangalos MN (2012) Treatment strategies targeting amyloid β-protein. Cold Spring Harb Perspect Med 2: a006387. doi:10.1101/cshperspect.a006387
Shi M, Caudle WM, Zhang J (2009) Biomarker discovery in neurodegenerative diseases: a proteomic approach. Neurobiol Dis 35: 157–164. doi:10.1016/j.nbd.2008.09.004
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. doi:10.1126/science.1072994
Pallàs M, Camins A (2006) Molecular and biochemical features in Alzheimer’s disease. Curr Pharm Des 12:4389–4408
Kumar GP, Khanum F (2012) Neuroprotective potential of phytochemicals. Pharmacogn Rev 6:81–90. doi:10.4103/0973-7847. 99898
Berrocal R, Vasudevaraju P, Indi SS, Sambasiva Rao KR, Rao KS (2014) In vitro evidence that an aqueous extract of Centella asiatica modulates α-synuclein aggregation dynamics. J Alzheimers Dis 39(2):457–465. doi:10.3233/JAD-131187
Ramesh BN, Indi SS, Rao KS (2010) Anti-amyloidogenic property of leaf aqueous extract of Caesalpinia crista. Neurosci Lett 475:110– 114. doi:10.1016/j.neulet.2010.03.062
Park SY (2010) Potential therapeutic agents against Alzheimer’s disease from natural sources. Arch Pharm Res 33:1589–1609. doi: 10.1007/s12272-010-1010-y
Fujiwara H, Tabuchi M, Yamaguchi T, Iwasaki K, Furukawa K, Sekiguchi K, Ikarashi Y, Kudo Y, Higuchi M, Saido T, Maeda S, Takashima A, Hara M, Yaegashi N, Kase Y, Arai H (2009) A traditional medicinal herb Paeonia suffruticosa and its active constituent 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose have potent anti-aggregation effects on Alzheimer’s amyloid beta proteins in vitro and in vivo. J Neurochem 169:1648–1657
Pérez-Jiménez J, Neveu V, Vos F, Scalbert A (2010) Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem 58:4959–4969. doi:10.1021/jf100128b
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835. doi:10.1016/j.foodchem.2010. 12.026
Royer M, Diouf PN, Stevanovic T (2011) Polyphenol contents and radical scavenging capacities of red maple (Acer rubrum L.) extracts. Food Chem Toxicol 49:2180–2188. doi:10.1016/j.fct.2011. 06.003
Ghosh D,McGhie TK, Zhang J, Adaim A, SkinnerM(2006) Effects of anthocyanins and other phenolics of boysenberry and blackcurrant as inhibitors of oxidative stress and damage to cellular DNA in SHSY5Y and HL-60 cells. J Sci Food Agric 86:678–686. doi:10.1002/ jsfa.2409
Hwang SL, Yen GC (2008) Neuroprotective effects of the citrus flavanones againstH2O2-induced cytotoxicity in PC12 cells. JAgric Food Chem 56:859–864. doi:10.1021/jf072826r
Heo HJ, Kim DO, Shin SC, Kim MJ, Kim BG, Shin DH (2004) Effect of antioxidant flavanone, naringenin, from Citrus junos on neuroprotection. J Agric Food Chem 52:1520–1525. doi:10.1021/ jf035079g
Murillo E, Britton GB, Durant AA (2012) Antioxidant activity and polyphenol content in cultivated and wild edible fruits grown in Panama. J Pharm Bioall Sci 4:313–317. doi:10.4103/0975-7406. 103261
Candiracci M, Piatti E, Dominguez-Barragán M, García-Antrás D, Morgado B, Ruano D, Gutiérrez JF, Parrado J, Castaño A (2012) Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J Agric Food Chem 60:12304–12311
Andrade JE, Burgess JR (2007) Effect of the citrus flavanone naringenin on oxidative stress in rats. J Agric Food Chem 55: 2142–2148. doi:10.1021/jf061714h
Cieslik E, Greda A, Adamus W (2006) Contents of polyphenols in fruit and vegetables. Food Chem 94:135–142. doi:10.1016/j. foodchem.2004.11.015
Zettersten C, Co M, Wende S, Turner C, Nyholm L, Sjöberg PJR (2009) Identification and characterization of polyphenolic antioxidants using on-line liquid chromatography, electrochemistry, and electrospray ionization tandem mass spectrometry. Anal Chem 81: 8968–8977. doi:10.1021/ac901397c
Tsao R,Yang R (2003) Optimization of a newmobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromatography. J Chromatogr A 1018:29–40. doi:10.1016/j.chroma.2003.08.034
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–333
Singh MA, Arseneault MA, Sanderson T, Murthy VEN, Ramassamy C (2008) Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem 56:4855–4873
Hegde ML, Bharathi P, Suram A, Venugopal C, Jagannathan R, Poddar P, Srinivas P, Sambamurti K, Rao KS, Scancar J,Messori L, Zecca L, Zatta P (2009) Challenges associated with metal chelation therapy in Alzheimer’s disease. J Alzheimers Dis 17:457–468. doi: 10.3233/JAD-2009-1068
Jez JM, Bowman ME, Noel JP (2002) Expanding the biosynthetic repertoire of plant type III polyketide synthases by altering starter molecule specificity. Proc Natl Acad Sci 99:5319–5324. doi:10. 1073/pnas.082590499
Chemler JA, Yan Y, Koffas MAG (2006) Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb Cell Fact 5:20. doi:10.1186/
Tian L, Pang Y, Dixon RA (2008) Biosynthesis and genetic engineering of proanthocyanidins and (iso)flavonoids. Phytochem Rev 7:445–465. doi:10.1007/s11101-007-9076-y
Gao X,Wang P, Tang Y (2010) Engineered polyketide biosynthesis and biocatalysis in Escherichia coli. Appl Microbiol Biotechnol 88: 1233–1242. doi:10.1007/s00253-010-2860-4
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34. doi:10. 1146/annurev.nutr.22.111401.144957
Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S (2006) Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem 54:9966–9977. doi:10. 1021/jf061478a
Vauzour D (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. OxidMed Cell Longev 2012:1–16. doi: 10.1155/2012/914273
Wolfe KL, Liu RH (2008) Structure–activity relationships of flavonoids in the cellular antioxidant activity assay. J Agric Food Chem 56:8404–8411. doi:10.1021/jf8013074
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79: 727–747
El Gharras H (2009) Polyphenols: food sources, properties and applications—a review. Int J Food Sci Technol 44:2512–2518. doi:10.1111/j.1365-2621.2009.02077.x
Zapata-Torres G, Opazo F, Salgado C, Muñoz JP, Krautwurst H, Mascayano C, Sepúlveda-Boza S, Maccioni RB, Cassels BK (2004) Effects of natural flavones and flavonols on the kinase activity of Cdk5. J Nat Prod 67:416–420. doi:10. 1021/np034011s
Santos-Buelga C, Scalbert A (2000) Proanthocyanidins and tanninlike compounds—nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric 80(7):1094–1117
Valls J, Millán S, Martí MP, Borràs E, Arola L (2009) Advanced separationmethods of food anthocyanins, isoflavones and flavanols. J Chromatogr A 1216:7143–7172. doi:10.1016/j.chroma.2009.07.030
De Brito ES, De Araújo MCP, Alves RE, Carkeet C, Clevidence BA, Novotny JA (2007) Anthocyanins present in selected tropical fruits: acerola, jambolão, jussara, and guajiru. J Agric Food Chem 55:9389–9394. doi:10.1021/jf0715020
Qin CG, Li Y, NiuW, Ding Y, Shang X, Xu C (2011) Composition analysis and structural identification of anthocyanins in fruit of waxberry. Czech J Food Sci 29:171–180
Chen W, Müller D, Richling E, Wink M (2013) Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J Agric Food Chem 61:3047–3053
Ruberto G, Renda A, Daquino C,Amico V, Spatafora C, Tringali C, De Tommasi N (2007) Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem 100:203–210. doi:10.1016/j.foodchem. 2005.09.041
Shih PH, Wu CH, Yeh CT, Yen GC (2011) Protective effects of anthocyanins against amyloid β-peptide-induced damage in neuro- 2A cells. J Agric Food Chem 59:1683–1689. doi:10.1021/ jf302972b
Wang JF, Lu R, Wang YZ (2010) Regulation of β cleavage of amyloid precursor protein. Neurosci Bull 26:417–427. doi:10. 1007/s12264-010-0515-1
Esler WP, Wolfe MS (2001) A portrait of Alzheimer secretases— new features and familiar faces. Science 293:1449–1454. doi:10. 1126/science.1064638
Tang BL (2005) Alzheimer’s disease: channeling APP to nonamyloidogenic processing. Biochem Biophys Res Commun 331: 375–378. doi:10.1016/j.bbrc.2005.03.074
Venugopal C, Demos CM, Rao KS, Pappolla MA (2008) Betasecretase: structure, function, and evolution. CNS Neurol Disord Drug Targets 7:278–294
Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K (2008) Geranylgeranyl pyrophosphate stimulates γ-secretase to increase the generation ofAβ andAPP-CTFγ. FedAm Soc Exp Biol J 22:47–54. doi:10.1096/fj.07-8175com
Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knölker H-J, Simons K (2008) Efficient inhibition of the Alzheimer’s disease β-secretase by membrane targeting. Science 320:520–523. doi:10.1126/science.1156609
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis J-C, Collins F, Treanor J, Rogers G, Citron M (1999) β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741. doi:10.1126/science.286.5440.735
Vidal R, Sammeta N, Garringer HJ, Sambamurti K, Miravalle L, Lamb BT, Ghetti B (2012) The Psen1-L166P-knock-in mutation leads to amyloid deposition in human wild-type amyloid precursor protein YAC transgenicmice. Fed AmSoc Exp Biol 26:2899–2910. doi:10.1096/fj.12-205542
Tiedt H, Lueschow A, Winter P, Müller U (2013) Previously not recognized deletion in presenilin-1 (p.Leu174del.) in a patient with early-onset familial Alzheimer’s disease. Neurosci Lett 544:115– 118. doi:10.1016/j.neulet.2013.03.056
Spasic D, Tolia A, Dillen K, Baert V, De Strooper B, Vrijens S, Annaert W (2006) Presenilin-1 maintains a nine-transmembrane topology throughout the secretory pathway. J Biol Chem 281: 26569–26577. doi:10.1074/jbc.M600592200
Utsuki T, Shoaib M, Holloway H, Ingram D, Wallace W, Haroutunian V, Sambamurti K, Lahiri D, Greig N (2002) Nicotine lowers the secretion of the Alzheimer’s amyloid beta-protein precursor that contains amyloid beta-peptide in rat. J Alzheimers Dis 4: 405
Hirohata M, Hasegawa K, Tsutsumi-Yasuhara S, Ohhashi Y, Ookoshi T, Ono K, Yamada M, Naiki H (2007) The antiamyloidogenic effect is exerted against Alzheimer’s β-amyloid fibrils in vitro by preferential and reversible binding of flavonoids to the amyloid fibril structure. Biochemistry 46:1888–1899. doi:10. 1021/bi061540x
Ge JF, Qiao JP, Qi CC, Wang CW, Zhou JN (2012) The binding of resveratrol to monomer and fibril amyloid beta. Neurochem Int 61: 1192–1201. doi:10.1016/j.neuint.2012.08.012
Porat Y, Abramowitz A, Gazit E (2006) Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des 67:27–37. doi:10.1111/j.1747-0285.2005.00318.x
Jung HA, Oh SH, Choi JS (2010) Molecular docking studies of phlorotannins fromEisenia bicyclis with BACE1 inhibitory activity. Bioorg Med Chem Lett 20:3211–3215. doi:10.1016/j.bmcl.2010. 04.093
Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F (2004) A disintegrinmetalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest 113:1456–1464. doi:10.1172/JCI200420864.1456
Endres K, Fahrenholz F (2012) Regulation of alpha-secretase ADAM10 expression and activity. Exp Brain Res 217:343–352. doi:10.1007/s00221-011-2885-7
Endres K, Fahrenholz F (2010) Upregulation of the α-secretase ADAM10—risk or reason for hope? Fed Eur Biochem Soc J 277: 1585–1596. doi:10.1111/j.1742-4658.2010.07566.x
SkovronskyDM,Moore DB, MillaME, Doms RW, LeeVM(2000) Protein kinase C-dependent α-secretase competes with β-secretase for cleavage of amyloid-β precursor protein in the trans-golgi network. J Biol Chem 275:2568–2575
Tian S, JianhuaW(2010) Comparative study of the binding pockets of mammalian proprotein convertases and its implications for the design of specific small molecule inhibitors. Int J Biol Sci 6:89–95
Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F (2001) Regulation of the α-secretase ADAM10 by its prodomain and proprotein convertases. Fed Am Soc Exp Biol J 15:1837–1839. doi:10.1096/fj.01
Edwards DR, Handsley MM, Pennington CJ (2008) The ADAM metalloproteinases. Mol Aspects Med 29:258–289. doi:10.1016/j. mam.2008.08.001
ZhongM, Munzer JS, Basak A, Benjannet S,Mowla SJ, Decroly E, Chrétien M, Seidah NG (1999) The prosegments of furin and PC7 as potent inhibitors of proprotein convertases. In vitro and ex vivo assessment of their efficacy and selectivity. J Biol Chem 274: 33913–33920
Kang IJ, Jang BG, In S, Choi B, Kim M, Kim MJ (2013) Phlorotannin-rich Ecklonia cava reduces the production of betaamyloid bymodulating alpha- and gamma-secretase expression and activity. Neurotoxicology 34:16–24. doi:10.1016/j.neuro.2012.09. 013
Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J,Morgan D, Hardy J, Town T, Tan J (2005) Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci 25:8807–8814. doi:10. 1523/JNEUROSCI.1521-05.2005
Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM (2006) Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol Dis 24:506–515. doi:10.1016/j.nbd. 2006.08.006
Vestling M, Cedazo-Mínguez Á, Adem A, Wiehager B, Racchi M, Lannfelt L, Cowburn RF (1999) Protein kinase C and amyloid precursor protein processing in skin fibroblasts from sporadic and familial Alzheimer’s disease cases. Biochim Biophys Acta 1453: 341–350
Narasingapa RB, Jargaval MR, Pullabhatla S, Htoo HH, Rao KS, Hernandez JF, Govitrapong P, Vincent B (2012) Activation of α- secretase by curcumin-aminoacid conjugates. Biochem Biophys Res Commun 424:691–696. doi:10.1016/j.bbrc.2012.07.010
Levites Y, Amit T, Mandel S, Youdim MBH (2003) Neuroprotection and neurorescue against Aβ toxicity and PKCdependent release of non-amyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. Fed Am Soc Exp Biol J 17:952–958
Mancini F, De Simone A, Andrisano V (2011) Beta-secretase as a target for Alzheimer’s disease drug discovery: an overview of in vitro methods for characterization of inhibitors. Anal Bioanal Chem 400:1979–1996. doi:10.1007/s00216-011-4963-x
Shimizu H, Tosaki A, Kaneko K, Hisano T, Sakurai T, Nukina N (2008) Crystal structure of an active form of BACE1, an enzyme responsible for amyloid β protein production. Mol Cell Biol 28: 3663–3671. doi:10.1128/MCB.02185-07
Yu N, Hayik SA,Wang B, Liao N, Reynolds CH,Merz KM(2006) Assigning the protonation states of the key aspartates in β-secretase using QM/MMX-ray structure refinement. J Chem Theory Comput 2:1057–1069. doi:10.1021/ct0600060
Stachel SJ, Coburn CA, Steele TG, Jones KG, Loutzenhiser EF, Gregro AR, Rajapakse HA, Lai MT, Crouthamel MC, Xu M, Tugusheva K, Lineberger JE, Pietrak BL, Espeseth AS, Shi XP, Chen-Dodson E, Holloway MK, Munshi S, Simon AJ, Kuo L, Vacca JP (2004) Structure-based design of potent and selective cell-permeable inhibitors of human β-secretase (BACE-1). J Med Chem 47:6447–6450. doi:10.1021/jm049379g
Chakraborty S, Kumar S, Basu S (2011) Conformational transition in the substrate binding domain of β-secretase exploited by NMA and its implication in inhibitor recognition: BACE1-myricetin a case study. Neurochem Int 58:914–923. doi:10.1016/j.neuint.2011. 02.021
Marcinkeviciene J, Luo Y, Graciani NR, Combs AP, Copeland RA (2001) Mechanism of inhibition of β-site amyloid precursor protein-cleaving enzyme (BACE) by a statine-based peptide. J Biol Chem 276:23790–23794. doi:10.1074/jbc.M101896200
Haass C, De Strooper B (1999) The presenilins in Alzheimer’s disease—proteolysis holds the key. Science 286:916–919. doi:10. 1126/science.286.5441.916
Gazit E (2002) A possible role for π-stacking in the self-assembly of amyloid fibrils. Fed Am Soc Exp Biol J 16:77–83. doi:10.1096/fj. 01-0442hyp
Doran TM, Anderson EA, Latchney SE, Opanashuk LA, Nilsson BL (2012) An azobenzene photoswitch sheds light on turn nucleation in amyloid-β self-assembly. ACS Chem Neurosci 3:211–220. doi:10.1021/cn2001188
Bett CK, Ngunjiri JN, Serem WK, Fontenot KR, Hammer RP, McCarley RL, Garno JC (2010) Structure-activity relationships in peptide modulators of β-amyloid protein aggregation: variation in α, α-disubstitution results in altered aggregate size andmorphology. ACS Chem Neurosci 1:608–626. doi:10.1021/cn100045q
Tycko R, Wickner RB (2013) Molecular structures of amyloid and prion fibrils: consensus versus controversy. Acc Chem Res 46: 1487–1496. doi:10.1021/ar300282r
Wang Q, Yu X, Patal K, Hu R, Chuang S, Zhang G, Zheng J (2013) Tanshinones inhibit amyloid aggregation by amyloid-β peptide, disaggregate amyloid fibrils, and protect cultured cells. ACS Chem Neurosci 4:1004–1015. doi:10.1021/cn400051e
Ashburn TT, Han H, McGuinness BF, Lansbury PT (1996) Amyloid probes based on Congo Red distinguish between fibrils comprising different peptides. Chem Biol 3:351–358
Wetzel R, Shivaprasad S,Williams AD (2007) Plasticity of amyloid fibrils. Biochemistry 46:1–10
Ma J, Komatsu H, Kim YS, Liu L, Hochstrasser RM, Axelsen PH (2013) Intrinsic structural heterogeneity and long-term maturation of amyloid β peptide fibrils. ACS Chem Neurosci 4:1236–1243. doi:10.1021/cn400092v
Sambamurti K, Rao KS, Pappolla MA (2009) Frontiers in the pathogenesis of Alzheimer’s disease. Indian J Psychiatry 51:S56–S60
Bett CK, Serem WK, Fontenot KR, Hammer RP, Garno JC (2010) Effects of peptides derived from terminal modifications of the Aβ central hydrophobic core on Aβ fibrillization. ACS Chem Neurosci 1:661–678. doi:10.1021/cn900019r
Jagota S, Rajadas J (2011) The role of Pro, Gly Lys, and Arg containing peptides on amyloid-beta aggregation. Int J Pept Res Ther 18:53–61. doi:10.1007/s10989-011-9278-4
Geng J, Li M,Wu L, Ren J, Qu X (2012) Liberation of copper from amyloid plaques: making a risk factor useful for Alzheimer’s disease treatment. J Med Chem 55:9146–9155. doi:10.1021/ jm3003813
Glabe CC (2005) Amyloid accumulation and pathogensis of Alzheimer’s disease: significance of monomeric, oligomeric and fibrillar Aβ. Subcell Biochem 38:167–177
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen P, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005) Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901. doi:10.1074/jbc.M404751200
Gupta VB, Rao KS (2007) Anti-amyloidogenic activity of S-allyl- L-cysteine and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Neurosci Lett 429:75–80. doi:10.1016/j.neulet. 2007.09.042
Chromy BA,Nowak RJ, LambertMP,Viola KL,Chang L,Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL (2003) Self-assembly of Aβ1-42 into globular neurotoxins. Biochemistry 42:12749–12760. doi:10.1021/bi030029q
Yamaguchi T, Yagi H, Goto Y, Matsuzaki K, Hoshino M (2010) A disulfide-linked amyloid-β peptide dimer forms a protofibril-like oligomer through a distinct pathway from amyloid fibril formation. Biochemistry 49:7100–7107
Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov 9:387–398. doi:10.1038/nrd2896
Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Dawson KA, Linse S (2010) Dual effect of amino modified polystyrene nanoparticles on amyloid β protein fibrillation. ACS ChemNeurosci 1:279–287. doi: 10.1021/cn900027u
Necula M, Kayed R, Milton S, Glabe CG (2007) Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J Biol Chem 282:10311–10324. doi:10.1074/jbc.M608207200
Chaudhary N, Singh S, Nagaraj R (2011) Aggregation properties of a short peptide that mediates amyloid fibril formation in model proteins unrelated to disease. J Biosci 36:679–689. doi:10.1007/ s12038-011-9104-3
Sinha S, Lopes DHJ, Bitan G (2012) A key role for lysine residues in amyloid β-protein folding, assembly, and toxicity. ACS Chem Neurosci 3:473–481
Bazoti FN, Bergquist J, Markides K, Tsarbopoulos A (2008) Localization of the noncovalent binding site between amyloid-β- peptide and oleuropein using electrospray ionization FT-ICR mass spectrometry. J Am Soc Mass Spectrom 19:1078–1085. doi:10. 1016/j.jasms.2008.03.011
Hudson SA, Ecroyd H, Dehle FC, Musgrave IF, Carver JA (2009) (—)-Epigallocatechin-3-Gallate (EGCG) maintains κ-casein in its pre-fibrillar state without redirecting its aggregation pathway. J Mol Biol 392:689–700
Dolai S, ShiW, Corbo C, Sun C,Averick S, ObeysekeraD, Farid M, Alonso A, Banerjee P, Raja K (2011) “Clicked” sugar–curcumin conjugate: modulator of amyloid-β and tau peptide aggregation at ultralow concentrations. ACS Chem Neurosci 2:694–699
Porat Y,Mazor Y, Efrat S, Gazit E (2004) Inhibition of islet amyloid polypeptide fibril formation: a potential role for heteroaromatic interactions. Biochemistry 43:14454–14462
Convertino M, Pellarin R, Catto M, Carotti A, Caflisch A (2009) 9, 10-Anthraquinone hinders beta β-aggregation: how does a small molecule interfere with Aβ-peptide amyloid fibrillation? Protein Sci 18:792–800. doi:10.1002/pro.87
Rivière C, Delaunay JC, Immel F, Cullin C, Monti JP (2009) The polyphenol piceid destabilizes preformed amyloid fibrils and oligomers in vitro: hypothesis on possible molecular mechanisms. Neurochem Res 34:1120–1128. doi:10.1007/s11064-008-9883-6
Wong HE, Qi W, Choi HM, Fernandez EJ, Kwon I (2011) A safe, blood-brain barrier permeable triphenylmethane dye inhibits amyloid-β neurotoxicity by generating nontoxic aggregates. ACS Chem Neurosci 2:645–657. doi:10.1021/cn200056g
Katayama S, Ogawa H, Nakamura S (2011) Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J Agric Food Chem 59:12691–12696. doi:10.1021/jf203654c
Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju S, Pollard A, Fenech M, Zhou XF (2009) Consumption of grape seed extract prevents amyloid-β deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox Res 15:3–14. doi:10. 1007/s12640-009-9000-x
Marin E, Briceño MI, Caballero-George C (2013) Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomedicine 8:3071–3091. doi:10.2147/IJN.S47186
Feng Y, Wang XP, Yang SG, Wang YJ, Zhang X, Du XT, Sun XX, Zhao M, Huang L, Liu RT (2009) Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 30:986–995. doi:10.1016/j.neuro.2009.08.013
Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M (2003) Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem 87:172–181. doi:10. 1046/j.1471-4159.2003.01976.x
Rivière C, Richard T, Vitrac X, Mérillon JM, Valls J, Monti JP (2008) New polyphenols active on β-amyloid aggregation. Bioorg Med Chem Lett 18:828–831. doi:10.1016/j.bmcl.2007.11.028
Shoval H, Weiner L, Gazit E, Levy M, Pinchuk I, Lichtenberg D (2008) Polyphenol-induced dissociation of various amyloid fibrils results in a methionine-independent formation of ROS. Biochim Biophys Acta 1784:1570–1577. doi:10.1016/j.bbapap.2008.08.007
Akaishi T, Morimoto T, Shibao M, Watanabe S, Sakai-Kato K, Utsunomiya-Tate N, Abe K (2008) Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid β protein. Neurosci Lett 444:280–285. doi:10.1016/j.neulet.2008.08.052
Henry-Vitrac C, Berbille H, Mérillon JM, Vitrac X (2010) Soy isoflavones as potential inhibitors of Alzheimer β-amyloid fibril aggregation in vitro. Food Res Int 43:2176–2178. doi:10.1016/j. foodres.2010.07.032
Carver JA, Duggan PJ, Ecroyd H, Liu Y, Meyer AG, Tranberg CE (2010) Carboxymethylated-k-casein: a convenient tool for the identification of polyphenolic inhibitors of amyloid fibril formation. Bioorg Med Chem 18:222–228. doi:10.1016/j.bmc.2009.10.063
Rivière C, Richard T, Quentin L, Krisa S, Mérillon JM, Monti JP (2007) Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorg Med Chem 15:1160–1167. doi:10.1016/j. bmc.2006.09.069
Rivière C, Papastamoulis Y, Fortin PY, Delchier N, Andriamanarivo S, Waffo-Teguo P, Kapche GD, Amira-Guebalia H, Delaunay JC, Mérillon JM, Richard T, Monti JP (2010) New stilbene dimers against amyloid fibril formation. Bioorg Med Chem Lett 20:3441– 3443. doi:10.1016/j.bmcl.2009.09.074
Ono K, Hamaguchi T, Naiki H, Yamada M (2006) Antiamyloidogenic effects of antioxidants: implications for the prevention and therapeutics of Alzheimer’s disease. Biochim Biophys Acta 1762:575–586. doi:10.1016/j.bbadis.2006.03. 002
Ono K, Hasegawa K, Naiki H, Yamada M (2004) Antiamyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Biochim Biophys Acta 1690:193–202
OnoK,YamadaM(2006) Antioxidant compounds have potent antifibrillogenic and fibril-destabilizing effects for α-synuclein fibrils in vitro. J Neurochem 97:105–115. doi:10.1111/j.1471-4159.2006. 03707.x
Ono K,Naiki H,YamadaM(2006) The development of preventives and therapeutics for Alzheimer’s disease that inhibit the formation of β-amyloid fibrils (fAβ), as well as destabilize preformed fAβ. Curr Pharm Des 12:4357–4375
Richard T, Poupard P, Nassra M, Papastamoulis Y, Iglésias ML, Krisa S, Waffo-Teguo P, Mérillon JM, Monti JP (2011) Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg Med Chem 19:3152–3155. doi:10.1016/j.bmc. 2011.04.001
Frid P, Anisimov SV, Popovic N (2007) Congo red and protein aggregation in neurodegenerative diseases. Brain Res Rev 53:135– 160. doi:10.1016/j.brainresrev.2006.08.001
Richard T, Papastamoulis Y, Waffo-Teguo P, Monti JP (2013) 3D NMR structure of a complex between the amyloid beta peptide (1– 40) and the polyphenol ε-viniferin glucoside: implications in Alzheimer’s disease. Biochim Biophys Acta 1830:5068–5074. doi:10.1016/j.bbagen.2013.06.031
D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R (2010) Bioavailability of the polyphenols: status and controversies. Int J Mol Sci 11:1321–1342. doi:10.3390/ijms11041321

Return to POLYPHENOLS
Since 1-28-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |