Resveratrol Improves Health and Survival
of Mice on a High-calorie DietThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Nature 2006 (Nov 16); 444 (7117): 337–342
Joseph A. Baur, Kevin J. Pearson, Nathan L. Price, Hamish A. Jamieson,
Carles Lerin, Avash Kalra, Vinayakumar V. Prabhu, Joanne S. Allard,
Guillermo Lopez-Lluch, Kaitlyn Lewis, Paul J. Pistell
Department of Pathology,
Paul F. Glenn Laboratories for the Biological Mechanisms of Aging,
Harvard Medical School,
77 Avenue Louis Pasteur,
Boston, Massachusetts 02115, USAResveratrol (3,5,4'-trihydroxystilbene) extends the lifespan of diverse species including Saccharomyces cerevisiae, Caenorhabditis elegans and Drosophila melanogaster. In these organisms, lifespan extension is dependent on Sir2, a conserved deacetylase proposed to underlie the beneficial effects of caloric restriction. Here we show that resveratrol shifts the physiology of middle-aged mice on a high-calorie diet towards that of mice on a standard diet and significantly increases their survival. Resveratrol produces changes associated with longer lifespan, including increased insulin sensitivity, reduced insulin-like growth factor-1 (IGF-I) levels, increased AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) activity, increased mitochondrial number, and improved motor function. Parametric analysis of gene set enrichment revealed that resveratrol opposed the effects of the high-calorie diet in 144 out of 153 significantly altered pathways. These data show that improving general health in mammals using small molecules is an attainable goal, and point to new approaches for treating obesity-related disorders and diseases of ageing.
From the FULL TEXT Article:
Background
The number of overweight individuals worldwide has reached 2.1 billion, leading to an explosion of obesity-related health problems associated with increased morbidity and mortality. [1, 2] Although the association of obesity with increased risk of cardiovascular disease and diabetes is well known, it is often under-appreciated that the risks of other age-related diseases, such as cancer and inflammatory disorders, are also increased. At the other end of the spectrum, reducing caloric intake by,40% below that of ad libitum-fed animals (caloric restriction) is the most robust and reproducible way to delay agerelated diseases and extend lifespan in mammals. [3, 4]
Experiments with Saccharomyces cerevisiae and Drosophila melanogaster have implicated the sirtuin/Sir2 family of NAD1-dependent deacetylases and mono-ADP-ribosyltransferases as mediators of the physiological effects of caloric restriction5. In mammals, seven sirtuin genes have been identified (SIRT1–7). SIRT1 regulates such processes as glucose and insulin production, fat metabolism, and cell survival, leading to speculation that sirtuins might mediate effects of caloric restriction in mammals. [5] We previously screened over 20,000 molecules to identify, 25 that enhance SIRT1 activity in vitro. [6] Resveratrol, a molecule produced by a variety of plants in response to stress, emerged as the most potent.
Resveratrol has since been shown to extend the lifespan of evolutionarily distant species including S. cerevisiae, C. elegans and D. melanogaster in a Sir2-dependent manner.6–9] A recent study found that resveratrol improves health and extends maximum lifespan by 59% in a vertebrate fish. [10] In mammalian cells, resveratrol produces SIRT1-dependent effects that are consistent with improved cellular function and organismal health. [11–15] Whether resveratrol acts directly or indirectly through Sir2 in vivo is currently a subject of debate. [16]
On the basis of the unprecedented ability of resveratrol to improve health and extend lifespan in simple organisms, we have asked whether it has similar effects in mice. We hypothesized that resveratrol might shift the physiology of mice on a high-calorie diet towards that of mice on a standard diet and provide the associated health benefits without the mice having to reduce calorie intake. Cohorts of middle-aged (one-year-old) male C57BL/6NIA mice were provided with either a standard diet (SD) or an otherwise equivalent highcalorie diet (60% of calories from fat, HC) for the remainder of their lives. To each of the diets, we added resveratrol at two concentrations that provided an average of 5.2 ± 0.1 and 22.4 ± 0.4 mg kg–1 day–1, which are feasible daily doses for humans. After 6 months of treatment, there was a clear trend towards increased survival and insulin sensitivity. Because the effects were more prominent in the higher dose (22.4 ± 0.4 mg kg–1 day–1, HCR), we initially focused our resources on this group and present the results of those analyses herein. Analyses of the other groups will be presented at a later date.
Increased survival
Mice on the HC diet steadily gained weight until ~75 weeks of age, after which average weight slowly declined (Fig. 1a). Although mice on the HCR diet were slightly lighter than the HC mice during the initial months, there was no significant weight difference between the groups from 18–24months, when most of our analyses were performed. There was also no difference in body temperature (Table 1), food consumption (Supplementary Fig. 1a, b), total faecal output or lipid content (Supplementary Fig. 1c, d), or post-mortem body fat distribution (Supplementary Fig. 2).
Table 1. Effects of a high-fat diet and resveratrol on various biomarkers in plasma

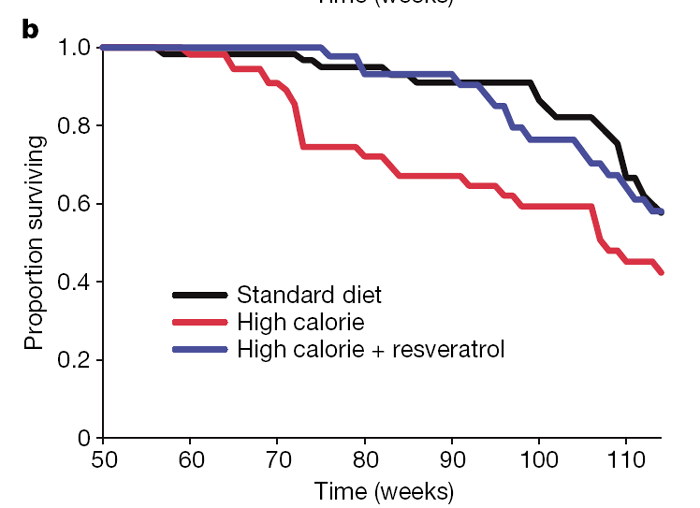
At 60 weeks of age, the survival curves of the HC and HCR groups began to diverge and have remained separated by a 3–4-month interval (Fig. 1b). A similar effect on survival was observed in a previous study of one-year-old C57BL/6 mice on caloric restriction, ultimately resulting in a 20% extension of mean lifespan. [17] With the present age of the colony at 114 weeks, 58% of the HC control animals have died (median lifespan 108 weeks), as compared to 42% of the HCR group and 42% of the SD controls. Although we cannot yet confidently predict the ultimate mean lifespan extension, Cox proportional hazards regression shows that resveratrol reduced the risk of death from the HC diet by 31% (hazard ratio = 0.69, P = 0.020), to a point where it was not significantly different from the SD group (hazard ratio = 1.03, P = 0.88).
Although resveratrol increased survival, it was important to ascertain whether quality of life was maintained. One way to assess this was to measure balance and motor coordination, which we did by examining the ability to perform on a rotarod. Surprisingly, the resveratrol- fed HC mice steadily improved their motor skills as they aged, to the point where they were indistinguishable from the SD group (Fig. 1c). It is possible that the improved rotarod performance might have been due to minor differences in body weight but we view this as unlikely because we found no correlation between body weight and performance within groups and rotarod performance was also improved for resveratrol-treated SD mice (R.deC. and K.P., unpublished data). These data are reminiscent of the resveratrol-mediated increase in motor activity in older individuals of the vertebrate fish species Nothobranchius furzeri. [10]
Increased insulin sensitivity
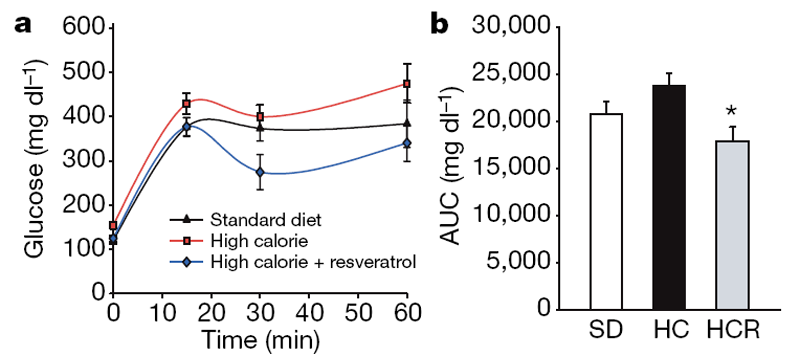
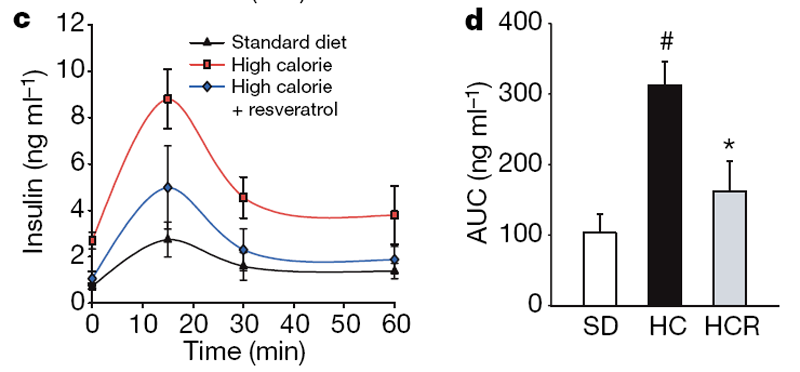
In humans, high-calorie diets cause numerous pathological conditions including increased glucose and insulin levels leading to diabetes, cardiovascular disease and non-alcoholic fatty liver disease, a condition for which there is no effective treatment. [18] The HC-fed mice had alterations in plasma levels of markers that predict the onset of diabetes and a shorter lifespan, including increased levels of insulin, glucose and IGF-1 (Table 1). The HCR group had significantly lower levels of these markers, paralleling the SD group. An oral glucose tolerance test indicated that the insulin sensitivity of the resveratrol- treated mice was considerably higher than controls (Fig. 2a–d). Homeostatic model assessment, which is used to quantify insulin resistance, gave scores of 2.5 for SD, 8.8 for HC and 3.5 for HCR, confirming improved sensitivity (HCR versus HC, P50.01). Although the persistence of high glucose levels for more than 60 min following an oral dose is unusual for young mice, it is typical for older animals. [19] Compared to the HC controls, the areas under the curves for both glucose and insulin levels were significantly decreased in the resveratrol-fed HC group and were not significantly different from mice in the SD group (Fig. 2b, d).
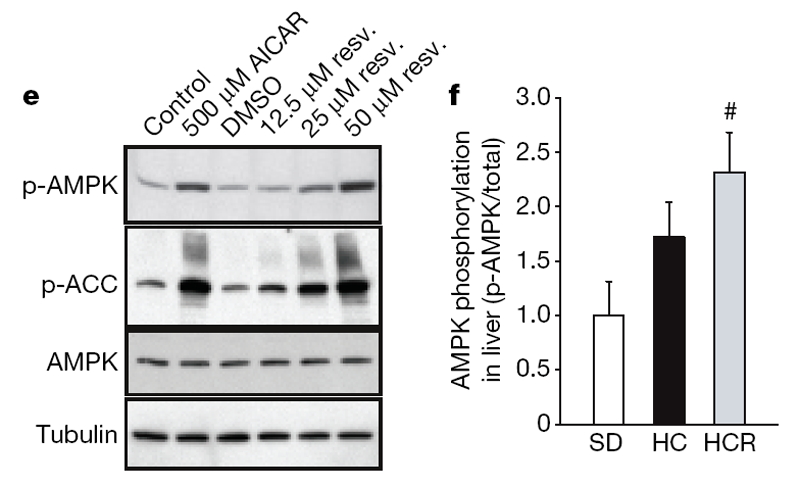
Next, we investigated possible mechanisms behind these metabolic effects. AMPK is a metabolic regulator that promotes insulin sensitivity and fatty acid oxidation. Its activity correlates tightly with phosphorylation at Thr 172 (p-AMPK). Chronic activation of AMPK occurs on a calorically restriction diet and has been proposed as a longevity strategy for mammals. [20] Consistent with this idea, additional copies of the AMPK gene are sufficient to extend lifespan in C. elegans. [21] Because we and others [22] have observed that resveratrol can activate AMPK in cultured cells through an indirect mechanism (Fig. 2e; see also Supplementary 3a–d), we examined whether AMPK activation occurred in the livers of the resveratrol-fed group. Resveratrol showed a strong tendency towards inducing phosphorylation of AMPK (Fig. 2f), as well as two downstream indicators of activity, namely phosphorylation of acetyl-coA carboxylase at Ser 79 and decreased expression of fatty acid synthase (Supplementary Fig. 3e, f).
Decreased organ pathology
At 18 months of age it was apparent that the high-calorie diet greatly increased the size and weight of livers and that resveratrol prevented these changes (Fig. 3a–c; see also Supplementary Fig. 4a, b) without altering plasma lipid levels (Table 1). Histological examination of liver sections by staining with haematoxylin and eosin or oil red O revealed a loss of cellular integrity and the accumulation of large lipid droplets in the livers of the HC but not the HCR group. Blinded scoring of the liver sections for overall pathology on a scale of 0–4 (with 4 being the most severe) gave mean values of 1.3 for the SD group, 2.8 for the HC group and 0.8 for the HCR group (Fig. 3b). Plasma amylase, which can indicate pancreatic damage, was elevated in the HC group and was significantly reduced by resveratrol (Table 1). The reasons for the elevation of plasma amylase levels in the HC group are unclear given that pancreatic sections of all animals revealed no damage to the pancreas or decrease in islet area (data not shown). Differences in the weights of other organs did not reach statistical significance.
Figure 3. Resveratrol improves liver histology, increases mitochondrial number
and decreases acetylation of PGC-1a.
a–c, Resveratrol prevents the development of fatty liver, as assessed by organ size (a), overall pathology (b) and decreased fat accumulation asmeasured by oil red O staining (c). AU, arbitrary units. d, Pathology of heart sections. Additional histology of liver, heart and aorta is shown in Supplementary Fig. 4. e, f, Transmission electron microscopy of liver sections (e) and mitochondrial counts (f). g, h,Mitochondrial number in HeLa cells treated with serumfromad libitumfed (AL) or calorically restricted (CR) rats, or resveratrol, and stained with Mitotracker green FM. i, j, Resveratrol reduces the acetylation of PGC-1a, a known SIRT1 target and regulator ofmitochondrial biogenesis, in vivo. PGC-1a was immunoprecipitated from liver extracts then blotted for acetyl lysine (i) and quantified (j). Asterisk, P,0.05 versus HC; hash, P,0.05 versus SD. n55 for b and d; n53 for f and j. Error bars indicate s.e.m.
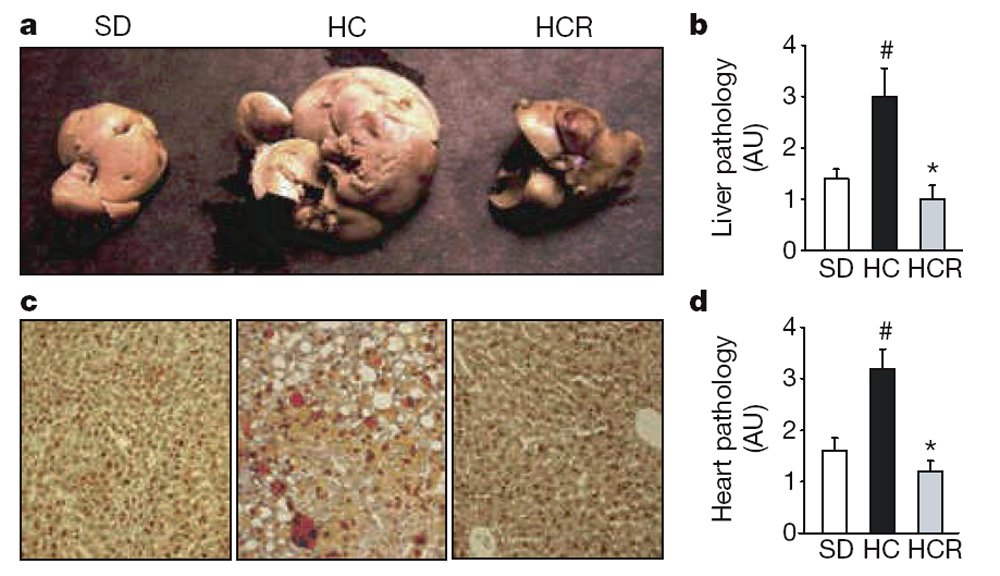

The ability of resveratrol to improve motor function and increase insulin sensitivity indicated that its effects were not confined to the liver. To test directly whether other organs also benefited, we examined heart tissue of the SD, HC and HCR mice. Blinded scoring of overall pathology—taking into account subtle changes in the abundance of fatty lesions, cardiac muscle vacuolization, degeneration and inflammation—on a relative scale of 0–4 (with 4 being the most severe) gave mean values of 1.6 for the SD group, 3.2 for the HC group and 1.2 for HCR group (Fig. 3d; see also Supplementary Fig. 4c). Improvements in the morphology of the aortic elastic lamina were also apparent (Supplementary Fig. 4d).
Increased mitochondria
Exercise and reduced caloric intake increase hepatic mitochondrial number [23, 24] and we wondered whether resveratrol might produce the same effect. The livers of the resveratrol-treated mice had considerably more mitochondria than those of HC controls and were not significantly different compared to those of the SD group (Fig. 3e, f). There was also a trend towards higher citrate synthase activity in the resveratrol-fed mice (an indicator of increased mitochondrial content) although the effect was not significant (Table 1). Culturing FaO hepatoma or HeLa cells in the presence of resveratrol increased mitochondrial number (Fig. 3g, h), similar to the previously reported effect of culturing cells in serum from calorically restricted rats24.
Mitochondrial biogenesis in liver and muscle is controlled, in large part, by the transcriptional coactivator PGC-1a [25, 26], the activity of which, in turn, is positively regulated by SIRT1-mediated deacetylation 27,28. Hence, the acetylation status of PGC-1a is considered a marker of SIRT1 activity in vivo. [27] Because this assay required more tissue than was available, we examined a separate cohort of one-yearold mice on the HC diet that had been treated with resveratrol for 6 weeks at 186 mg kg –1 d–1. The acetylation status of PGC-1a in the resveratrol-fed mice was threefold lower than the diet-matched controls (Fig. 3i, j). There was no detectable increase in SIRT1 protein levels in resveratrol-treated mice (data not shown), suggesting that SIRT1 enzymatic activity was enhanced by resveratrol.
Microarrays and pathway analysis
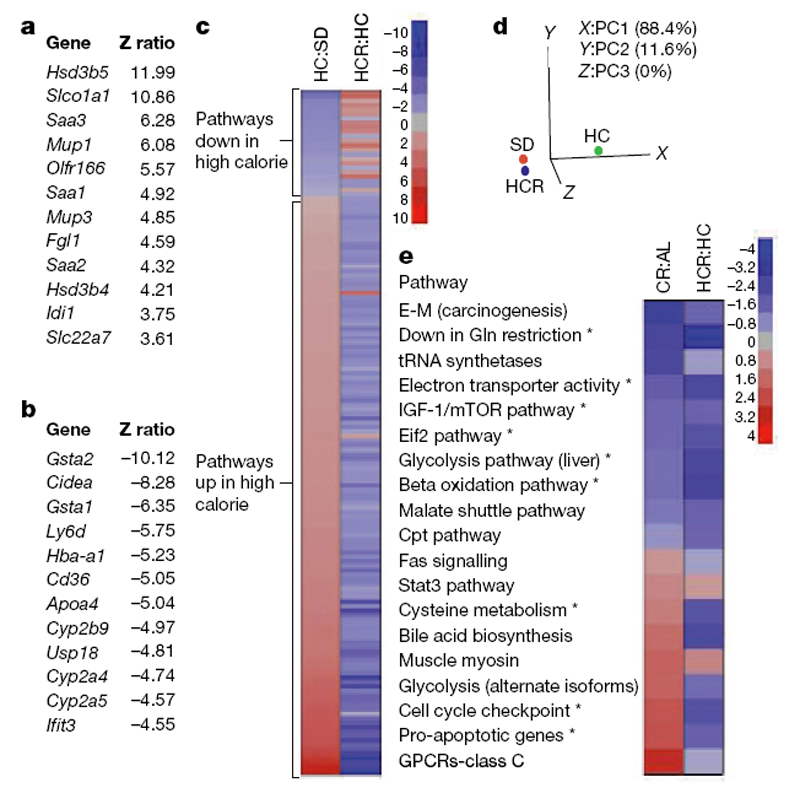
These data demonstrate that resveratrol can alleviate the negative impact of a high-calorie diet on overall health and lifespan. To determine to what extent resveratrol had shifted the physiology of the high-calorie group towards the lower calorie group, we performed whole-genome microarrays and pathway analysis on liver samples. Z ratios were calculated as described previously [29] and a subset of expression changes was verified by polymerase chain reaction with reverse transcription (RT–PCR) (Supplementary Fig. 5). In the HCR group, expression patterns for 782 out of 41,534 (,2%) individual genes changed significantly relative to the diet-matched controls (Fig. 4a, b). Notably, within the top 12 most highly elevated transcripts were serum amyloid proteins (Saa1–3), major urinary proteins (Mup1 and Mup3), and both forms of hydroxysteroid dehydrogenase that degrade testosterone (Hsd3b4, Hsd3b5). The list of 12 most highly downregulated transcripts included three cytochrome p450 enzymes (Cyp2a4, Cyp2a5 and Cyp2b9) that are known to activate pro-carcinogens. [30]
The complete data set is available at
http://www.grc.nia.nih.gov/branches/rrb/dna/index/dnapubs.htm#2.
Figure 4. Resveratrol shifts expression patterns of mice on a high-calorie diet towards those on a standard diet.
a, b, The most highly significant upregulated (a) and downregulated (b) genes in livers of HC and HCR groups are shown. c, Parametric analysis of gene-set enrichment (PAGE) comparing every pathway significantly upregulated (red) or downregulated (blue) by either the HC diet or resveratrol (153 in total, with 144 showing opposing effects). d, Principal component analysis of PAGE data. The first principal component (PC1) is dominant, with 88.4% variability, and shows HCR to be more similar to SD than HC. e, Comparison of pathways significantly altered by resveratrol treatment and caloric restriction using data from the AGEMAP caloric restriction study. Pathways with significant differences between HC and HCR are indicated by an asterisk. Complete pathway listings are in Supplementary Fig. 7. Asterisk, P,0.05 versus HC. n55 for SD and HC; n54 for HCR.
We next performed parametric analysis of gene set enrichment (PAGE), a computational method that determines differences between pathways using a priori defined gene sets. [31, 32] PAGE analysis indicated that resveratrol caused a significant alteration in 127 pathways, including the TCA cycle, glycolysis, the classic and alternative complement pathways, butanoate and propanoate metabolism, sterol biosynthesis and Stat3 signalling (Supplementary Fig. 6; for a complete list see Supplementary Fig. 7). Some of the most highly downregulated pathways in the resveratrol-fed group are known to extend lifespan in model organisms when attenuated, including insulin signalling, IGF-1 and mTOR signalling, oxidative phosphorylation and electron transport. [33–36] Downregulation of glycolysis is a well known marker of caloric restriction37 and has been proposed as a mechanism by which caloric restriction works. [38] The increase in Stat3, a transcription factor involved in cell survival and liver regeneration [39], is of note because its activity is known to be suppressed in the liver by high caloric diets and shows an age-related decline in activity that is attenuated by caloric restriction. [40, 41]
A few of the pathway changes were unanticipated. Although we had observed an increase in mitochondrial number in the HCR group, there was a decrease in the transcription of numerous mitochondrial genes, suggesting that the turnover of mitochondrial proteins was reduced. This result was unexpected, but is consistent with a previous report showing that SIRT1-dependent activation of PGC- 1a does not enhance transcription of mitochondrial genes. [27] Upregulation of complement, which occurs in obese and aged mice, was also observed in the HCR group for reasons that are currently unclear.
It is notable that resveratrol opposed the effects of high caloric intake in 144 out of 153 significantly altered pathways (Fig. 4c). In fact, the PAGE signature of the HCR group was considerably more similar to that of the SD group than the HC controls. Principal component analysis yielded values of 21.82 (SD), 21.41 (HCR) and 3.22 (HC), with 88.4% of the variability assigned to the first principal component, making the HC group the clear outlier (Fig. 4d).
We next compared our PAGE results to a pre-existing caloric restriction data set for C57BL/6 mice known as AGEMAP, hypothesizing that the comparison of changes induced by these two paradigms might reveal pathways common to the enhancement of health and longevity. Of the 36 different pathways identified by AGEMAP as being significantly altered by caloric restriction, there was sufficient overlap to compare 19 of them to our data (Fig. 4e). Pathways altered in the same direction by caloric restriction and resveratrol included the downregulation of IGF-1 and mTOR signalling, downregulation of glycolysis, and upregulation of Stat3 signalling. One interesting difference was that cell cycle checkpoint and apoptotic pathways were elevated in the caloric restriction group but downregulated by resveratrol. We do not favour the interpretation that the resveratrol-treated livers were undergoing less apoptosis because levels of AST and ALT, two indicators of hepatic apoptosis, were unchanged (see Table 1). Perhaps the downregulation of cell cycle checkpoints is linked to the recent discovery that inhibition of checkpoint function in C. elegans increases stress resistance and lifespan. [42] Although the statistical power of this analysis is limited by the overlap in data sets, the results suggest that more comprehensive comparisons of the effects of resveratrol and caloric restriction are warranted.
Discussion
The ability of resveratrol to prevent the deleterious effects of excess caloric intake and modulate known longevity pathways suggests that resveratrol and molecules with similar properties might be valuable tools in the search for key regulators of energy balance, health and longevity. As a case in point, the most highly upregulated gene in the HC group and second most highly downregulated gene in the HCR group was Cidea, which regulates energy balance in brown fat and provides resistance to obesity and diabetes when knocked out. [43]
Taken together, the findings in this study show that resveratrol shifts the physiology of mice consuming excess calories towards that of mice on a standard diet, modulates known longevity pathways, and improves health, as indicated by a variety of measures including survival, motor function, insulin sensitivity, organ pathology, PGC- 1a activity, and mitochondrial number. Notably, all these changes occurred without a significant reduction in body weight. Whether these effects are due to resveratrol working primarily through SIRT1, which is the case for simpler metazoans, or through a combination of interactions, as predicted by the xenohormesis hypothesis [6, 44], remains to be determined. In either case, this study shows that an orally available small molecule at doses achievable in humans can safely reduce many of the negative consequences of excess caloric intake, with an overall improvement in health and survival.
METHODS
Animals and diets. Animal housing and diets are described in Supplementary Information. Briefly, one-year-old male C57BL/6NIA mice were maintained on AIN-93G standard diet (SD), AIN-93G modified to provide 60% of calories from fat (HC), or HC diet with the addition of 0.04% resveratrol (HCR).
Electron microscopy. Tissues were processed as previously reported, imaged at 31,500 on a Jeol 1210 transmission microscope, and photographed using a Gatan US 4000 MP Digital camera. Mitochondria per cell and hepatocyte size were quantified using grid counting by three blinded raters.
PAGE analysis. RNA extracted from the livers of five 18-month-old mice per group was hybridized to Agilent 44k whole-genome microarrays following protocols listed on the Gene Expression and Genomics Unit website at the National Institute on Aging (http://www.grc.nia.nih.gov/branches/rrb/dna/index/protocols. htm). One array from the HCR group was discarded owing to RNA degradation. Raw data were subjected to Z normalization and tested for significant changes as previously described. [29] For parametric analysis of gene set enrichment (PAGE), a complete set of 522 pathways in the cell was obtained from http:// www.broad.mit.edu/gsea/msigdb/msigdb_index.html. Details of the method are described in Supplementary Information and elsewhere. [31] Principal component analysis was performed on the replicate average for the three groups. These tools are part of DIANE 1.0, a program developed by V.V.P and available at http:// www.grc.nia.nih.gov/branches/rrb/dna/diane_software.pdf. Caloric restriction data were extracted from the AGEMAP project.
Cell-based mitochondrial assays. Mitochondrial mass was determined using the mitochondria-specific fluorescent dye Mitotracker greenFMafter fixation of cells, with minor modifications. Staining after fixation permits determination of the incorporation of the dye due to mitochondrial mass independently of the mitochondrial membrane potential (Dym).
Serum markers and hormones. Details of biochemical assays are described in Supplementary Information.
AMPK and PGC-1a analysis. Extraction of tissues and cells for western blotting were performed using standard techniques. Details and antibodies are outlined in Supplementary Information.
Histology. Eighteen-month-old mice were fasted overnight and fixed using Streck fixative (Streck). Organs were sectioned and stained with haematoxylin and eosin or frozen and stained with oil red O lipid stain, then scored blindly for overall pathology.
Rotarod. Results shown are the average of three trials per mouse, measuring time to fall from an accelerating rotarod (4–40 r.p.m. over 5 min). Data from animals that survived to 24 months are shown.
Statistical analyses. Single factor analysis of variance followed by Fisher’s posthoc tests were used for all comparisons, except where noted. Details are provided in Supplementary Information.
References:
Olshansky, S. J. Projecting the future of U.S. health and longevity. Health Aff. (Millwood) 24 (suppl. 2), W5R86–W5R89 (2005).
Li, Z., Bowerman, S. & Heber, D. Health ramifications of the obesity epidemic. Surg. Clin. North Am. 85, 681–701 (2005).
Ingram, D. K. et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann. NY Acad. Sci. 1019, 412–423 (2004).
Sinclair, D. A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 126, 987–1002 (2005).
Guarente, L. & Picard, F. Calorie restriction—the SIR2 connection. Cell 120, 473–482 (2005).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004).
Viswanathan, M., Kim, S. K., Berdichevsky, A. & Guarente, L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell 9, 605–615 (2005).
Jarolim, S. et al. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 5, 169–177 (2004).
Valenzano, D. R. et al. Resveratrol prolongs lifespan and retards the onset of agerelated markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 (2006).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-c. Nature 429, 771–776 (2004).
Chen, J. et al. SIRT1 protects against microglia-dependent amyloid-b toxicity through inhibiting NF-kB signaling. J. Biol. Chem. 280, 40364–40374 (2005).
Kolthur-Seetharam, U., Dantzer, F., McBurney, M. W., de Murcia, G. & Sassone-Corsi, P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle 5, 873–877 (2006).
Raval, A. P., Dave, K. R. & Perez-Pinzon, M. A. Resveratrol mimics ischemic preconditioning in the brain. J. Cereb. Blood Flow Metab. 26, 1141–1147 (2006).
Frescas, D., Valenti, L. & Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 (2005).
Denu, J. M. The Sir2 family of protein deacetylases. Curr. Opin. Chem. Biol. 9, 431–440 (2005).
Weindruch, R. & Walford, R. L. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215, 1415–1418 (1982).
Siebler, J. & Galle, P. R. Treatment of nonalcoholic fatty liver disease. World J. Gastroenterol. 12, 2161–2167 (2006).
Scrocchi, L. A. & Drucker, D. J. Effects of aging and a high fat diet on body weight and glucose tolerance in glucagon-like peptide-1 receptor2/2 mice. Endocrinology 139, 3127–3132 (1998).
McCarty, M. F. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Med. Hypotheses 63, 334–339 (2004).
Apfeld, J., O’Connor, G., McDonagh, T., DiStefano, P. S. & Curtis, R. The AMPactivated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009 (2004).
Zang, M. et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55, 2180–2191 (2006).
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005).
Lopez-Lluch, G. et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl Acad. Sci. USA 103, 1768–1773 (2006).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Yoon, J. C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1a and SIRT1. Nature 434, 113–118 (2005).
Lerin, C. et al. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1a. Cell Metab. 3, 429–438 (2006).
Cheadle, C., Vawter, M. P., Freed, W. J.& Becker, K. G. Analysis of microarray data using Z score transformation. J. Mol. Diagn. 5, 73–81 (2003).
Baur, J. A. & Sinclair, D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev. Drug Discov. 5, 493–506 (2006).
Kim, S. Y. & Volsky, D. J. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics 6, 144 (2005).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Feng, J., Bussiere, F. & Hekimi, S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1, 633–644 (2001).
Kenyon, C. A conserved regulatory mechanism for aging. Cell 105, 165–168 (2001).
Kapahi, P. et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885–890 (2004).
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 (2005).
Ingram, D. K. et al. Calorie restriction mimetics: an emerging research field. Aging Cell 5, 97–108 (2006).
Hipkiss, A. R. Does chronic glycolysis accelerate aging? Could this explain how dietary restriction works? Ann. NY Acad. Sci. 1067, 361–368 (2006).
Taub, R. Liver regeneration: from myth to mechanism. Nature Rev. Mol. Cell Biol. 5, 836–847 (2004).
Xu, X. & Sonntag, W. E. Growth hormone-induced nuclear translocation of Stat-3 decreases with age: modulation by caloric restriction. Am. J. Physiol. 271, E903–E909 (1996).
Wilsey, J. & Scarpace, P. J. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J. Endocrinol. 181, 297–306 (2004).
Olsen, A., Vantipalli, M. C. & Lithgow, G. J. Checkpoint proteins control survival of the postmitotic cells in Caenorhabditis elegans. Science 312, 1381–1385 (2006).
Zhou, Z. et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nature Genet. 35, 49–56 (2003).
Howitz, K. T. & Sinclair, D. A. in Handbook of the Biology of Aging (eds Masoro, E. J. & Austad, S. N.) 63–104 (Elsevier, Boston, 2006).

Return to RESVERATROL
Since 8-31-2008


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |