FROM:
Archives of Gerontology and Geriatrics 2004; (9): 393–402 ~ FULL TEXT
C. Smorgon, E. Mari, A.R. Atti, E. Dalla Nora, P.F. Zamboni, F. Calzoni, A. Passaro* And R. Fellin
Second Department of Internal Medicine,
University of Ferrara,
Via Savonarola, 9 - 44100
Ferrara, Italy.
Dementia is one of the most pressing public health problems with social and economic implication. The form called cognitive impairment non-dementia (CIND)represents a subclinical phase of dementia. Different studies have shown a possible effect of micro- and macro-nutrients on cognitive function. Trace elements, being involved in metabolic processes and redox reactions in the central nervous system (CNS), could influence the cognitive functions. This study evaluated the presence of an eventual correlation between serum trace element concentrations and cognitive function in a group of subjects with CIND and manifest dementia (Alzheimer dementia = AD, and vascular dementia = VaD), and compared them with a control group. Thirty -five patients were enrolled in this study. Each patient underwent a clinical and biochemical examination. We also performed a neuropsychological and functional assessment (the Milan overall dementia assessment = MODA, activities of daily living = ADL, and instrumental activities of daily living = IADL), and a computerized tomographic (CT) cerebral scan. Patients were than divided in 4 groups according to the obtained diagnosis (Controls, CIND, AD, VaD).
The presence of any acute or chronic conditions, affecting cognitive functions, was considered as exclusion criteria. A blood sample was collected to determine iron (Fe), zinc (Zn), manganese (Mn), selenium (Se), cobalt (Co), chromium (Cr), copper (Cu), molybdenum (Mo) and aluminium (Al) serum concentrations (chromatographic, spectrophotometric methods). In our cohort we found a positive correlation between cognitive function, expressed as the MODA score, and Se, Cr, Co and Fe serum levels, while a negative correlation was observed between MODA score, Cu and Al serum levels. Moreover, some statistically significant differences in Se, Cr, Co, Cu and Al concentrations were found among the groups. According to these results, we may suppose that Se, Cr and Co protect cognitive function, Cu influences the evolution of cognitive impairment, while Al contributes to the pathogenesis of AD.
Keywords: dementia, cognitive impairment non-dementia (CIND), cognitive function, trace elements, selenium, chromium, cobalt, copper, aluminium
From the FULL TEXT Article:
INTRODUCTION
Dementia is one of the most pressing public health problems with social and economic
implication. The form called cognitive impairment non-dementia (CIND) [Graham et al,
1997] represents a sub-clinical phase of dementia, defined as short and long-term memory
impairment only, with no functional disabilities and not affecting the autonomy of daily living
and the global cognitive function.
The brain possesses resident immune effector cells, called microglia. Whereas alterations
in peripheral immune functions, with usual aging, are well established, the involvement
of the immune system in brain aging, is not clear. In contrast to normal aging, there is
abundant evidence that inflammation is associated with, and contribute to, the
neurodegenerative process in Alzheimer disease (AD) and other age-related neurodegenerative
disorders [Kalaria et al., 1996; Wisniewski and Frangione, 1996; Zielasek and
Hartung, 1996]. Cells, in nervous system, produce a variety of proteins, which serve the
function of promoting neuronal survival and outgrowth and protecting neurons against injury
and death. There appear to be two general mechanisms by which neurotrophic factors
prevent neuronal degeneration: increasing cellular resistance to oxidative stress, and stabi -
lizing cellular calcium homeostasis. These actions of neurotrophic factors appear to result
from induction of the expression of antioxidant enzymes (e.g., superoxide dismutases and
glutathione peroxidase) and calcium regulating proteins (e.g., the calcium -binding protein
calbindin) [Disterhoft et al., 1994].
Aging and AD are characterized by an impairment of blood brain barrier function. With
aging, and especially in AD, oxidative damage to endothelial cells results from exposure to
oxidized low-density lipoproteins and amyloid ˛-peptide (A˛). Oxidative stress impairs
functions of endothelial cells and promotes penetration of macrophages into the brain
parenchyma: reduces neurons nutrient availability and stimulates inflammatory processes
[Kalaria et al., 1996; Blanc et al., 1997; Markesbery, 1997].
Different studies showed possible effects of micro- and macro-nutrients in affecting
cognitive function. Trace elements are found in tissue and cells as components of the active
site of enzymes or as regulators of enzymatic activity. As components of enzymes and
proteins, trace elements are frequently key elements in red-ox reaction. It is assumed that
the antioxidant system is widely involved in aging process, as well in carcinogenesis (such
as Cu/Zn-SOD) [Floyd, 1990; Paustenbach et al., 1996; Speich et al., 2001]. Cellular
oxidative stress results in an impaired function of plasma membrane glucose and glutamate
transporters and in failure of mitochondrial electron transport and ATP production.
Moreover, the important calcium-sequestering function of mitochondria may be
compromised as a result of age-related DNA damage and this may increase neuronal
vulnerability to excitotoxicity and metabolic insults [Blass, 1993; Bowling and Beal, 1995].
Thus, microelements, being involved in metabolic processes and redox reactions in
the central nervous system (CNS), could influence cognitive function [Berr et al., 2000]
optimizing many cellular protein functions.
Finally, inflammation could be the trade union between AD and VaD. The primary role
of the inflammation in the pathogenesis of arteriosclerosis is well known, but there are also
several studies supporting a possible role of inflammations in the clinical course of AD. In
fact, the rate of cognitive decline in AD is accelerated in those subjects showing higher
interleukin -1 (IL-1) concentrations [Murphy et al., 2001]. It is still unclear if an interaction
between genes and inflammatory cytokines (e.g., IL-1) could play a pathogenetic role on
cognitive impairment, especially in AD [Sheng et al., 1998].
The aim of this pilot study was to evaluate the presence of an eventual correlation
between serum trace-element concentrations and cognitive function in a group of subjects
with CIND and dementia (AD e VaD) and to compare them with a control group.
SUBJECTS AND METHODS
In this study we enrolled 35 patients. Each patient underwent a clinical and biochemical
examination. We also performed a neuropsychological and functional assessment by
using the Milan overall dementia assessment (MODA) [Brazzelli et al., 1994] the activities
of daily living (ADL) [Katz et al., 1970], the instrumental activities of daily living (IADL)
[Lawton and Brody, 1969], and a computerized tomographic (CT) cerebral scan. Patients
were then divided in 4 groups according to the obtained diagnosis: Controls, CIND, AD and
VaD. The presence of any acute or chronic conditions, affecting cognitive function, was
considered as exclusion criteria (such as, chronic heart failure, chronic renal failure,
anemia, vitamin B12 and folate deficit, depression, cancer, impairment of glucose tolerance).
A blood sample was collected to determine iron (Fe), zinc (Zn), manganese (Mn), selenium
(Se), cobalt (Co), chromium (Cr), copper (Cu), molybden (Mo) and aluminium (AI) serum
concentrations, by means of chromatographic, or spectrophotometric methods [Eitenmiller
and Landen, 1999].
 Table 1
Table 1
 Table 2
Table 2
 Table 3
Table 3
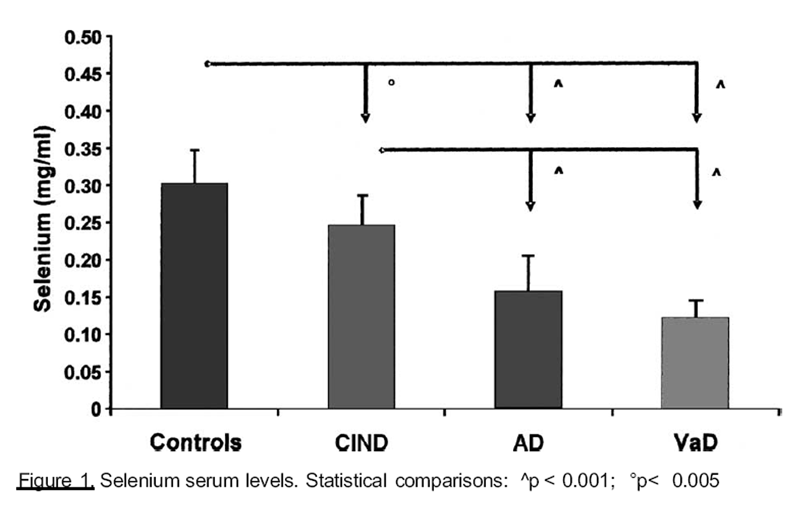 Figure 1
Figure 1
 Figure 2
Figure 2
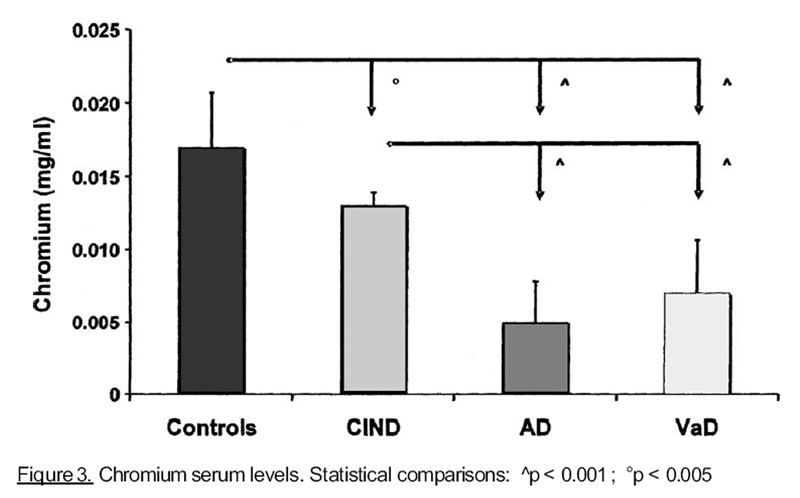 Figure 3
Figure 3
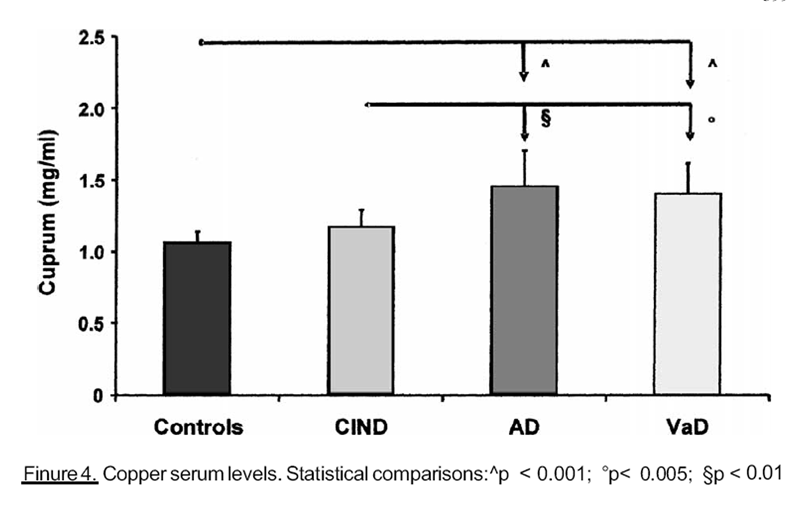 Figure 4
Figure 4
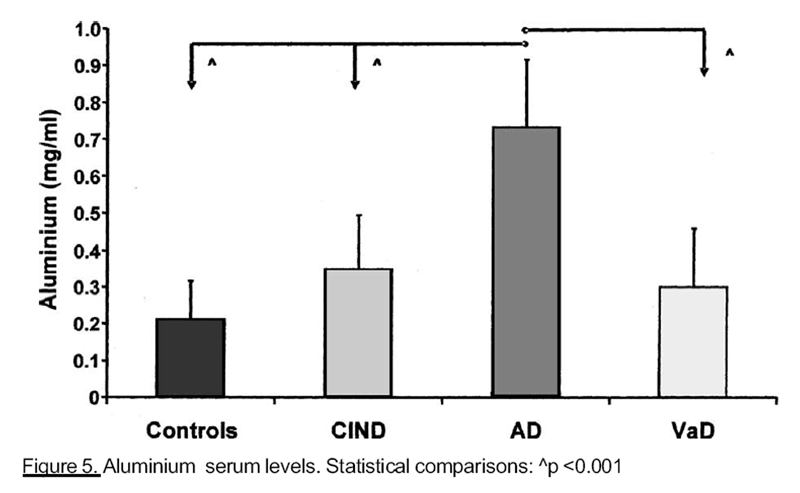 Figure 5
Figure 5
|
RESULTS
Clinical and biochemical characteristics of the four groups are described in the Tables
I and II. Trace element serum concentrations were in a normality range in all subjects. We
observed a positive linear correlation between MODA score and serum concentration of Se
(r = 0.776; P 0.001), Co (r = 0.634; p < 0.001), Cr (r = 0.840; P 0.001) and Fe (r = 0.364;
p < 0.04). On the other hand, we found a negative correlation between MODA score and Cu
(r= -0.913; p < 0.001) as well as AI (r = -0.628 ; p< 0.001) concentration.
As shown in Table Ill and Figures 1-5, we found significantly different concentrations
of some trace elements in the different groups. Selenium (Se) serum levels (Figure 1) were
significantly lower in patients with dementia, than in control and ClND groups. Similar
results were found for cobalt and chromium, as shown, respectively, in Figures 2 and 3. On
the other hand, we obtained higher copper serum levels (Figure 4) in subjects with
advanced impaired cognitive function, than in controls and ClND patients. Finally subjects
with AD showed higher AI concentrations (Figure 5) than any other group .moment, there are no clinical studies about the possible correlation between Co and cognitive function.
Our findings are similar for Cr, that was shown to be essential for optimal peripheral
insulin action with respect to glucose uptake, but it improves also glucose uptake
independently from insulin. A number of studies have shown that impaired glucose
tolerance or type 2 diabetes is associated with impaired cognitive function in older subjects
(Messier and Gagnon, 2000) and it has been demonstrated that, in a group of elderly
humans with type 2 diabetes, a period of chromium supplementation improve the glucose
tolerance [Berdanier, 1998]. The positive relation between Cr levels and good cognitive
performance could be understood because of a better glucose intake in CNS [Blass, 1993;
Shrivastava et al., 2002].
In our population there was a negative correlation between Cu levels and brain performance.
Consistently with this result, we may suppose that Cu could predict the evolution
from ClND to dementia. It plays a role in the expression of the gene for the cysteine-rich
metallothionein, being part of a DNA-binding protein, which stimulates transcription.
Moreover, Cu cross links DNA with a covalent binding, producing non-functional DNA or
DNA which is unable to repair itself, and this could be a cause of cellular alterations and
neuronal death. Cu is also required, as a cofactor, in several enzymes, especially in the
mitochondrial respiratory enzymes cytochrome-oxidase. CNS degeneration can be related
to a decline in respiratory chain activity and ATP synthesis, which is essential to neural
activity. Our results are consistent with some studies about Cu functions in vitro and on rats
[Hoshi et al., 1993; Chen et al., 1995; Matz et al., 1995], but we have not found any data in
humans.
In our population, serum AI concentration is strictly positively correlated with AD; in
fact, it has already been known as a toxic element for CNS, because it leads to an impaired
glucose metabolism and it stimulates the fibrillar ˛-amyloid production [Marcus et al., 1992].
Our results confirm those several evidences in the literature about its possible ethiopathogenetic
role for AD.
On the basis of our findings, we may suppose that Se, Cr and Co could play a protective
role in cognitive function, while Cu could accelerate the evolution of cognitive impairment.
AI concentrations, we have found significantly higher in AD, than in all other groups,
suggest a possible ethiopathogenetic role of this element in AD.
References
Berdanier, C. D. (1998): Advanced Nutrition. Micronutrients. CRC Press, Boca Raton, pp.
15-17, and 215.
Berr, C., Balansard, B., Arnaud, J., Roussel, A.M. and Alperovitch, A. (2000): Cognitive
decline is associated with systemic oxidative stress: the EVA study. (Etude du
Vieillissement Arteriel). J. Am. Geriatr. Soc., 48, 1285-1291.
Blanc, E.M., Toborek, M., Mark, R.J., Hennig, B. and Mattson, M.P. (1997): Amyloid ˛-
peptide induces cell monolayer albumin permeability, impairs glucose transport, and
induces apoptosis in vascular endothelial cells. J. Neurochem., 68, 1870-1881.
Blass, J.P. (1993): Metabolic alterations common to neural and non-neural cells in Alzheimer’s
disease. Hippocampus, 3 (Spec. Issue), 45-53.
Bowling, A.C. and Beal, M.F. (1995): Bioenergetic and oxidative stress in neurodegenerative
diseases. Life Sci., 56, 1151-1171.
Brazzelli, M., Capitani, E., Della Sala, S., Spinnler, H. and Zuffi, M. (1994): A neuropsy -
chological instrument adding to the description of patients with suspected cortical
dementia: the Milan overall dementia assessment. J. Neurol. Neurosurg. Psychiatry,
Chen,Y., Saari, J. T. and Kang, Y.J. (1995): Copper deficiency increases metallothionein-l
mRNA content selectively in rat liver, J. Nutr. Biochem., 6, 572-576.
Chu, F.F. (1994): The human glutathione peroxidase genes GPX2, GPX3 and GPX4 map
to chromosome 14, 5 and 19 respectively, Cytogenet. Cell Genet., 66, 96-98.
Cohen, H.J., Brown, M.R., Hamilton, D., Lyons-Patterson, J., Avessar, N., and Liegey, P.
(1 989): Glutathion peroxidase and selenium deficiency in patients receiving home
parenteral nutrition. Time course for development of deficiency and repletion of
enzyme activity in plasma and blood cells. Am. J. Clin. Nutr., 49, 132-139.
Disterhoft, J.F., Moyer, J.R. Jr. and Thompson, L.T. (1994): The calcium rationale in aging
and Alzheimer’s disease. Evidence from an animal model of normal aging. Ann. N.Y.
Acad. Sci., 747, 382-406.
Eitenmiller, R.R. and Landen, W.O.Jr. (1999): Vitamin Analysis for the Health and Food
Sciences, CRC Press, Boca Raton.
Floyd, R.A. (1990): Role of oxygen radicals in carcinogens and brain ischemia. FASEB J.,
Graham, J.E., Rockwood, K., Beattle, B.L., Eastwood, R., Gauthier, S., Tuokko, H., Mc
Dowell, I. (1997): Prevalence and severity of cognitive impairment with and without
dementia in an elderly population, Lancet, 349, 1793-1796.
Hoshi, Y., Hazeky, O. and Tamura, M. (1993): Oxygen dependence of redox state of copper
in cytochrome oxidase in vitro, J. Appl. Physiol., 74, 1622-1627.
Kalaria, R.N., Harshbarger-Kelly, M., Cohen, D.L. and Premkumar, D.R. (1996): Molecular
aspects of inflammatory and immune responses in Alzheimer’s disease. Neurobiol.
Aging, 17, 687-693.
Katz, S., Downs, T.D., Cash, H.R. and Grotz, R.C. (1970): Progress in development of the
index of ADL. Gerontologist, 10, 20-30.
Lawton, M.P. and Brody, E.M. (1969): Assessment of older people: self-maintaining and
instrumental activities of daily living. Gerontologist, 9, 179-18 6.
Marcus, D.L, Wong, S. and Freedman, M.L. (1992): Dietary aluminium and Alzheimer’s
disease. J. Nutr. Elder., 12, 55-61.
Markesbery, W.R. (1997): Oxidative stress hypothesis in Alzheimer's disease. Free. Radic.
Biol. Med., 23, 134-147.
Matz, J.M., Saari, J.T. and Bode, A.M. (1995): Functional aspects of oxidative phospho -
rylation and electron transport in cardiac mitochondria of copper deficient rats, J. Nutr.
Biochem., 6, 644-652.
Messier, C. and Gagnon, M. (2000): Glucose regulation and brain aging. J. Nutr. Health
Aging, 4, 208-213.
Murphy, G.M.Jr., Claassen, J.D., DeVoss, J.J., Pascoe, N., Taylor, J., Tinklenberg, J.R. and
Yesavage, J.A. (2001): Rate of cognitive decline in AD is accelerated by the
interleukin-1 alpha-889*1 allele. Neurology, 56, 1595-1 597.
Paustenbach, D.J., Finley, B.L. and Kacew, S. (1996): Biological relevance and consequen -
ces of chemical or metal-induced DNA cross linking. Proc. Soc. Biol. Med., 21 1, 21 1-
217.
Sheng, J.G., Griffin, W.S., Royston, M.C. and Mrak, R.E. (1998): Distribuition of interleukin-
1-immunoreactive microglia in cerebral cortical layers: implications for neuritic plaque
formation in Alzheimer’s disease. Neuropathol. Appl. Neurobiol., 24, 278-283.
Shrivastava, R., Upreti, R.K., Seth, P.K. and Chaturvedi, U.C. (2002): Effects of Chromium
on the immune system. FEMS Immunol. Med. Microbiol., 34,1-7.
Speich, M., Pineau, A. and Ballereau, F. (2001): Minerals, trace elements and related biolo -
gical variables in athletes and during physical activity. Clin. Chim. Acta, 312, 1-11.
Strain, J.J. (1991): Disturbances of micronutrient and antioxidant status in diabetes. Proc.
Nutr. Soc., 50, 591-604.
Vogt, J.G., and Vogt, D. (1990): Biochemistry. John Wiley and Sons, New York, pp.: 771-
986 and 1086-1 178.
Wisniewski, T. and Frangione, B. (1996): Molecular biology of. brain aging and neurodegenerative
disorders. Acta Neurobiol. Exp. (Warsz), 56, 267-271.
Zielasek, J. and Hartung, H.P. (1 996): Molecular mechanisms of microglial activation.
Adv. Neuroimmunol., 6,191-222.

Return to ALZHEIMER's
Since 4-14-2020
|















