| PMC full text: | Published online 2019 Jan 3. doi: 10.3390/nu11010079
|
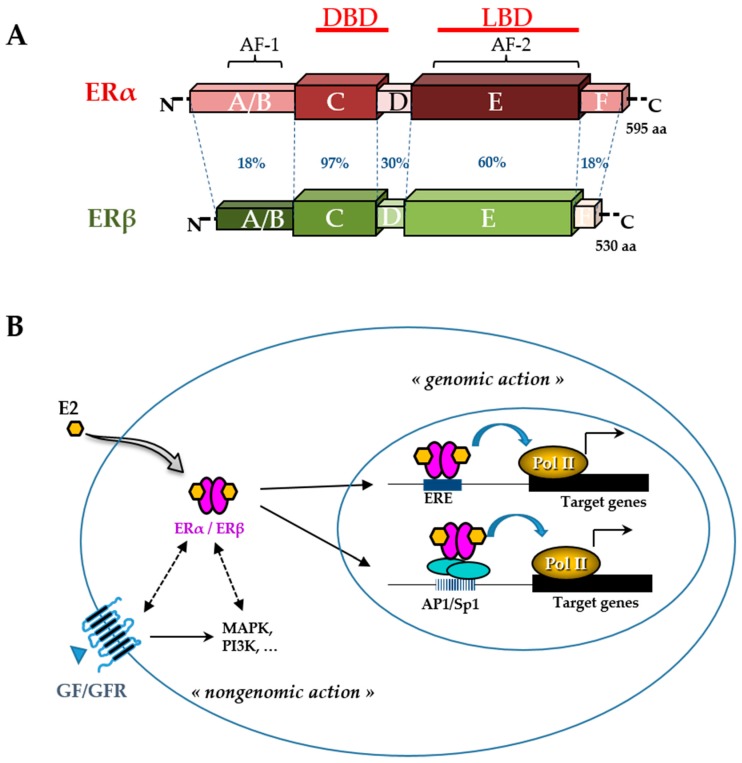
Figure 3
Estrogen receptor (ER) structure and action. The schematic structures of the two human ERα and ERβ and the percentage of homology between the different domains (annotated by the letters A to F) are indicated (A). Domains involved in DNA binding (DBD), ligand binding (LBD), ligand-independent transactivation function 1 (AF-1), and ligand-dependent transactivation function 2 (AF-2) are shown. The number of amino acids for each receptor is also indicated on the right side. Estradiol (E2) mediates numerous phenotypic effects in cells by binding to and activating ERs (B). E2 enters the cell through the lipid membranes and binds ER, which can be present in the cytoplasm and the nucleus. The activated ER forms a dimer to tightly fix chromatin directly at the estrogen-responsive element (ERE) sites or indirectly at activator protein 1 (AP1) or specificity protein 1 (Sp1) sites. ER is then able to remodel chromatin by recruiting cofactors and activating RNA polymerase II (Pol II), at target genes (genomic action). Besides, ERs can use rapid non-genomic action through the interaction with intracellular kinases (mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K),…) and the growth factor (GF) receptor (GFR) pathways.