

Is There a Difference in Head Posture and
Cervical Spine Movement in Children
With and Without Pediatric Headache?This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: European Journal of Pediatrics 2013 (Oct); 172 (10): 1349–1356 ~ FULL TEXT
OPEN ACCESS Kim Budelmann, Harry von Piekartz, Toby Hall
University of Applied Science,
Osnabrück, Germany,
k.b.budelmann@gmail.comPediatric headache is an increasingly reported phenomenon. Cervicogenic headache (CGH) is a subgroup of headache, but there is limited information about cervical spine physical examination signs in children with CGH. Therefore, a cross-sectional study was designed to investigate cervical spine physical examination signs including active range of motion (ROM), posture determined by the craniovertebral angle (CVA), and upper cervical ROM determined by the flexion-rotation test (FRT) in children aged between 6 and 12 years. An additional purpose was to determine the degree of pain provoked by the FRT.
Thirty children (mean age 120.70 months [SD 15.14]) with features of CGH and 34 (mean age 125.38 months [13.14]) age-matched asymptomatic controls participated in the study. When compared to asymptomatic controls, symptomatic children had a significantly smaller CVA (p < 0.001), significantly less active ROM in all cardinal planes (p < 0.001), and significantly less ROM during the FRT (p < 0.001), especially towards the dominant headache side (p < 0.001).
In addition, symptomatic subjects reported more pain during the FRT (p < 0.001) and there was a significant negative correlation (r = -0.758, p < 0.001) between the range recorded during the FRT towards the dominant headache side and FRT pain intensity score. This study found evidence of impaired function of the upper cervical spine in children with CGH and provides evidence of the clinical utility of the FRT when examining children with CGH.
From the FULL TEXT Article:
Introduction
Headache is the most frequently reported pain in children [28], with an even sex distribution up to the age of 12 [2, 25, 28], after which more females than males suffer. [25, 49] Pediatric headache prevalence rates are 50% during school years, increasing during adolescence to 80%. [41] Studies have shown that children with more severe headache report lower quality in life, in general [5], while early onset headache can be predictive of ongoing problems during adolescence and adult life [8, 18], indicating the importance of diagnosis and management.
Cognitive, behavioral, and emotional factors have been shown to play important roles in generating headache in children. [4, 33] In addition, physical factors, such as schoolwork, increased forward head posture, and prolonged static postures of the head [11, 34, 49], have also been shown to play a role in triggering headache. Hence, headache diagnosis is important, particularly for physiotherapists who have to consider whether physical treatment may be helpful to alleviate symptoms.
There are numerous structures and disorders capable of causing headache. [22] The International Headache Society [20] has formulated the International Classification of Headache Disorders (ICHD) to enable differentiation of primary and secondary headache disorders. One form of secondary headache is cervicogenic headache (CGH), where pain is believed to originate from a disorder in the neck. [20] The anatomical basis for pain perceived in the head is due to the convergence of afferent impulses from the upper three cervical nerve roots with the trigeminal nerve in the trigeminocervical nucleus. [7, 22] The ICHD [20] is commonly used to diagnose headache in adults and relies mainly on subjective descriptors from the patient. [40] In pediatric headache, such subjective differentiation is more difficult [27, 49] and physical signs become increasingly important to identify CGH.
Physical examination has been shown to be successful in distinguishing CGH fromother headache forms in adults. [23] Physical signs characteristic of CGH in adults include impaired range of rotation in the upper cervical spine identified by the flexion–rotation test (FRT) [12, 15, 35, 45], decreased active range of motion (ROM) [23, 51, 52], increased forward head posture [48], upper cervical joint dysfunction [16], and impaired cervical muscle function. [21, 22] To date, few studies have investigated these or other factors in children who suffer from headache [47, 49]. Published normal values for active cardinal plane ROM in asymptomatic children indicate larger ranges than adults [3, 29], thus warning of the difficulty of using adult values when examining children.
The therapist examining children with purported CGH requires a good knowledge of the musculoskeletal characteristics of the cervical spine of asymptomatic children in order to identify differences and potential impairments. Recent literature advocates the use of the FRT as a useful means of identification of impairment of the upper cervical spine and CGH diagnosis in adults. [14, 16, 17] For this test, the subject's neck is positioned in end range flexion, which blocks as much rotational movement as possible in the cervical spine below and above C1/C2 and helps to identify dysfunctions in the upper cervical spine. [12, 35] In asymptomatic adults, normal values for ROM during the FRT are reported as 38° (36) and 45° (13) to each side, while range is less than 32° is the positive cutoff value. [16] However, this test has not been evaluated in children. Furthermore, no studies have examined the relationship between ROM of the upper cervical spine and other measures of musculoskeletal function of the cervical spine in children with headache. Specifically, there are no studies that have determined the relationship between cervical posture and ROM of the upper cervical spine. Indeed, there is very little information regarding the presence of impairments of the cervical spine in pediatric headache, in general, or CGH, in particular. Therefore, the aim of this study was to investigate active ROM of the cervical spine, forward head posture identified by the craniovertebral angle (CVA), and the FRT in asymptomatic children and children with purported CGH in order to detect possible differences between groups.
Methods
A cross-sectional study was designed to assess active ROMof the cervical spine, the CVA, and the FRT in 30 children with purported CGH and 34 age-matched asymptomatic children.
Subjects
Due to logistical reasons, asymptomatic subjects were recruited from a high school and handball club in Bremen/Germany, whereas the subjects with purported CGH were recruited from three physiotherapy departments in the Netherlands. One examiner lived in the Netherlands and had contact with three physiotherapy departments that treat children, whereas the second examiner lived in Germany. This approach allowed a more practical recruitment of a higher number of feasible subjects. All children were recruited after consultation and after written informed consent was provided by their parents. All potential subjects had been informed of their right to refuse to participate in the study or to withdraw consent to participate at any time without reprisal. In addition, the rights of the children were protected at all times. Thus, the protocol for this study followed the ethical principles of the Declaration of Helsinki of theWorld Medical Association.
To be included in the asymptomatic group, volunteers were required to be asymptomatic and between the ages 6 and 12 years. Subjects were excluded if they had headache more than once per month, any history of cervical spine surgery, a diagnosis of Down's syndrome or rheumatoid arthritis, and inability to tolerate the FRT.

Table 1 Symptomatic children were interviewed and included in the purported CGH group if they met the inclusion criteria based on the description outlined by Antonaci et al. [1] All children were required to fulfill all five criteria derived from the original diagnostic criteria for CGH proposed by Sjaastad et al. [43], thus indicating “probable” CGH (Table 1). To be included in the symptomatic group, the children had to have unilateral or side-dominant headache without side shift [43], associated neck pain or stiffness [6, 43], headache precipitated by neck movement or postures [42], headache frequency of at least an average of one per week, and history of episodic semicontinuous or continuous headache for at least the previous 3 months. Previous studies [12, 15] have used these criteria and showed differences in FRT ROM values between symptomatic and asymptomatic groups of adults.
Potential subjects with CGH were put forward by the physiotherapy clinics for potential recruitment and the subjects were then interviewed by one of the examiners. In total, 46 children were interviewed and of these, 30 children were found to be suitable for inclusion in the study. Consequently, 16 children did not meet the inclusion criteria and were not assessed.
Instrumentation

Figure 1 The Keno®-cervical measurement instrument (Kuntoväline Oy & David Fitness & Medical Ltd., Helsinki, Finland) was used to measure cardinal plane active cervical ROM during flexion, extension, lateral bending, and rotation. The Keno®- cervical measurement helmet (Figure 1) consists of a plastic frame with two adjustable gravity goniometers, a compass, and two spirit levels attached to the frame. A previous study has found a standard error of measurement (SEM) of at most 4° [10] for a similar measurement device for measuring cervical ROM. Intrarater reliability has been reported as good, with intraclass correlation coefficient's [ICC] of 0.64–0.90 [36], while interrater reliability ICC's range from 0.61 to 0.95. [36]

Figure 2
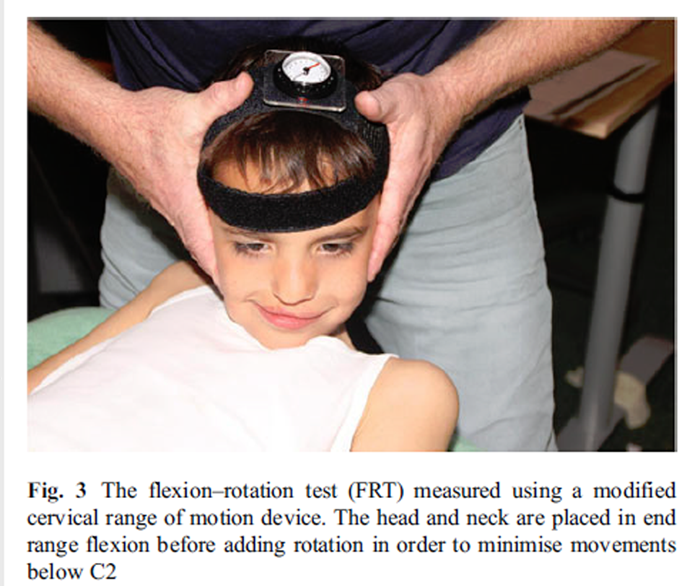
Figure 3 The photometry program designed by the Cranio Facial Therapy Academy (CRAFTA) was used to determine the CVA from a digital photograph (Figure 2). The CVA is the angle formed by a horizontal line drawn through the spinous process of the seventh cervical vertebra (C7) and a line joining the spinous process of C7 vertebra with the tragus of the ear. [38, 46] This measurement has shown to be a reliable indicator for identifying head and neck posture (ICC 0.84) and has a minimal detectable change of 3.6°. [26, 50]
A compass goniometer fixed to the subject's head with elasticated Velcro straps was used to measure ROM during the FRT (Plastimo Airguide, Inc. (compasses), 1110 Lake Cook Road, Buffalo Groove, IL 60089, USA) (Figure 3) according to a previously reported method. [12] This measurement method has been shown to be reliable, even when used by inexperienced examiners. [14] Intrarater reliability is reported as 0.95 (95% CI: 0.90–0.98) [16] and 0.93 (95% CI: 0.87–0.96) [14], while the SEM is at most 1.0° [14]. Range was recorded to the left and right and separately towards the dominant and nondominant headache sides.
Pain responses associated with the FRT were assessed with the colored analog scale (CAS). This scale has a colored triangle on the front with gradations in length and color, which helps children to estimate their pain intensity, whereas the reverse side shows numerical ratings between 0 and 10. The CAS has been found to be an accurate and valid measuring instrument for measuring pain in children 5 years and older. [32]
Procedures
Prior to the main study, an interrater reliability study was conducted. Two examiners, physiotherapists with more than 4 years experience, carried out all tests, one for the asymptomatic group and one for the group with purported CGH. To determine interrater reliability, eight volunteers were tested according to the examination procedure by each examiner. Subjects were examined independently, with each examiner blind to the other's measured values. Subjects were tested 5 min apart.
In the main study, all measurements were assessed in a standardized manner to ensure reproducibility. The CVA was determined first. Before the subject's photograph was taken, the camera was fixed to a tripod set 2 m from the subject. The tripod was equipped with two spirit levels to ensure horizontal alignment of the camera. The photographed image section included the lateral view of the head and shoulder girdle down to the insertion of the deltoid muscle. Each child was barefoot and asked to stand comfortably in a relaxed stance on a 70- cm-long and 30-cm-wide piece of carpet.
Following this, each child was given a practical demonstration of the assessment procedure for all six active ROM tests. They were also given a trial practice run to warrant familiarity with the testing protocol. Each child was instructed to sit with their trunk stationary in an erect posture on a plinth, with the arms relaxed at their sides. If necessary, the movement was corrected by the examiner to ensure movement of the head in only one plane. The child was asked to move their head to the maximum comfortable range. Following each movement, subjects were asked to return to the starting position. Each cardinal plane movement was performed only once.
Subsequently, the FRT was performed while the child was positioned in supine. This procedure was based on the description of Hall and Robinson [12] and Hall et al. [16] Each child lay supine on an examination table with their hands relaxing on their abdomen with the neck passively placed in end-range flexion. In this position, the head was rotated to each side to the maximum comfortable range until the examiner noticed firm resistance or the child requested the movement to be stopped because of pain. In all cases, resistance rather than pain limited the movement. Immediately following the FRT, each child was asked to rate the discomfort felt during the FRT on the CAS.
Data analysis
All data were analyzed using Statistical Package for Social Sciences version IBM SPSS Statistics 19. In all cases, alpha was set at the 0.05 level. Interrater reliability was determined by an average measure intraclass correlation coefficient (ICC). The Shapiro–Wilk's test was used to determine normality of data distribution. Data was analyzed using an unpaired t test or Mann–Whitney U test to compare mean values. An unpaired t test was used for normally distributed data and the Mann–Whitney U test used when this was not the case. Spearman's rank correlation was used to determine the relationship between ROM on the FRT and pain recorded by the CAS as well as ROM on the FRT and the CVA. The purpose of this analysis was to identify any possible relationship between impairment measures in children with purported CGH.
Results

Table 2 Interrater reliability for ROM recorded during the FRT was high with an ICC of 0.93 (95% CI: 0.69–0.99) and moderate to high for the CVAwith an ICC of 0.88 (95% CI: 0.51– 0.97), indicating at least good reliability for these measures. The asymptomatic group consisted of 34 children (26 females; mean age 125.38 months [SD 13.14]), whereas the group with purported CGH consisted of 30 children with a mean duration of symptoms of 20.7 months (19 females; mean age 120.70 months [SD 15.14]). An unpaired t test revealed no significant difference for age between groups (p=0.58). In the symptomatic group, headache was more frequently reported as dominant on the right side (19/30, 63.3%) compared to the left (11/30, 36.7%). Means, standard deviations (SD), ranges in degrees and level of significance of the variables age, CVA, pain intensity, and cervical movements are outlined in Table 2.
The CVA of the asymptomatic children and symptomatic children were 51.26° (SD 4.78) and 47.27° (SD 2.36), respectively. An unpaired t test revealed a significant difference of 3.99° in CVA between groups (p<0.001).Similarly, a Mann–Whitney U test revealed a significant difference between groups for each active cervical ROM (p<0.001).
The asymptomatic subjects had significantly greater ROM, as well as cardinal plane ROM differences, recorded during the FRT to the right and left when compared to the symptomatic children (p<0.001). Mean ranges of rotation to the right (52.97/SD 4.65) and left (52.38/SD5.47) were not significantly different within the asymptomatic group (p= 0.370). However, ranges recorded during the FRT to the right (34.53/SD 8.11) and left (42.63/SD 7.91) differed significantly within the symptomatic group (p<0.01). Furthermore, ROM recorded during the FRT towards the dominant headache side (33.36/SD 6.57) was significantly less than the nondominant headache side (43.80/SD 7.93) (p<0.01).
The asymptomatic children had no significant increase in pain (p=0.378) as a result of performing the FRT. However, this was not the case in the symptomatic group, where subjects showed a significant increase in pain (p<0.001) after applying the FRT. Pain intensity scores are shown in Table 2. The higher pain intensities recorded during the FRT to the right in the symptomatic group may be due to the higher prevalence of right-sided headache in this group (19/30, 63.3% had right-sided headache).
A Spearman's rank correlation was used to determine the relationship between ROM on the FRT and pain recorded by the CAS. This analysis revealed a highly significant negative correlation between the range recorded during the FRT towards the dominant headache side and the post-FRT pain intensity score (r=–0.758, p<0.001) with r2 value of 0.574, indicating that 57.4% of the variance of FRT ROM towards the dominant headache side is explained by variability in the CAS pain score. Generally speaking, the lower the ROM towards the dominant headache side, the higher the post- FRT pain intensity score.
In addition, the relationship was sought between combined left and right ROM recorded during the FRT and the CVA. This analysis revealed a significant positive correlation (r=0.421, p<0.05) with a r2 value of 0.177, indicating that only 17.7% of the variance of combined FRT ROM is predicted by variability in the CVA.
Discussion
The results of this study show significant differences in all variables, despite no difference in age and similarity in distribution of gender. Previous reports indicated a higher prevalence of headache in girls [24, 25, 49], which is reflected in our sample of children with headache who were predominantly female.
Cervical range of motion (ROM) in each cardinal plane was significantly less in the children with purported cervicogenic headache (CGH) compared to those without headache (Table 1). ROM values recorded in the asymptomatic group are comparable with a previous report for children. [3] While no previous studies have reported ROM values for children with CGH, these results are consistent with reports in adult populations. [23, 51, 52] Interestingly, ROM does not appear to be restricted in all directions in adults with headache [23, 51, 52], but the explanation for this is not clear. This study finding of reduced ROM in children with purported CGH supports the current criteria for CGH diagnosis. [20, 44]
In addition to differences in ROM, our study found children with purported CGH had significantly different posture to asymptomatic children as identified by the craniovertebral angle (CVA). Children with purported CGH had a significantly smaller CVA and, therefore, increased forward head posture when compared with asymptomatic children (Table 1). The mean CVA of the asymptomatic group is comparable to a previous report of 55° (SD 9.02) in children whose mean age was 12 years. [39] The difference between groups was 4°, more than the minimal detectable change of 3.6° for this measurement method. [26] This finding is consistent with one previous report in adults with headache [48] and neck pain [26], but in contrast to other reports, which found no difference in posture between people with and without headache. [9, 51] Previously, only one study has investigated the CVA in symptomatic children and those with neck pain and/or headache. [49] In that study, no difference was found in CVA between 52 adolescents with pain and 75 adolescents without pain. Taken as a whole, itwould appear that postural change in subjects with purported CGH remains equivocal and further research is required in this area.
To our knowledge, this is the first study investigating the flexion–rotation test (FRT) in children with purported CGH. The results revealed three interesting aspects for discussion. Firstly, the mean range recorded during the FRT in the asymptomatic group was approximately 8° more than that reported for asymptomatic adults. [12] Secondly, the symptomatic group had significantly less range when compared to the asymptomatic group. The difference in mean range recorded towards the dominant headache side and the range in asymptomatic children was 19°. Lastly, ranges recorded to the right and left were dissimilar in range in children with headache, with approximately 8° difference between sides. One explanation for this could be that of the 30 children with headache, 19 children reported right-side dominant symptoms, while only 11 reported the left side as dominant. Data for ROM towards the dominant and nondominant headache sides was very similar to range to the left and the right. The mean difference of 19°, between children with and without headache, further highlights the usefulness of the FRT in CGH diagnosis. However, it is important to recognize that previous reports of a positive cutoff point of 32–33° reported for the FRT in adults [17, 35] should not be used in children because of their greater mobility. Further studies are required to identify the positive cutoff value in children.
It is unclear as to why cardinal plane movement as well as movement during the FRT is altered in children with purported CGH. It is clear that degeneration of the cervical spine is not a factor in this age group. An alternative explanation may be the presence of altered muscle activation in the cervical spine associatedwith CGH[26, 51] A recent study [19] found massage of the cervical muscles immediately improved range of motion recorded during the FRT in adults. Similarly, a Mulligan mobilization with movement technique also gained immediate range recorded by the FRT. [13] Interestingly, we found a strong negative correlation between range recorded towards the dominant headache side and the pain intensity scores recorded after the FRT (r=–0.758, p<0.001). In adults, the presence of headache pain at the time of testing and the presence of subclinical pain significantly influences the range recorded during the FRT. [16, 45] Hence, pain and associated muscle activity may be important limiting factors influencing upper cervical mobility and the FRT.
In addition to the correlation between the ROM recorded during the FRTand pain intensity scores, we found amoderate positive correlation between the ROM recorded during the FRT and the CVA (r=0.421, p<0.05). This indicates that a relatively small proportion of the FRT ROM could be explained by the CVA. One explanation could be the starting position of the FRT. In contrast to increased forward head posture where the upper cervical segments are positioned in extension, the FRT puts the upper cervical spine into full flexion (13). Consequently, altered head posture and reduced ROMof the upper cervical spine do not appear to be related in children with purported CGH. This finding is consistent with that of adults [37], which found ROM recorded during the FRT was only weakly associated with forward head posture. To our knowledge, this is the first study to investigate pain provocation during the FRT. Pain levels after the FRT in the asymptomatic group were very low with a maximum of 2/10 on the CAS. In contrast, following the FRT, pain levels were much higher in the children with headache. This difference may be explained by chronically altered tissue sensitivity in the children with headache who had a mean history of headache for 20.7 months.
We acknowledge a number of limitations of this study. Firstly, a different examiner was used to examine each group. This was done for logistical reasons with children with headache all recruited from physiotherapy practices in the Netherlands, while asymptomatic children were recruited from Germany. This meant that examiners were not blind to the subject's group allocation, but they were trained in the measurement methods. Previously, it has been reported that when using the FRT, even inexperienced examiners have good reliability when compared with experienced examiners. [14] Secondly, the majority of the asymptomatic children were recruited from a sports club. Each child has a different pain perception depending on the personality, learning, expectations, and previous pain experiences. [30, 31] Consequently, active children who play sport may have different range of motion, posture, and responses to testing than less active children.
Conclusion
This study found evidence of impaired function of the cervical spine in children with purported CGH. When compared with an asymptomatic group of children, those with headache had significantly reduced active ROM in all directions, significantly less range recorded during the FRT, significantly higher pain scores following the FRT, and significantly greater forward head posture. This information may be useful to clinicians in the identification of children with suspected CGH. Decreased ROM and pain provocation during the FRT appears to have potential diagnostic value. This study sets the groundwork for future studies investigating headache in children. Future studies should investigate the diagnostic value of these tests in the identification of CGH from other headache forms such as migraine or tension-type headache. In addition, impairments of the cervical spine as a contributing factor to different pediatric headache forms needs to be clarified in more detail.
Conflicts of interest
None.
Glossary
CAS = Colored analog scale
CGH = Cervicogenic headache
CI = Confidence interval
CVA = Craniovertebral angle
FRT = Flexion rotation test
ICC = Intraclass correlation coefficient
ROM = Range of motion
SD = Standard deviation
References:
Antonaci F, Ghirmai S, Bono G, Sandrini G, Nappi G (2001)
Cervicogenic headache: evaluation of the original diagnostic criteria.
Cephalalgia 21:573–583Aromaa M, Rautava P, Helenius H, Sillanpaa ML (1997)
Factors of early life as predictors of headache in children at school entry.
Headache 38:23–30Arbogast KB, Gholve PA, Friedman JE, Maltese MR, Tomasello MF, Dormans JP (2007)
Normal cervical spine range of motion in children 3–12 years old.
Spine 32:E309–E315Bandell-Hoekstra IE, Abu-Saad HH, Passchier J, Frederiks CM, Feron FJ, Knipschild P (2001)
Prevalence and characteristics of headache in Dutch schoolchildren.
Eur J Pain 5:145–153Bandell-Hoekstra IE, Abu-Saad HH, Passchier J, Frederiks CM, Feron FJ, Knipchild P (2002)
Coping and quality of life in relation to headache in Dutch schoolchildren.
Eur J Pain 6:315–321Bogduk N (1994)
Cervical causes of headache and dizziness. In: Boyling JD, Palastanga N (eds)
Grieve’s modern manual therapy,
2nd edn. Churchill Livingstone, EdinburghBogduk, N and Govind, J.
Cervicogenic Headache: An Assessment of the Evidence on Clinical Diagnosis,
Invasive Tests, and Treatment
Lancet Neurol. 2009 (Oct); 8 (10): 959–968Brattberg G (2004)
Do pain problems in young school children persists into early adulthood? A 13-year follow-up.
Eur J Pain 8:187–199Dumas J, Arsenault A, Boudreau G, Magnoux E, Lepage Y, Bellavance A, Loisel P (2001)
Physical impairments in cervicogenic headache—traumatic vs. nontraumatic onset.
Cephalalgia 21:884–893Fletcher JP, Bandy WD (2008)
Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain.
J Orthop Sports Phys Ther 38(10):640–645Geldhof E, Clerk D, Bourdeaudhuij I, Cardon G (2007)
Classroom postures of 8–12 year old children.
Ergonomics 50(10):1571–1581Hall T, Robinson K (2004)
The flexion–rotation test and active cervical mobility—a comparative measurement study in cervicogenic headache.
Man Ther 9:197–202Hall T, Chan HT, Christensen L, Odenthal B, Wells C, Robinson K (2007)
Efficacy of a C1–C2 self-sustained natural apophyseal glide (SNAG) in the management of cervicogenic headache.
J Orthop Sports Phys Ther 37(3):100–107Hall TM, Robinson KW, Fujinawa O, Akasaka K, Pyne EA.
Intertester Reliability and Diagnostic Validity of the Cervical Flexion-Rotation Test
J Manipulative Physiol Ther 2008 (May); 31 (4): 293–300Hall T, Briffa K, Hopper D (2010)
The influence of lower cervical joint pain on range of motion and interpretation of the flexion–rotation test.
J Man Manip Ther 18(3):126–202Hall T, Briffa K, Hopper D, Robinson K (2010)
The relationship between cervicogenic headache and impairment determined by the flexion–rotation test.
J Manipulative Physiol Ther 33(9):666–671Hall T, Briffa K, Hopper D, Robinson K (2010)
Long-term stability and minimal detectable change of the cervical flexion–rotation test.
J Orthop Sports Phys Ther 40(4):225–229Hernandez-Latorre MA, Roig M (2000)
Natural history of migraine in childhood.
Cephalalgia 20:573–579Hopper D, Bajaj Y, Choi CK, Jan H, Hall T, Robinson K, Briffa K (2012)
A pilot study to investigate the short-term effects of specific soft tissue massage on upper cervical movement impairment in patients with cervicogenic headache.
J Man Manip Ther (accepted for publication).Society IH (2004)
The international classification of headache disorders. 2nd edition.
Cephalalgia 24(1):9–160Jull G, Barrett C, Magee R, Ho P (1999)
Further Clinical Clarification of the Muscle Dysfunction in Cervical Headache
Cephalalgia 1999 (Apr); 19 (3): 179–185Jull G, Niere KR (2004)
The cervical spine and headache.
In: Boyling JD (ed) Grieve’s modern manual therapy, chapter 21.
Churchill Livingstone, Edinburgh, pp 291–309Jull G, AmiriM, Bullock-Saxton J, Darnell R, Lander C (2007)
Cervical musculoskeletal impairment in frequent intermittent headache. Part 1:
subjects with single headaches.
Cephalalgia 27(7):793–802Knackstedt H, Bansevicius D, Aaseth K, Grande RB, Lundqvist C, RussellMB (2010)
Cervicogenic Headache in the General Population:
The Akershus Study of Chronic Headache
Cephalalgia. 2010 (Dec); 30 (12): 1468–1476Kröner-Herwig B, Heinrich M, Morris L (2007)
Headache in German children and adolescents: a population-based epidemiological study.
Cephalalgia 27:519–527Lau HC, Chiu TW, Lam TH (2010)
Measurement of craniovertebral angle with electronic head posture instrument: criterion validity.
J Rehabil Res Dev 47(9):911–918Laurell K, Larsson B, Eeg-Olofsson O (2003)
Headache in schoolchildren: agreement between different sources of information.
Cephalalgia 23:420–428Laurell K, Larsson B, Eeg-Olofsson (2005)
Headache in schoolchildren: association with other pain, family history and psychosocial factors.
Pain 119:150–158Lynch-Caris T, Majeske KD, Brelin-Fornari J, Nashi S (2008)
Establishing reference values for cervical spine range of motion in pre-pubescent children.
J Biomech 41:2714–2719Mathews L (2011)
Pain in children: neglected, unaddressed and mismanaged.
Indian Journal of Palliative Care 17(4):70–73McGrath PA (1990)
Pain in children: nature, assessment and treatment.
Guilford, New YorkMcGrath PA, Seifert C, Speechley K, Booth J, Stitt L, Gibson M (1996)
A new analogue scale for assessing children's pain. An initial validation study
Pain 64:435–443McGrath PA, Hillier LM (2001)
Recurrent headache: triggers, causes and contributing factors.
In: McGrath, Hillier LM (eds)
The child with headache—diagnosis and treatment.
International Association for the Study of Pain, SeattleMurphy S, Buckle P, Stubbs D (2004)
Classroom posture and selfreported back and neck pain in schoolchildren.
Appl Ergon 35:113–120Ogince M, Hall T, Robinson K, Blackmore AM (2007)
The diagnostic validity of cervical flexion–rotation test in C1/C2-related cervicogenic headache.
Man Ther 12:256–262Poelsson A, Hedlund R, Ertzgaard S, Öberg B (2000)
Intra- and intertester reliability and range of motion of the neck.
Physiother Can 52:233–242Quek J, Pua YH, Clark R, Bryant AL (2013)
Effects of thoracic kyphosis and forward head posture on cervical range of motion in older adults.
Man Ther 18(1):65–71Raine S, Twomey L (1997)
Head and shoulder posture variations in 160 asymptomatic women and men.
Arch Phys Med Rehabil 78:1215–1223Ramprasad M, Alias J, Raghuveer AK (2010)
Effect of Backpack Weight on Postural Angles in Preadolescent Children
Indian Pediatr. 2010 (Jul 7); 47 (7): 575–580Seshia SS, Wolstein JR, Adams C, Booth FA, Reggin JD (1994)
International headache society criteria an childhood headache.
Dev Med Child Neurol 36(5):419–428Sillanpa M, Abu-Arafeh I (2002)
Epidemiology of recurrent headache in children.
In: Abu Arafeh I (ed) Childhood headache.
MacKeith, LondonSjaastad O, Fredriksen TA, Sandt ST, Antonaci F (1989)
The localization of the initial pain of attack: a comparison between classic migraine and cervicogenic headache.
Funct Neurol 6:93–100Sjaastad O, Fredriksen TA, Pfaffenrath V (1990)
Cervicogenic headache: diagnostic criteria.
Headache 30:725–726Sjaastad O, Frederiksen T, Pfaffenrath V (1998)
Cervicogenic headache—diagnostic criteria.
Headache 38:442–445Smith K, Hall T, Robinson K (2007)
The influence of age, gender, lifestyle factors and sub-clinical neck pain on cervical range of motion.
Man Ther 13:552–559Visscher CM, De Boer W, Lobbezoo F, Habets L, Naeije M (2002)
Is there a relationship between head posture and craniomandibular pain?
J Oral Rehabil 29:1030–1036Von Piekartz H, Schouten S, Aufdemkampe G (2007)
Neurodynamic responses in children with migraine or cervicogenic headache versus a control group—a comparative study.
Man Ther 12:153–160Watson DH, Trott PH (1993)
Cervical headache. An investigation of natural head posture and upper cervical flexor muscle performance.
Cephalalgia 13:272–284Weber Hellstenius SA (2009)
Recurrent Neck Pain and Headaches in Preadolescents Associated
with Mechanical Dysfunction of the Cervical Spine:
A Cross-Sectional Observational Study
with 131 Students
J Manipulative Physiol Ther 2009 (Oct); 32 (8): 625—634Yip T, Tai Wing Chui T, Tung Kuen Poon T (2008)
The Relationship Between Head Posture and Severity
and Disability of Patients With Neck Pain
Manual Therapy 2008 (May); 13 (2): 148—154Zito G, Jull G, Story I (2006)
Clinical Test of Musculoskeletal Dysfunction in the Diagnosis of Cervicogenic Headache
Manual Therapy 2006 (May); 11 (2): 91–166Zwart JA (1997)
Neck mobility in different headache disorders.
Headache 37:6–11
Return to HEADACHE
Return to PEDIATRICS
Return to FORWARD HEAD POSTURE
Since 8-26-2016


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |