Measuring Musculoskeletal Pain in Infants, Children, and Adolescents
Abstract
Synopsis
Accurate, reliable, and timely assessment of pain is critical for effective management of musculoskeletal pain conditions. The assessment of pain in infants, children, and adolescents with and without cognitive impairment can be particularly challenging to clinicians for a number of reasons, including factors related to the consultation (eg, heterogeneous patient population, time constraints), the clinician (eg, awareness/knowledge of available pain scales), standardized assessment scales (eg, availability, psychometric properties, and application of each scale), the patient (eg, developmental stage, ability to communicate), and the context in which the interaction took place (eg, familiarity with the setting and physiological and psychological state). As a result, pain is frequently not assessed or measured during the consultation and, in many instances, underestimated and undertreated in this population. The purpose of this article is to provide clinicians with an overview of scales that may be used to measure pain in infants, children, and adolescents. Specifically, the paper reviews the various approaches to measure pain intensity; identifies factors that can influence the pain experience, expression, and assessment in infants, children, and adolescents; provides age-appropriate suggestions for measuring pain intensity in patients with and without cognitive impairment; and identifies ways to assess the impact of pain using multidimensional pain scales. J Orthop Sports Phys Ther 2017;47(10):712–730. doi:10.2519/jospt.2017.7469
Musculoskeletal pain is common in children and adolescents. As many as half of all children and adolescents report experiencing musculoskeletal pain at least once a month, and as many as a third report persistent or recurrent musculoskeletal pain.70,75 Musculoskeletal pain is known to have a substantial impact on the everyday life of children and adolescents. For example, in those who report musculoskeletal pain, approximately 40% report that it interferes with daily activities and sports participation, 20% report missing school/work, 20% to 30% take medication, and more than half seek health care, all of which are associated with significant health care costs, especially for those who experience persistent pain.52,58,100,109,110 Critical to the effective management of musculoskeletal pain by clinicians is accurate, reliable, and timely assessment, which is a pivotal component of evidence-based medicine. Specifically, the valid and reliable measurement of pain is helpful in understanding a person's pain experience, identifying appropriate treatment options, and monitoring change in a person's pain condition, minimizing potential adverse physiological and psychological consequences of unrelieved or inadequately managed pain.3,18,34,93,107,111,146
Fundamental differences exist between the expressions of the pain experience in infants, children, adolescents, and adults, which highlights the need to assess and interpret pain in a way that is specific to each age group. For example, while the definition of pain is universal, “A distressing experience associated with actual or potential tissue damage with sensory, emotional, cognitive and social components,”159 the way in which these components interact with environmental, developmental, sociocultural, and contextual factors suggests that the way in which infants, children, adolescents, and adults conceptualize, understand, and communicate pain is distinctly different.34,35,56,70 For example, the vocabulary by which infants identify pain emerges at around 18 months (eg, “ouch,” “ow,” “hurt”) and continues to develop until they are around 5 years of age, when it can reliably be used. Similarly, this is the time at which a child begins to develop an understanding of the causes and consequences of pain and the ability to control its expression.28,56,127 In the absence of intellectual or cognitive deficit, a child's age may serve as a reasonable and easily measured indicator of level of development, and should be used to guide the way in which pain is measured in children and adolescents.56,127,151
The assessment and measurement of pain in infants, children, and adolescents can be a challenge to clinicians.55,140 Reasons for this include factors related to the consultation (eg, heterogeneous patient population, time constraints), the clinician (eg, awareness/knowledge of available pain scales), standardized assessment scales (eg, availability, psychometric properties, and application of each scale), the patient (eg, developmental stage, ability to communicate), and the context in which the interaction took place (eg, familiarity with the setting and physiological and psychological state). As a result, pain is frequently not formally assessed or measured during the consultation, with more informal questioning used (eg, questions such as “Are you ok?” or “Feeling better?”) and the presence of pain validated through observation of behavioral cues, such as crying or grimacing.103,128,146 Even in populations who are at a higher risk of experiencing musculoskeletal pain (eg, children with cerebral palsy), there are data to suggest that pain is assessed using validated tools in less than 10% of encounters.99 The inconsistency in assessment, measurement, and documentation of pain means that, in many instances, pain may be underestimated and undertreated in this population.146 Reports of hospital audit data suggest that a third of children experience moderate to severe pain during their hospital admission, and documentation of pain assessment varies between 12% and 100% of the time across hospital settings and between clinicians. All too frequently, the assessment, measurement, and documentation of pain do not meet hospital or professional guidelines.99,103,111 Encouragingly, clinical practice has been responsive to knowledge translation and implementation strategies aimed at improving the assessment, measurement, and documentation of pain in children and adolescents.53,76 Further work is needed to understand the frequency at which the assessment and measurement of pain are conducted in other health care settings, such as in primary care and community facilities.
Pain is recognized as a core outcome domain by a number of national and international initiatives, such as the Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials Consensus Group, the Society for Pediatric Pain Medicine Assessment Task Force, and the National Institutes of Health Toolbox.32,57,93 These initiatives promote the use of evidence-based measures of pain intensity and impact in clinical practice and research. Recommendations are the results of formal collaborative processes and methodologies that combine empirical evidence, expert opinion, and clinical utility. The purpose of this paper was to provide clinicians with an overview of scales that can be used to measure musculoskeletal pain in infants, children, and adolescents in a way that is quick, accurate, and reliable. However, few pain scales have specifically been evaluated for this purpose, and suggestions are based on scales that have been evaluated to measure procedural (eg, immunization pain) and nonspecific (eg, musculoskeletal pain) pain, with emphasis on clinical utility. Using the social communication model of pain as a framework,35,55 the present article specifically considers factors that may influence the pain experience and expression, reviews the various approaches that can be used to assess and measure pain, provides age-appropriate suggestions for measuring pain intensity in patients with and without cognitive impairment, and identifies ways to assess the impact of pain using multidimensional pain scales. The article incorporates and extends the work of the previous collaborations outlined above, by identifying and integrating evidence from more recent publications into the measurement of pain in infants, children, and adolescents. Scales designed for use in the intensive care setting (eg, to assess pain in the intubated patient) or solely to assess postoperative pain are not reported in this paper, unless specifically stated. Also, the authors acknowledge that there are aspects of pain beyond pain intensity, such as affective (emotional, unpleasantness) and evaluative (cognitive processes [eg, appraisal of pain]) dimensions, that are not addressed comprehensively in this article.
The Social Communication Model of Pain
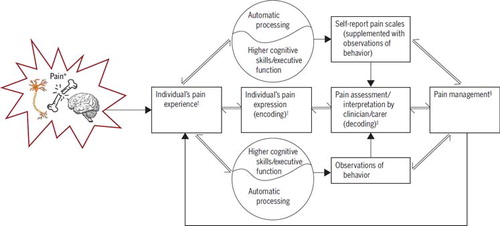
The social communication model of pain (FIGURE 1) is a theoretical model that can be used as a framework from which to examine, understand, and approach the assessment and management of pain in individuals of all ages and for those with cognitive impairments.35,55 This comprehensive model highlights a number of factors (biological, psychological, and social) related to the individual in pain and the treating clinician that influence how pain is experienced, expressed, and interpreted, and the effectiveness of this communication. The model also considers the social context (interpersonal context) in which the communication of pain occurs and, importantly, recognizes how the communication of pain may differ, whether initiated by the person in pain or elicited by an observer's questioning at presentation or after treatment, when a socially desirable response may be provided. This model places assessment and measurement at the heart of understanding a person's pain experience and highlights the importance of how that information is obtained. The remainder of this paper will consider the measurement and assessment of pain in children and adolescents using the social communication model as a guide.
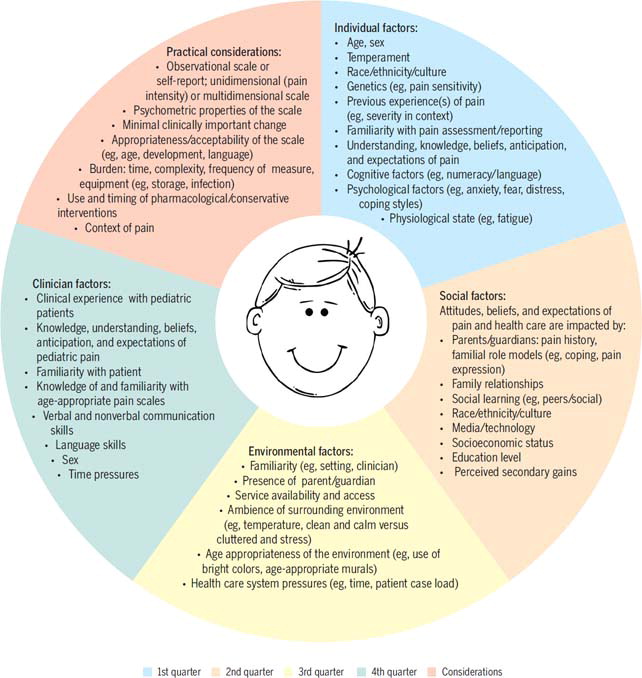
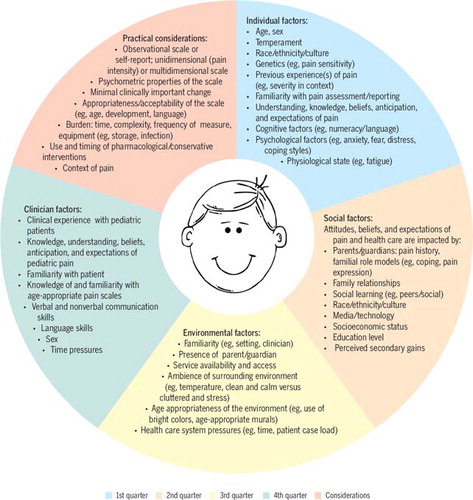
As suggested by the social communication of pain model, a number of factors related to the individual in pain, clinician, and context can influence the experience and communication (expression, interpretation) and management of pain. In FIGURE 2, we outline some of the key factors that are relevant to the assessment and measurement of pain in children, for example, the cognitive ability of the child to understand concepts such as pain severity or intensity, the burden that a child may feel when questioned by a clinician, and the potential influences of parents who are present within the consultation.
Approaches to Measuring Pain Intensity in Children
The 3 main approaches to measuring pain intensity in children are physiological (how the child's body reacts), observations of behavior (how the child reacts), and self-report (what the child says). It is important to note that the choice of approach will depend on the age and abilities of the child, and that the different approaches are not interchangeable and typically only correlate poorly to moderately with each other.49,157 Ideally, information from each approach may be used simultaneously to provide a detailed understanding of the pain experience, with consideration of both automatic processing and higher cognitive function (FIGURE 1).35,49,55,157
Physiological indicators (eg, increased heart rate, blood pressure, sweating) are associated with generalized (nonspecific) stress reaction and more strongly associated with distress and anxiety than self-report pain measures.22 For this reason, physiological indicators should not be used in isolation to estimate presence, quality, or intensity of pain. Further, these indicators habituate over time and are therefore not appropriate for use in acute pain that is continuous or in those with chronic pain.62
Observational measures involve observing an individual's nonverbal behavior (eg, crying, facial expression, torso and limb movements) and interactions (eg, social, appetite). The behavioral response to pain is recognized to be more of an automatic and reflexive response to actual or potential tissue damage. Parents and carers can often provide specific and helpful information about typical and idiosyncratic pain-related behaviors that reflect different quality or intensity of pain in their child. This information can then be used to inform the selection of appropriate pain management or prevention strategies.35,55,153 As cognitive skills and function increase with age, along with the ability to control (eg, suppress, exaggerate, or feign) behavior, observational measures should be used when possible to complement self-report measures of pain (FIGURE 1). Observational measures are particularly useful for assessing pain in children aged less than 4 years, who do not have the language skills necessary to communicate pain or lack the comprehension necessary for self-report measures; patients with cognitive or communication impairments (eg, cerebral palsy); and situations in which valid self-report is not possible (eg, extreme distress) or the credibility of the self-report is in doubt.55
The most direct and reliable approach to measuring pain in those who are able to communicate their experience is self-report.129,140 The ability of a child to understand and report the presence and intensity of pain requires cognitive skills, including receptive language and understanding, knowledge and memory of pain, executive function (eg, cognitive flexibility, working memory), and the ability to understand and estimate magnitudes and symbolic processing.28,66,151,152 These skills begin to emerge as early as 3 years of age and gradually develop to enable the accurate and reliable self-reporting of pain intensity by children aged 5 years (on average) or older. While screening tasks (eg, counting, comprehension, and seriation) are available, these are time consuming and do not predict a child's ability to accurately and reliably self-report pain beyond age alone.28,155 The association between a child's age and cognitive skills highlights the need to measure pain using different pain scales in children of different ages, such as a more simplified scale (with fewer response options) for younger children.28,152
Proxy Report While input from parents/guardians has a place in the assessment of pain in children, clinicians should be mindful of overreliance on this information. Numerous studies have shown discrepancies between reports of pain from parents and children. Studies in the general population of healthy children show that parents typically underreport pain in their children.30,69,79 In contrast, studies in children with painful health conditions typically report better concordance, although with a tendency for parents to overestimate pain severity, compared to the child's report.33,149
Age-Appropriate Scales
Single-item scales of pain intensity are most commonly used to measure pain because they are fast, easy to administer, and closely correlated with the impact of pain on the individual (eg, activity limitations, health care seeking, medication use).141 Reported in TABLE 1 are operational definitions of the psychometric properties considered in this paper. TABLES 2 through 4 outline available pain intensity scales, and TABLE 5 details multidimensional pain scales, with each table including a general description of the scale, age range, psychometric properties, and practical considerations for use. These tables synthesize evidence from several systematic reviews,23,32,37,38,43,62,77,83,129,142,153 practice guidelines,111 and peer-reviewed articles. The evidence outlined in TABLES 2 through 4 was used to identify an appropriate pain-intensity scale for each age group, with the suggested scales summarized in TABLE 6. The scales that have been suggested for use are based on the authors' judgments, along with consideration of the psychometric properties of the scale, type of pain (eg, procedural versus nonspecific pain), population, and context in which the scale has been evaluated.
| Term | Operational Definition |
|---|---|
| Reliability | The reproducibility of a measure over different occasions, concerned with minimizing sources of random error so that measures are reproducible. In general, acceptable reliability coefficients for research and clinical purposes are 0.7 or greater and 0.9 or greater, respectively134 |
| Test-retest | The agreement between observations with the same individuals on at least 2 occasions134 |
| Interrater | The agreement between different raters/observers of an observational measure of pain134 |
| Validity | Used to assess whether the scale measures what it intends to measure134 |
| Face | Whether the pain scale includes appropriate items that appear to measure what they are proposing to measure134 |
| Content | The assessment of whether the items in the pain measure include the appropriate information and content134 |
| Criterion | Includes concurrent validity and predictive validity. In concurrent validity, a new pain measure is correlated with a gold standard measure, which is administered at the same time. In general, correlations between the new measure and the gold standard should be at least r ≥0.3–0.5. The magnitudes of the coefficients are hypothesis dependent but should not be so high as to make the new measure redundant. In predictive validity, the correlation of the measure to the criterion variable is determined later134 |
| Construct | Determines the validity of abstract variables that cannot be directly observed, such as pain. These constructs are assessed by their relationships with other variables134 |
| Convergent | Evaluates how well items on a pain scale correlate with other measures of the same construct or related variables. In general, correlations between the measure and another measure of the same construct should be r ≥0.3–0.5; however, the magnitudes of the coefficients are hypothesis dependent134 |
| Discriminant | Evaluates how items on a pain scale correlate with other measures that are unrelated. In general, correlations between the measure and another unrelated measure should be r <0.3; however, the magnitudes of the coefficients are hypothesis dependent134 |
| Responsiveness | Measures whether the measure is able to identify changes in pain over time when change is expected (eg, after analgesia) (COSMIN taxonomy) |
| Interpretability | The meaningfulness of the scores obtained from a pain measure |
| Feasibility | How easily a pain measure can be scored and interpreted |
| Minimal clinically important change | The smallest difference in score in the domain of interest that patients perceive as beneficial and that would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient's management68 |
TABLE 2
Observational Scales Used to Measure Pain in Infants and Children 3 Years or Younger
| Pain Scale | Description | Age Range | Evidence Available | Strengths | Limitations |
|---|---|---|---|---|---|
| Face, Legs, Activity, Cry and Consolability scale5,37,48,93,94,136 | 5-item scale measures facial expression, leg movements, activity, cry, and consolability. Each item is scored from 0 to 2. Total score range, 0–10 Originally validated for postoperative pain in children aged 2 mo to 7 y | IAR: 2 mo to 7 y SAR: 2 mo to 16 y | Reliability: intrarater, interrater Validity: convergent, discriminant, criterion Responsiveness Practical considerations: feasibility, user preference |
| |
| Child Facial Coding System17,32,54 | Frequency and intensity of 13 facial actions; scored from 0 (no action) to 2 (distinct action [eg, squinting, brow lowering, nostril flare, mouth stretching]) | IAR: 2–5 y SAR: 3–7 y | Reliability: interrater Validity: criterion Practical considerations: none |
|
|
| Children's Hospital of Eastern Ontario Pain Scale36,37,41 | 6-item scale (cry, facial expression, verbalization, touching, torso and leg movements) rated on a 4-point scale. Total score range, 4–13 | IAR: 6 mo to 6 y SAR: 6 mo to 12 y | Reliability: none Validity: convergent Practical considerations: none |
|
|
| Modified Behavioral Pain Scale136,137 | 3-item scale measures facial expression, cry, and movements. Facial expression and movement scored on a 0-to-3 scale and cry on a 0-to-4 scale. Total score range, 0–10 | IAR: 4–6 mo | Reliability: intrarater, interrater Validity: convergent, criterion Responsiveness Practical considerations: feasibility, user preference |
|
|
| Neonatal Infant Pain Scale41,84,136 | 6-item scale (facial expression, breathing patterns, cry, arm movement, arousal, leg movement). All items scored from 0 (absent/relaxed) to 1 (change from normal), except cry (0–2) | IAR: 0–2 mo | Reliability: interrater Validity: convergent, content, criterion Responsiveness Practical considerations: feasibility, training |
|
|
| Pain Scale | Description | Age Range | Evidence Available | Strengths | Limitations |
|---|---|---|---|---|---|
| Pieces of Hurt Tool (Hester's poker-chip tool)47,50,60,133,139 | The child is asked, “Did it hurt?” If the child responds yes, then he or she is given 4 chips (“pieces of hurt”). The child is told, “These are pieces of hurt: 1 chip is a little bit of hurt, and 4 chips are the most hurt you could ever have. Do you have 1, 2, 3, or 4 pieces of hurt?” The number of chips is recorded | IAR: 4–7 y SAR: 3–18 y | Reliability: intrarater, interrater Validity: convergent, discriminant Responsiveness Practical considerations: acceptable to carers |
|
|
| Faces Pain Scale-Revised11,24,61,95,96,106,115–117,129,142–145 | 6 line-drawn faces aligned horizontally from an expression of “no pain” (left) to “most pain possible” (right). The child points to the face that shows his or her pain. Standardized instructions are used The original Faces Pain Scale had 7 faces scored on a 0-to-6 scale. It was revised to be compatible with other 0-to-10 scales | IAR: 4–12 y SAR: 3–18 y | Reliability: intrarater Validity: convergent, discriminant, content, criterion Responsiveness Practical considerations: feasibility, interpretability, acceptable to carers |
| |
| Verbal NRS-116,23,24,39,95,113,115–117,154 | The child is asked, “On a scale of 0 to 10, where 0 is no pain and 10 is the worst possible pain, tell me what number best represents your pain.” The individual responds with a number that reflects his or her pain | IAR: 8–18 y SAR: 6–18 y | Reliability: intrarater Validity: convergent, discriminant, content, criterion Responsiveness Practical considerations: interpretability, acceptable to carers |
|
|
| Color Analog Scale20,21,24,91,92,113,115–117,143–145 | VAS with a mechanical device: a plastic slider over a 143-mm-long tetragon, varying from narrow (10 mm) and white (labeled “no pain”) to wide (30 mm) and dark red at the end (labeled “most pain”). Range, 0–10 | IAR: 5–17 y SAR: 3–18 y | Reliability: intrarater Validity: convergent, discriminant, content, criterion Responsiveness Practical considerations: feasibility, interpretability, user preference, acceptable to carers |
| |
| OUCHER (NRS and photographic scale)9,10,87,142 | 0-to-10 NRS aligned vertically next to 6 photographs ranging from “no hurt at all” at the bottom (0) to the “biggest hurt you could ever have” at the top (10). (Prior to 2009, scoring was from 0 to 100.) | IAR: 3–7 y SAR: 3–18 y | Reliability: intrarater, interrater Validity: convergent, discriminant, content, criterion Responsiveness Practical considerations: interpretability, acceptable to carers |
|
|
| VAS6,8,24,87,116,117,121,122,160 | 10-cm vertical/horizontal line with anchors (eg, “no pain,” “worst possible pain”). The child marks along the line to indicate the intensity of the pain | IAR: 2–17 y SAR: 3–18 y | Reliability: intrarater, interrater Validity: convergent, discriminant, content, criterion Responsiveness Practical considerations: interpretability, acceptable to carers |
| |
| Wong-Baker FACES scale26,32,47,74,87,129,142,160 | 6 line-drawn faces aligned horizontally, from a smiling “no hurt” face (left) to a crying “hurts worst” face (right). Range, 0–5 (or 0–10 if each face is 2 points) | IAR: 3–18 y SAR: 9 mo to 18 y | Reliability: intrarater, interrater Validity: convergent, discriminant, criterion Responsiveness Practical considerations: feasibility, interpretability, user preference, acceptable to carers |
| |
| Word descriptor scales/word graphic scale47,74,138,160 | 5 to 6 words to describe pain, from “no pain” to “worst pain.” A word is selected to best describe the pain Each word has a number for scoring. Range is from 0 (no pain) to 5 (worst pain) | IAR: 8–17 y SAR: 3–18 y | Reliability: intrarater Validity: convergent, discriminant, criterion Practical considerations: acceptable to carers |
|
TABLE 4
Scales Used to Measure Pain in Children and Adolescents With a Cognitive Impairment
| Pain Scale | Description | Age Range, y | Evidence Available | Strengths | Limitations |
|---|---|---|---|---|---|
| Revised FLACC scale29,38,45,88,104–106,150 | 5-item scale measures facial expression, leg movements, activity, cry, and consolability. Each item is scored from 0 to 2 (total range, 0–10). Needs input from parent/guardian to identify “baseline” behaviors Includes open-ended descriptor for individual pain behaviors | IAR: 4–19 SAR: 3–18 | Reliability: intrarater, interrater Validity: convergent, discriminant, criterion Responsiveness Practical considerations: feasibility, interpretability, user preference, acceptable to carers |
|
|
| Individualized numeric rating scale38,106,124,125 | Carers provide word descriptors to be used as anchors for their child's pain behaviors from 0 (no pain) to 10 (worst possible pain) | IAR: 6–18 | Reliability: interrater Validity: convergent Responsiveness Practical considerations: acceptable to carers |
|
|
| Non-communicating Children's Pain Checklist-Revised13–16,38,90 | 30 items (6 subscales: vocal, social, facial, activity, body and limb, physiological). Frequency of each behavior from 0 (not at all) to 3 (very). Range, 0–90 Postoperative version: 27 items (total range, 0–81); does not include eat/sleep items | IAR: 3–18 | Reliability: intrarater Validity: criterion Responsiveness Practical considerations: interpretability |
|
|
| Pediatric Pain Profile29,38,45,63–65,104,106 | 20 items rated on 4-point Likert scales (0, not at all to 3, a great deal). Used by an observer familiar with the child. Observer completes the scale to establish baseline on a “good day,” which is then used as a benchmark for ongoing ratings | IAR: 1–18 | Reliability: intrarater, interrater Validity: convergent, criterion Responsiveness Practical considerations: feasibility, interpretability, training, user preference, acceptable to carers |
|
|
TABLE 5
Multidimensional Pain Scales That Measure the Impact of Pain in Infants, Children, and Adolescents
| Pain Scale | Description | Age Range, y | Evidence Available | Strengths | Limitations |
|---|---|---|---|---|---|
| Adolescent Pediatric Pain Tool46,67,118,119 | Pain intensity measured on 0-to-100-mm VAS, body chart, and 67 word descriptors to express sensory (37 words), evaluative (8 words), affective (11 words), and temporal (11 words) qualities 5 pain subscale scores: number of pain sites (from body chart), pain-intensity score, number of temporal descriptors (percent), total pain quality, and temporal descriptors | IAR: 8–18 SAR: 8–18 | Reliability: none Validity: none Responsiveness Practical considerations: none |
|
|
| Bath Adolescent Pain Questionnaire42,44,156 | 61 items in 7 domains: social functioning, physical functioning, depression, general anxiety, pain-specific anxiety, family functioning, and development. Each item rated on 5-point scale (0 is never, 4 is always), except the development subscale, which is rated from 0 (“very behind”) to 4 (“very ahead”). Range, 0–244 | IAR: 11–18 SAR: 10–18 | Reliability: intrarater Validity: convergent, discriminant, criterion Practical considerations: interpretability |
|
|
| Childhood Health Assessment Questionnaire19,78,97,112,123 | Includes disability and discomfort in the last week. Disability includes 30 items and 8 subscales: dressing, grooming, arising, eating, walking, reaching, grip, activities. Each item scored from 0 (no difficulty) to 3 (unable to do it, or “not applicable” if beyond development level). Disability score is the unweighted average of the 8 subscale scores. Discomfort: 10-cm VAS | IAR: 1–19 | Reliability: intrarater, interrater Validity: convergent, discriminant, criterion Responsiveness Practical considerations: interpretability |
|
|
| Child Activity Limitations Interview101,102 | 8 activities selected from list of 21 options found difficult or bothersome due to pain. Importance and difficulty over the last 4 wk are rated on a 5-point scale from 0 (not important/difficult) to 4 (extremely important). Ratings are summed; total score, 0–32 21-item version: participants report on limitations for all 21 activities. Ratings summed; total score, 0–84 | IAR: 8–18 | Reliability: interrater Validity: convergent, criterion Responsiveness Practical considerations: acceptable to patients |
|
|
| e-Ouch electronic pain diary86,129–131 | Electronic diary with real-time data: pain intensity, unpleasantness, and interference (with activity, mood, walking, stiffness, enjoyment of life, sleep, schoolwork, tiredness, relationships, and control over pain) using a sliding 0-to-100 VAS. Number of painful joints and pain words also selected. Pain ratings captured 3 times per day (on waking, after school, and before bed) | IAR: 9–18 | Reliability: none Validity: convergent, discriminant Responsiveness Practical considerations: acceptable to patients |
|
|
| Functional Disability Inventory31,73,158 | 15 items assessing everyday activities in the past 2 wk (eg, walking up stairs). Each item scored from 0 (no trouble) to 4 (impossible). Scores summed; total score, 0–60 | IAR: 8–17 | Reliability: intrarater, interrater Validity: convergent, discriminant, content, criterion Responsiveness Practical considerations: feasibility, interpretability |
|
|
| Pain Experience Questionnaire59 | 15-item questionnaire with 4 subscales: pain severity, pain-related interference, social support, and affective distress. Each item scored on 7-point Likert scale from “not at all” to “very much” | IAR: 7–18 | Reliability: interrater Validity: convergent, discriminant, content Practical considerations: none |
|
|
| Pain-QuILT80,81 (previously the Iconic Pain Assessment Tool Version 2) | Web-based tool for tracking pain (quality, intensity, location) using time-stamped records. Pain quality involves choosing from a validated library of pain icons (eg, a matchstick for “burning pain”). Pain intensity: 0-to-10 NRS from “no pain” to “worst pain imaginable.” Pain location: dragging and dropping pain icons onto a virtual body map | IAR: 12–18 | Reliability: none Validity: none Practical considerations: user preference, acceptable to patients |
|
|
| Pediatric Pain Assessment Tool12,86 | 32 word descriptors, 10-cm VAS; present and worst pain, pain coping strategies, and influence of pain on daily activities Modeled on McGill Pain Questionnaire, Pediatric Pain Questionnaire | IAR: 5–17 | Reliability: none Validity: convergent, discriminant, content Responsiveness Practical considerations: acceptable to patients |
|
|
| PROMIS Pediatric Pain Interference Scale40,72,147 | Pain interference bank contains 13 questions (8 in the short form). All questions use 7-d recall scored on a 5-point Likert scale, with anchors of 0 (“never”) to 4 (“almost always”). Raw score totals can be converted to a T score using reference tables | IAR: 8–18 | Reliability: none Validity: convergent, content, criterion Responsiveness Practical considerations: none |
|
|
| Teen Nordic Musculoskeletal Screening Questionnaire85 | 27-item questionnaire, dichotomous responses. The presence of musculoskeletal symptoms and their impact on school attendance, sports, and leisure activity participation over the past 6 mo | IAR: 6–18 | Reliability: intrarater Validity: criterion Practical considerations: none |
|
|
| Varni/Thompson Pediatric Pain Questionnaire7,32,51,77,86,108,148 | Assesses chronic pain intensity and location and sensory, evaluative, and affective qualities. Pain intensity measured on 10-cm VAS, body chart (location and number of pain sites), and with 46 word descriptors to assess sensory, evaluative, and affective qualities of pain Modeled on the McGill Pain Questionnaire | IAR: 4–18 SAR: 6–16 | Reliability: intrarater, interrater Validity: convergent Practical considerations: training |
|
|
| Young Spine Questionnaire82 | For each region of the spine (cervical, thoracic, lumbar): pain presence, frequency, and intensity (Faces Pain Scale-Revised). Also includes function at school, recreation, treatment, and family history of pain. No summary score | IAR: 9–11 | Reliability: none Validity: convergent Practical considerations: none |
|
|
TABLE 6
Summary of the Scales That Have Been Suggested to Measure Pain Intensity for Each Age Group
| Age Group | Recommended Scale | Type of Scale | Psychometric Properties Evaluated | Strengths | Limitations |
|---|---|---|---|---|---|
| Infant (3 y or younger) | FLACC scale5,37,48,93,94,136 | Observational | Reliability, validity, responsiveness, user preference, patient preference |
|
|
| Preschool child (3–5 y) | Pieces of Hurt Tool (Hester's poker-chip tool)47,50,60,133,139 | Self-report | Reliability, validity, responsiveness, patient preference |
| |
| Child (6–11 y) | Faces Pain Scale-Revised11,24,61,95,96,106,115–117,129,142–145 | Self-report | Reliability, validity, responsiveness, patient preference |
|
|
| Adolescent (12–18 y) | Verbal numeric rating scale-116,23,24,39,95,113,115–117,154 | Self-report | Reliability, validity, responsiveness, patient preference |
|
|
| Cognitive impairment (4–19 y) | Revised FLACC scale29,38,45,88,104–106,150 | Observational | Reliability, validity, responsiveness, user preference, carer preference |
|
|
Infant (3 Years or Younger)
Observations of behavior are most commonly used in this age group, which typically manifests as crying, facial expression, verbalization, and torso and leg movements. It is important to note that no scale has been comprehensively evaluated to assess pain in children aged 3 years or younger in primary care settings or in children with chronic or persistent pain.37,153 The majority of observational scales have been developed to measure postoperative pain in the hospital setting, but a number of these scales have since been used to assess brief pain associated with medical procedures (eg, venipuncture, immunizations). In the absence of more robust evidence, the scales that have been validated to assess procedural pain are reported in TABLE 2.36 Scales that only evaluate postoperative pain or incorporate physiological measures (eg, blood pressure, oxygen levels) have not been included, as this information is not readily available or feasible for use by many clinicians.
Suggested Scale: The Face, Legs, Activity, Cry and Consolability (FLACC) Scale The FLACC scale was originally designed and validated for use in infants and children aged 2 months to 7 years to measure postoperative pain.94 Since its original development, the scale has been used to measure acute and procedural pain in emergency departments, immunization centers, and various clinical settings (eg, radiology, ambulatory, dental) and in research.37 The FLACC scale is recommended to measure pain in infants (aged 3 years or younger) on the basis that it has been validated to measure acute procedural pain in a variety of settings (eg, outpatient pediatric clinic, emergency department, immunization clinic) and there is no other scale as comprehensively evaluated.
A score of 0, 1, or 2 is given for each of the 5 items. Descriptions of typical behaviors are provided for each item (eg, for the legs, “normal or relaxed position” is a score of 0; “uneasy, restless, tense” is a score of 1; and “kicking, or leg drawn up” is a score of 2). Item scores are summed to provide a total score from 0 to 10. TABLE 2 contains additional details about the FLACC scale and other observational scales to measure pain intensity in children who are unable to self-report.
Preschool Child (3–5 Years)
Age is the strongest predictor of a child's ability to understand and use self-report pain scales.127,155 It is noted, however, that the rate of development is varied. While preschool-aged children (3–5 years of age) are generally less likely to be able to understand self-reported pain scales than older children, some will be able to do so. Experience of pain and prior use of a scale appear to influence a child's ability to use a pain scale reliably, emphasizing the need to measure pain consistently. It is recommended that pain intensity be captured through self-report in children of this age (if deemed appropriate), supplemented by information from parents/guardians of the child and observation of behavior (FLACC scale).
Suggested Scale: Pieces of Hurt Tool, Supplemented by Parent/Guardian Report and Observation The Pieces of Hurt Tool was designed and validated for use in children aged 4 to 7 years to measure procedural pain at immunization clinics.60 The scale has since been used to measure acute (eg, postoperative), procedural, chronic, and recurrent pain in hospitalized children as young as 3 years of age. The Pieces of Hurt Tool is the recommended pain scale for children aged 3 to 5 years.
The child is asked, “Does it hurt?” If the child says “no,” then zero is recorded. If the child responds “yes,” then the child is presented with 4 tokens (eg, poker chips) and it is explained that each token represents a “piece of hurt” (1 token is a little bit of hurt, 2 is a bit more, and 4 tokens represent the most hurt you could ever have). The child is then asked, “How many pieces of hurt do you have right now?” The number of tokens is then recorded. Additional details are reported in TABLE 3.
Child (6–11 Years)
Face scales are consistently preferred by children over numerical, analog, or word descriptor scales.142 Several versions are available that use either line drawings or photographs (eg, Faces Pain Scale-Revised [FPS-R], Wong-Baker FACES scale, OUCHER scale). While the scales perform similarly, they are not interchangeable due to their different anchors, highlighting the importance of using the same scale consistently. The main limitation of face scales is that pain intensity, a sensory component of pain, is being measured using faces that express the affective dimension of pain. The type of face anchors used by scales (eg, smiling versus neutral) has been found to influence children's responses.26,32,142
Suggested Scale: FPS-R The FPS-R was adapted from the original Faces Pain Scale11 and validated in children 4 to 12 years of age and undergoing a painful procedure (ear piercing), and in an inpatient clinical population.61 The FPS-R is the recommended pain scale for children aged 6 to 11 years based on considerable evidence in support of its reliability and validity in this age group. The primary strength of the FPS-R compared to other face scales consists of the gender-neutral face anchors that do not convey the affective dimension of pain (eg, smiling, crying).26
The FPS-R consists of a set of 6 line-drawn faces with depictions of increasing levels of pain from left to right. Children are asked to specify which face best illustrates the amount of pain they are experiencing at that time. Each face is assigned an increasing score from left to right, either 0 to 5 or 0 to 10 (increments of 2). Electronic versions of the FPS-R have also been developed and validated (eg, the Sydney Animated Facial Expressions scale61 and Painometer app).115,116 Additional details are reported in TABLE 3.
Adolescent (12–18 Years)
Suggested Scale: Verbal Numeric Rating Scale-11 The numeric rating scale-11 is one of the most commonly used scales to measure pain intensity in both clinical and research settings, despite only recently undergoing appropriate psychometric evaluation in children and adolescents. The scale has been validated to measure acute, procedural pain, as well as chronic pain, in a wide range of settings, including schoolchildren receiving immunizations,154 outpatient pain clinics,113 and emergency departments.6 The numeric rating scale-11 is recommended to measure acute pain in children aged 12 to 18 years due to its simplicity, validity, reliability, and brevity as a pain assessment tool.
The individual is asked, “On a scale of 0 to 10, where 0 is no pain and 10 is the worst possible pain, tell me what number best represents your pain.” The individual responds with a number that reflects his or her pain. The numeric rating scale-11 has also been adapted and validated for use to capture pain intensity for both acute and chronic conditions by short message service and online.4,135 Additional details are reported in TABLE 3.
Children and Adolescents With Cognitive Impairment
Children and adolescents with cognitive impairments (eg, cerebral palsy) experience more significant and frequent pain than children without cognitive impairment, and are less likely to receive adequate pain management, indicating the need for specific and appropriate pain assessment measures.12,89,126 Pain behaviors displayed by children with cognitive impairment are not always comparable to those of children without cognitive impairment, although pain expression has been found to be consistent, observable, and reflective of the presence and severity of pain.12,111 Thus, pain measurement tools should be adaptable to reflect individual pain-related behaviors, but ideally also contain standardized items that enable their use in any setting.38 Very few scales are available to assess pain in children with cognitive impairment, and, as seen in TABLE 4, these have only been tested in postoperative, residential care, or school settings. No scale has been tested to measure brief procedural, chronic, or recurrent pain in children and adolescents with cognitive impairment. A recommended scale is the revised FLACC scale.
The revised FLACC scale was adapted from the FLACC scale94 for use in children and adolescents with cognitive impairment.88 The revised FLACC scale is suggested for children and adolescents with cognitive impairment, based on evidence demonstrating valid and reliable measurement of postoperative pain in a hospital setting, the ability of the scale to be individualized, and evidence of its clinical utility. This suggestion is made in the absence of any other more comprehensively evaluated scale.
The revised FLACC scale is essentially the same as the FLACC scale, but it also enables behaviors to be described that are unique to the respondent for each of the 5 behaviors (face, movement of the body and legs, cry, and consolability). Identifying pain behaviors that are unique to the individual requires input from a family member or carer.
Assessing the Broader Impact of Pain Using Multidimensional Pain Scales
While this paper has focused predominantly on the measurement of pain intensity, it is acknowledged that pain experience is complex and contains other dimensions, including affective and evaluative dimensions, as well as the impact pain has on everyday life, including an individual's physical, social, and emotional functioning and ability to fulfill his or her “role.” The social communication model presented at the start of this paper can still be used to conceptualize the communication of these other dimensions of pain, albeit using broader, multidimensional pain scales. Multidimensional pain scales are particularly useful for assessing recurrent and chronic pain, as they can capture various dimensions of the pain experience (including duration, frequency, location, nature, aggravating and easing factors) and how pain impacts everyday life (eg, interference with daily activity or participation in school and sport). This fills a well-accepted need to differentiate between low-intensity transient pain and more persistent pain that has substantial impact on life.100 Multidimensional scales differ with respect to the factors assessed (eg, psychosocial factors, situational factors, nature of disability) and period of time. Some of the most commonly used multidimensional pain scales for use in children and adolescents with chronic or recurring pain are outlined in TABLE 5. No specific scale has been suggested, as the choice will depend on the purpose of measurement and the health condition being measured (eg, region- or condition-specific scales). Common to many of the scales is identification of impact on school absenteeism, interference with sports participation, interference with activities of daily living, medication use, and health care utilization. These are acknowledged as important indicators of pain impact in pediatric populations.100
Another important dimension of pain assessment is fluctuation over time. In addition to the scales outlined in TABLE 5, pain diaries are often used to capture information about pain (eg, intensity, frequency, and location) and its effect on behavior over time. The information collected in a pain diary may provide a more accurate and reliable measure of pain, if completed on a regular basis, by minimizing recall bias. Recent advances in information and communication technology (eg, internet, smartphones) have permitted the development of electronic methods such as e-diaries. Advantages of this electronic approach over traditional paper-based techniques include minimizing errors in data transfer and transcription, ability to capture time-stamped data, ease of data sharing, increased compliance, and heightened patient satisfaction.81 Recently, Lalloo et al,80 Lalloo and Stinson,81 and Stinson and colleagues132 have validated a number of web-based and smartphone-based multidimensional electronic pain assessment tools, including e-Ouch, Standardized Universal Pain Evaluations for Rheumatology Providers for Children and Youth, and Pain-QuILT (freely available). These tools can be used in a variety of clinical settings to monitor musculoskeletal pain in children in real time.80,81
Good-Practice Points
Pain in children has been inadequately assessed, underestimated, and undertreated for many years.146 This situation can only be rectified through improved communication of pain from the patient to the clinician at every relevant clinical contact, until it becomes a part of routine care.111 Central to the social communication model of pain framework are the various intrapersonal and interpersonal factors that may influence the expression, assessment, and management of pain. Consideration of these factors and the context in which the communication occurs is vital for effective, accurate, and reliable communication of pain, early detection of pain, and timely management (including reassurance and advice). Most importantly, the improved communication of pain has been found to improve patient outcomes (eg, reduction in mean pain scores, improved satisfaction) and reduce health care costs (eg, reduction in length of stay).76,120
An individual's pain experience and expression are determined by a range of biopsychosocial factors that are specific to his or her developmental stage. The clarity of the pain expression can be optimized through the consistent use of valid and reliable pain scales that are age appropriate and meet the cognitive and communication capabilities of the individual, where available. This means using observational scales of behavior for infants, integrating both observational and self-report scales for children, and primarily using self-report scales for those in later childhood or their teenage years. In all cases, patient self-report of pain should be considered and interpreted alongside knowledge of the context, and supplemented with information gained from observation of behavior and input from parents and guardians, when appropriate. A child's ability to use a pain scale accurately and reliably increases with his or her familiarity with the scale, highlighting the need to introduce and educate children on the use of pain scales early in their life course. By providing children with the vocabulary and skills necessary to express their pain, the clarity of the pain expression can be improved, potentially reducing errors in the interpretation by the observer.
Effective pain assessment and management by clinicians can be enhanced through the consistent use of standardized pain scales within and across health care settings and by the accurate and timely documentation of assessment findings.61 Advantages of this approach include improved continuity of care for the individual and the ability to generate consistent data for longitudinal comparison of pain over time. Evidence from studies on global perceived effect scales shows that patient-reported outcomes taken over time provide a more accurate understanding of changes in a person's health status compared to recall of improvement/deterioration.71 The minimal clinically important change has been determined for a number of scales (reported in TABLE 3 where available), which can assist clinicians to determine the effectiveness of an intervention and provide insight into the meaningfulness of the change for the individual. Areas in need of further development include the assessment and measurement of pain resulting from other mechanisms, such as neuropathic and central pain, for which there are currently no scales validated for use in children and adolescents. Undoubtedly preferable to the timely assessment and management of pain are practices that can help minimize or prevent the experience of pain. In many instances, pain can be anticipated (eg, procedural, vaccinations, postoperative pain) and proactively managed by clinicians and parents/guardians, using both pharmacological (eg, topical analgesics) and nonpharmacological interventions (eg, distraction). The “It doesn't have to hurt” online video is one such example of providing clinicians and parents/guardians with effective, evidence-based information that can positively influence a child's experience of painful situations.25
Conclusion
To date, little research has been done to evaluate the use of measures designed for assessment of pain in infants, children, and adolescents outside the hospital setting. This constitutes an important evidence gap, given that primary care, community outpatient, and rehabilitation clinics have frequent contact with infants, children, and adolescents with pain. Though further work is needed, this paper provides clinicians with a pragmatic, evidence-based overview of scales that can be used to measure pain intensity in infants, children, and adolescents, with and without cognitive impairment, and to assess the impact of pain.