

Cannabis for Pain and Headaches: Primer This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Curr Pain Headache Rep. 2017 (Apr); 21 (4): 19 ~ FULL TEXT
Philip S. Kim, Michael A. Fishman
Center for Interventional Pain Spine and Ageless MD, LLC,
931 East Haverford Road, Suite 202,
Bryn Mawr, PA, 19010, USA.
phshkim@yahoo.com
PURPOSE OF REVIEW: Marijuana has been used both medicinally and recreationally since ancient times and interest in its compounds for pain relief has increased in recent years. The identification of our own intrinsic, endocannabinoid system has laid the foundation for further research.
RECENT FINDINGS: Synthetic cannabinoids are being developed and synthesized from the marijuana plant such as dronabinol and nabilone. The US Food and Drug Administration approved the use of dronabinol and nabilone for chemotherapy-associated nausea and vomiting and HIV (Human Immunodeficiency Virus) wasting. Nabiximols is a cannabis extract that is approved for the treatment of spasticity and intractable pain in Canada and the UK. Further clinical trials are studying the effect of marijuana extracts for seizure disorders. Phytocannabinoids have been identified as key compounds involved in analgesia and anti-inflammatory effects. Other compounds found in cannabis such as flavonoids and terpenes are also being investigated as to their individual or synergistic effects. This article will review relevant literature regarding medical use of marijuana and cannabinoid pharmaceuticals with an emphasis on pain and headaches.
KEYWORDS: Cannabinoids; Cannabis; Headache; Marijuana; Nociception; Pain; Tetrahydrocannabinols
Introduction
The medical use of cannabis has been documented in ancient Greece and China. [1] The most commonly used species of the plant are Cannabis sativa and Cannabis indica. Each variety has varying composition and relative concentrations of active compounds. In the last two centuries, Cannabis has been used and recommended by various physicians. Dr. William B. OShaughnessy, an Irish physician, introduced Indian hemp to Europe with reports of high rates of success for rheumatism, rabies, cholera, tetanus, cramps, and delirium. Dr. William Osler reported benefits of cannabis for various conditions including migraines and menstrual cramps. The Marihuana Tax Act of 1937 began the government intervention that lead to the downfall of cannabis for medical use. The removal of cannabis in 1940 from the US Pharmacopeia further compromised its medical use. In 1970, the Controlled Substance Act made cannabis a schedule I drug. Recently, the USA has opened the policies for medical marijuana by allowing the States to legislate medical marijuana laws. Currently, there are 23 states with medical marijuana laws. Interesting, the federal government applied for and was granted a patent on cannabinoids for antioxidant and neuroprotectant use in 2003.
Endocannabinoid System
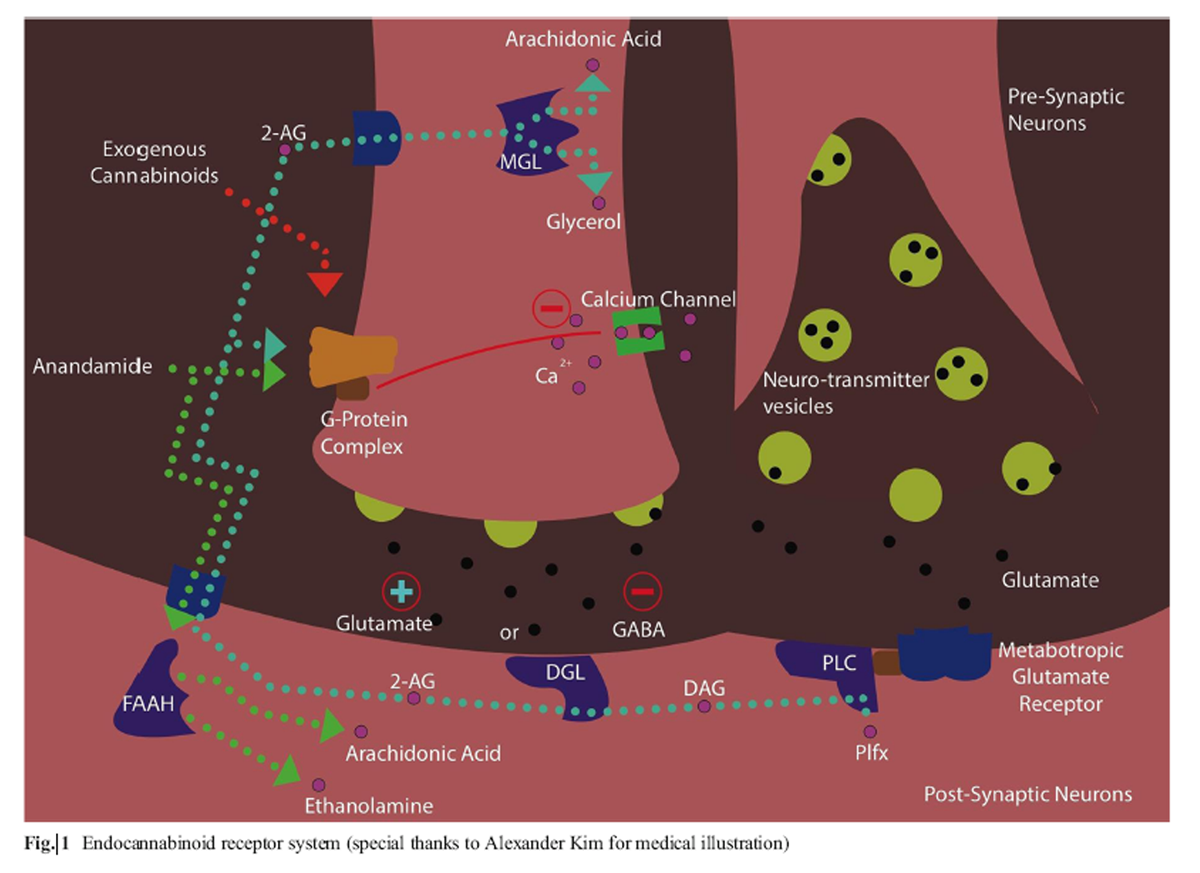
Figure 1 Cannabinoids and medical marijuana research follows a similar path to other plant-derived therapy. Opium poppy (Papaver Somniferum) led to the development of the standard narcotic analgesic morphine. The morphine alkaloid led to the development of various synthetic derivatives and discovery of the endogenous endorphin systems. Like morphine, the isolation and identification of the first cannabinoid Δ9- tetrahyrocannabinol (THC) in 1964 by Dr. Raphael Mechoulam led to discovery of the endogenous cannabinoid system. Endogenous cannabinoids are natural chemicals such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG). The basic function of the endocannabinoid system acts to modulate the sensitivity to many other neurotransmitters such as dopamine and serotonin in the central nervous system (Figure 1. The human experience of pain and response to stress involves the interaction of endocannabinoids through endorphins and cortisol release. From a global perspective, the endocannabinoid system functions are: relax, eat, sleep, forget, and protect. [2] AEA is hydrolyzed by the enzyme fatty acid amide hydrolase (FAAH) to arachidonic acid and ethanolamine; 2-AG is metabolized by monaoacylglycerol lipase (MGL) into arachidonic acid and glycerol. [3]
These enzymes, FAAH and MGL, represent potential therapeutic targets to modulate endogenous endocannabinoid levels or potential mechanisms of dysfunction in the development of various disease states. Currently, there are two well-defined cannabinoid receptors CB1 (Cannabinoid receptor 1) and CB2 (Cannabinoid receptor 2). [4] CB1 is a seventransmembrane spanning G protein-coupled receptor inhibiting cyclic AMP release. [4] CB1 is the primary neuromodulatory receptor accounting for the psychopharmacological effect of THC and most of its analgesic effects. Presynaptic activation of CB1 acts as a synaptic circuit breaker to inhibit neurotransmitters such as GABA (gamma- Aminobutyric acid) or glutamate. CB2 works primarily as an immunomodulatory receptor in the periphery. It is postulated that CB2 modulates persistent inflammatory and neuropathic pain conditions. [5] THC, the prototypical phytocannabinoid, is a weak partial agonist of both CB1 and CB2 receptors. [6]
Cannabinoids
Cannabinoids can be broken down into three subgroups: endogenous endocannabinoids, botanicals (phytocannabinoids), and synthetic derivatives. Over 60 different phytocannabinoids have been identified in the marijuana plant. [7] The principal cannabinoids appear to be delta-9-tetrahydrocannabinol andcannabidiol (CBD). Other potential cannabinoids with medical value include cannabigerol (CBG), Cannabinol (CBN), cannabichromene (CBC) and tetrahydrocannabivarin (THCV). [8] CBG is a product of delta-9-THC oxidation and displays potent GABA reuptake inhibition activity and phospholipaseA2 modulator. [9, 10] CBD lacks detectable psychoactivity and does not appear to bind to either CB1 or CB2 receptors in high concentrations. Rather, it displays activity at other targets such as ion channels, receptors and enzymes. Pre-clinical studies support anti-inflammatory, analgesic, anti-emetic, anti-psychotic, anti-ischemic, anxiolytic and anti-epileptiform activity. THCVacts as a CB1 receptor antagonist and CB2 receptor partial agonist with pre-clinical studies suggesting ant-epileptiform/anti-convulsant properties.
In the living plant, these phytocannabinoids exist as both inactivemonocarboxylic acids and an active decarboxylated forms. Heating above 120 °C promotes decarboxylation and results in biological activation. [7] Other constituents in cannabis with potential medical benefit include the following: terpenes, noncannabinoid phenols, flavonoids and vitamins. Further differences in the chemical constituents are noted in various cannabis species and extraction techniques. The terpenes and flavonoids are not yet well characterized, but they are believed to have a broad spectrum of potential anti-inflammatory, anti-oxidant, antibacterial and anti-neoplastic actions. An example is noted in myrecene, a terpenoid, with anti-inflammatory activity via PGE-2 and opioid type analgesic effect blocked by naloxone. The scientific and pharmaceutical approach to identify single ingredients and synthesize one compound, such as THC, for use may not offer full effect of the polypharmaceutical cannabis plant. The many constituentsmay work by multiple mechanisms to improve therapeutic activity either an additive or synergistic manner and mitigate the side effects if their predominant active ingredients. [11] An example is the co-administration of CBD and THC may result in attenuation or potentiation of some of the effects of THC through a pharmacodynamics mechanisms. [12] A ratio of CBD to THC of at least 8:1 attenuates THC induced effects, where as CBD potentiates THC at a ratio 2:1. Potentiation of THC may be caused by inhibition of THC metabolism in the liver.
Synthetic cannabinoids have been developed to mimic THC. Oral dronabinol (THC) has been available as Marinol® for nausea associated with chemotherapy and appetite stimulation with HIV/AIDS. In the USA, it is classified as a schedule III drug. A new liquid dronabinol formulation called Syndros® has recently been approved for the same indication. Nabilone is another formulation of synthetic THC marketed under the brand name Cesamet® and available as an anti-emetic for chemotherapy-associated nausea and is a schedule II substance. It is ten times more potent than dronabinol. Ajulemic acid (CT3) is a synthetic THC currently being studied in phase II randomized clinical trial in peripheral neuropathic pain. [13] Other synthetic cannabinoids are in development.
Biochemical and Neurophysiological Basis of Pain Control by Cannabinoids
Thorough reviews of pre-clinical and clinical studies support the therapeutic effects of cannabinoids in nociception. [14, 15] The endocannabinoid system is active in the central and peripheral nervous system at nociception centers such as the periaqueductal gray matter, ventroposterolateral nucleus of the thalamus and the spinal cord. In neuropathic pain states, endocannabinoids are involved in stress-induced analgesia, wind-up phenomena, and central sensitization. [16, 17] The periaqueductal gray region has also been implicated in migraine generation. [18] In the peripheral nervous system, the endocannabinoid system is active in suppressing hyperalgesia and allodynia. [19] Pathological pain states such Complex Regional Pain Syndrome (CRPS) has been postulated to arise and at least involve a dysregulation of the endocannabinoid system.
An endocannabinoid deficiency is theorized to underlie the pathophysiology of migraine or headaches. [20] Clinical studies suggest that the lower concentration of anandamide is found in the cerebral spinal fluid of migraineurs and the calcitonin gene-related peptide (CGRP) and nitric oxide (NO) levels are increased. [21, 22] In addition, the activity of the anandamide-degrading enzyme, FAAH, is significantly decreased in chronic migraineurs compared to controls. [23] It is also postulated that active migraines are aggravated by release of serotonin during migraine attacks. In one study, THC inhibited release of serotonin from platelets in a plasma sample taken during an active migraine episode. [24] The endocannabinoid system is active in the trigeminovascular system, which has been implicated in migraine pathogenesis at the vascular and neurochemical level. [25] Other postulated endocannabinoid-deficiency conditions include fibromyalgia, idiopathic bowl syndrome and endometriosis. [20] The endocannabinoid system is also very active in modulating nociceptive response in gastrointestinal and visceral sites. [26] Endocannabinoid modulators may help restore homeostasis and lead to normalization of function in pathophysiological conditions. [20]
Clinical Studies Review
The White House Office of National Drug Control recommends that the Institute of Medicine focus cannabis research on the following:
Physiologic effects of synthetic and phytocannabinoids
Development of new delivery systems
Psychological effect of cannabis
Health risks of smoked marijuana [27]

Table 1 High-quality clinical studies that are randomized, doubleblinded and placebo controlled are limited overall with small population and short duration of the study. Sample of these studies are listed in Table 1. The studies are broken down on the type of cannabinoid use with listing of type of patients. As we have discussed in the previous section, the type of cannabinoids; synthetic and phytocannabinoid and delivery system can affect response for various types of chronic pains.
Clinical Evidence of Synthetic Cannabinoids
The synthetic cannabinoids are delivery in oral formulation with mixed results for pain (see Table 1). The focus of synthetic THC has been on nausea associated with chemotherapy and appetite stimulation in HIV/AIDS. The studies with dronabinol show some benefit for multiple sclerosis but no benefit for postoperative pain. [28, 29] In the study of multiple sclerosis patients, 24 patients received benefit with 10 mg dronabinol compared to placebo with modest reduction of pain p = .02. [30] Dronabinol was assessed in 30 patients with chronic non-cancer pain in a double-blind cross-over single day sessions with improvement. [31] These were patients on high-dose opioids who noted 10 or 20 mg dronabinol added additional analgesia in combination with current opioids. Methodological issues are present where58% of patients correctly guessed the dronabinol dose on test day and 4 placebo patients had a positive THC assay.
Further studies were done using nabilone with mixed results for pain (Table 1). Nabilone is a semi-synthetic analogue of THC that is tenfold more potent and has a long duration of action. As with dronabinol, prominent sedation and dysphoria are noted side effects. In a postoperative pain study of 41 patients, postoperative pain scores actually increase compared to control group. [32] A small randomized control trial with 12 patients with spasticity showed a decrease in pain (p < .05) vs. placebo but no improvements such as spasticity, motor function, and activities of daily living. A double-blind randomized crossover comparison of nabilone to dihydrocodeine in chronic neuropathic pain, showed both drugs produced marginal benefit with dihydrocodeine superior in efficacy and side effect profile/ [33] In a randomized control trial (RCT), 40 patients noted benefit with nabilone with fibromyalgia with decrease in pain and anxiety. [34] Another fibromyalgia study compared nabilone vs. amitriptyline and noted nabilone yielded benefit for sleep but not pain, mood, or quality of life. [35]
Another synthetic THC analogue, ajulemic acid (CT3) is under review and has been utilized in a phase II RCT in 21 patients with peripheral neuropathic pain. [13] Pain relief is statistically significant (p = .02) and no major adverse side effects were noted.
Evidence for Smoked or Vaporized Cannabis

Table 2 Randomized controlled clinical trials are limited to studies with small patient populations and limited duration. The clinical trials in human volunteers with painful stimuli support analgesic benefit of smoked or vaporized cannabis (Table 2). One study shows that smoked cannabis increased pain tolerance to pressure algometer and that experienced users had greater effect compared to naïve users. [36] In a cold pressor test, volunteers experienced equal decreases in pain sensitivity and tolerance in smoked marijuana and 10 mg dronabinol when compared to placebo. [37] The dronabinol group experienced longer duration of effect. In a radiant heat stimulation test, the healthy volunteers experienced a dose-dependent response to 3.55% THC cannabis compared to placebo. [38 Naloxone administration did not alter the response to cannabis. Finally, an intradermal capsaicin test was done on human volunteers. [39] Interesting, the noxious stimulus was blunted with smoked cannabis 4% THC only. At 2% THC, no analgesic effect was noted and 8% THC caused increased pain from the stimuli suggesting a therapeutic window of analgesia.

Table 3 Clinical trials in human patients with smoked cannabis focus on chronic neuropathic pain and specifically the subset of patients with HIV neuropathy (Table 3). A HIV neuropathy group of 25 patients underwent a double blind RCT with 3.56% THC smoked vs. placebo cigarettes over 5 days. [40] The cannabis group noted a 30% reduction in VAS and hyperalgesia. The cannabis was well tolerated with sedation (54%), anxiety (25%) and disorientation (15%) as the most common side effects. Similar findings were noted in another HIV neuropathy study with a decrease in pain with cannabis 18% THC smoked. [41] The study was administered four times daily over 5 days with a 2-week washout period. These patients were considered experienced with cannabis. The cannabis was overall well-tolerated. In two different centers and investigators, similar methods with smoked cannabis on chronic neuropathic pain identified a significant analgesic response. [43, 44, ]
In the Canadian study, 23 subjects underwent a single 25 mg inhalation of various cannabis potencies (09.4% THC) three times daily 5 days. The most frequent side effects were headache, dry eyes, burning sensation, dizziness, numbness and cough. Wilsey et al. assessed medicinal cannabis in neuropathic pain using double-blind randomized crossover study design. All the Wilsey studies showed similar benefit with various concentrations of THC. The most important methodological measure was inclusion of specific objective neurological measures and measured impairment in attention, learning, and memory; most noted at 7% THC. In addition, the comparison of various concentration of THC showed that lower THC of 2.9 vs. 6.7% and 1.29 vs. 3.53% worked just as well for pain with fewer side effects.
Evidence for Cannabis-Based Extracts
Cannador® is a cannabis extract available as an oral capsule with various ratios of THC:CBD ratios. In the studies reviewed, the ratio was 2:1. Cannador was utilized in a phase III RCT for the treatment of spasticity. Six hundred thirty patients were studied with a noted decrease in pain related to spasms but no improvement in spasticity as measured by the Ashworth scale. [46] Cannador was evaluated in 65 patients with postherpetic neuralgia with no improvement. [47] In postoperative pain, a slight reduction in pain was noted in a single dose of 5, 10, and 15 mg. An incremental reduction in pain and reduction in opioid requirements was noted. A single case of a serious vasovagal adverse event was noted at 15 mg, which lead to study termination.
Nabiximols (Sativex®) is a cannabis extract delivered as an oral-mucosal spray at approximate THC:CBD ratio of 1:1. Multiple RCT studies have demonstrated pain relief in various chronic pain conditions. In a phase II study of 20 patients with neurogenic pain, significant improvement was noted with THC extract (Tetranabinex®) and THC:CBD extract (Sativex®,) compared to placebo. The nabiximols combination of THC and CBD offered better pain control than THC extract alone with fewer side effects of intoxication. Similar results were noted in another phase II study of intractable chronic pain where [48] THC:CBD were superior to THC alone. [49] Sixty-five patients in a phase III study of brachial plexus avulsion injury showed pain reduction with both THC and THC:CBD formulations. [50]
In a RCT study with multiple sclerosis, THC:CBD combination improved pain control over placebo. [51] Similar results were noted for pain relief in patients with peripheral neuropathies. The equal ratio of THC to CBD provided a reduction in dynamic and punctate allodynia. A safety extension study in 160 multiple sclerosis patients showed sustained improvement over the course of a year without significant tolerance to nabiximols. Spasticity and pain as measured by VAS were both significantly reduced. No significant adverse effects were seen on mood or cognition. Chronic pain related to rheumatoid arthritis also responded to nabiximols in RCTs. [52] Two well-designed RCTs showed statistically significant pain relief for intractable cancer pain. [53, 54] Another RCT with multiple sclerosis patient showed significant relief of bladder spasms and pain. [55] Overall, Nabiximols is well tolerated with reduction in pain but also improvement in sleep. [56 It is believed that the sleep is improved by reduction in pain and not as a hypnotic effect. The benign common adverse side effects of nabiximol include the following: bad taste, oral stinging, dry mouth, dizziness, nausea and fatigue. These side effects did not lead to discontinuation of therapy in any of these trials.
Pharmacology, Dosing, and Side Effects
The pharmacokinetics and pharmacodynamics of cannabinoids is complex as it on the interaction of various cannabinoids as well as on the route of administration. The actual temporal relationship between delta-9-THC and associated clinical/therapeutic benefits, psychotropic, cognitive and motor effects are not well established. Dosing for cannabis is highly variable due multitude of factors. Each individual may have interindividual (genetic) differences in the endocannabinoid system, metabolism (cytochrome P450system), previous exposure, and tolerance to cannabis. The various strains/formulations of cannabis display an array of potencies and other constituents that make it difficult to have a traditional uniform dosing scheduling. The dosing of any cannabis product is highly individualized and relies to a great extent on titration. For cannabis-naïve patients, it is recommended to start at the lowest dose and titrate to effect. Surveys suggest that the majority of people using smoked or orally ingested cannabis for medical purposes use between 10 and 20 g of cannabis per week or approximately 13 g of cannabis per day. [57] The standard cannabis cigarette contains approximately 750 mg of cannabis material.
Little is known in regard to conversion of smoked cannabis to an equivalent oral dose. [58] A formula exists where the smoked cannabis content of delta-9-THC is multiplied by 2.5 to correct for differences between the bioavailability of delta- 9-THC through smoked route (25%) vs. oral route (10%). [57, 58]
Smoked and Vaporized Cannabis
Smoking cannabis results in a very rapid onset of action (minutes) with higher blood levels of cannabinoids and short duration of pharmacodynamics effects compared to oral administration. [59] The amount of delta-9-THC is variable depending upon the composition of plant material and composition of the cigarette. [60] The level of inhalation, puff duration and breath hold make the bioavailability estimated at 256%. [57] Overall, about 2527% of the total amount of delta-9-THC is absorbed in the body. [57]
One of the concerns about smoked cannabis is the intoxication due to rapid absorption. Cannabis also presents with similar concerns as with cigarette smoking, including chronic cough and bronchitis symptoms. [61] Of equal concern is that cannabis smoke contains similar carcinogens and mutagens as tobacco smoke, including carbon monoxide, ammonia, benzene, acetaldehyde, polycyclic aromatic hydrocarbons, aromatic amines, and hydrogen cyanide. However, population-based studies have failed to show increased risk of chronic pulmonary disease or lung cancer associated with smoking cannabis. [62]
Nevertheless, the concerns of the carcinogens and mutagens with combustion of cannabis have led to the development of alternative routes of delivery. Vaporization is where cannabis is heated to a lower temperature that volatilizes THC and other components while mitigating the production of carbon monoxide, polycyclic aromatic hydrocarbons, and tar. The pharmacokinetics of vaporized cannabis is considered the same as smoked cannabis but further research is needed to completely elucidate this. [63]
Oral and Oral-Mucosal Cannabis
Oral THC has a wide variability due to gastric degradation, first-pass pharmacokinetics, and liver metabolism. [64] Studies suggest oral bioavailability may be 520%. [64] Oral cannabidiol is reported to be about 1319% bioavailable [65]. Compared to smoked cannabis, the peak plasma concentration of THC is 34 h, with duration of effect variable up to 8 h. Variable duration of effects is noted with all oral preparations of synthetic or phytocannabinoids. Interpretation of delta-9-THC levels is complicated by the presence of active psychoactive metabolites, such 11-hydroxy THC, which are found in higher concentrations after oral administration compared to inhalation [66]. In addition, a study on simulated gastric fluid shows cannabidiol can be converted to delta-9- THC and delta-8-THC [67].
The effect of cannabis varies amongst different patient populations. One study suggest female patients with high estrogen levels are more sensitive to medical cannabis in regard to pain, behavior, and reward [65]. Long-term use may be related to lower levels of luteinizing hormones, follicle-stimulating hormones, prolactin, and growth hormone. One retrospective study compared the analgesic, subjective and physiological effects of active cannabis (3.565.60% THC) and inactive cannabis in male and female cannabis smokers during a cold pressor test (CPT) [68]. Male smokers exhibited greater cannabis-induced analgesia compared to women.
Side Effects of Cannabis
The most common acute effects of cannabis use are impairments in memory, motor coordination, altered judgment, and in high doses, paranoia and psychosis [69]. In patients with cardiovascular conditions, an increased in heart rate, cardiac variability, and orthostatic hypotension has been reported. Precautions should be taken with vulnerable patients. Other physical effects can include conjunctival injection, decreased lacrimation, headache, nausea, and vomiting. Similar side effects are noted with use of the synthetic cannabinoids (dronabinol, nabilone and nabiximols) with the most common side effects reported including sedation, nausea, vomiting, dry mouth, and dizziness. Special precaution is necessary with concomitant administration of opiates, anti-depressants, benzodiazepines, and anti-psychotics as decreased alertness and over sedation may occur [70].
Death has not been associated with cannabis use, and the data on this is clear. Unlike opioids, the endocannabinoid system is not expressed in the respiratory center of the brain. Perhaps that explains why the LD-50 (the lethal dose in 50% of the population) for cannabis is high. In rats, the LD-50 of oral THC is 8001900 mg/kg and in monkeys it is 9000 mg/kg [71]. For an average 70 kg man, that means taking 6300 tablets of dronabinol (10 mg) or smoking 6300 joints (each joint is 1000 mg).
The chronic use of cannabis has been associated with development of dependence, chronic bronchitis, and increased risk of chronic psychotic disorders [69]. Multiple studies demonstrate that chronic cannabis use is associated with poor neuropsychological performance, especially in the areas of learning and memory recall [69]. The difficulty in long studies is differentiating from the effects of associated tobacco and/or alcohol use [69] When used in early adolescence, long-term and/or heavy cannabis use has been associated with altered brain development, poor educational outcomes, and cognitive impairment [72]. These same concerns can be applied to fetal development and early child development with cannabis use during pregnancy. It is also noted that chronic long-term use of cannabis may lead to decreased sperm count, motility, and abnormal sperm morphology [73].
The chronic effects of cannabis on the lungs have the same concerns as tobacco as it relates to increased risk of bronchitis, emphysema, airflow obstruction, and carcinogen exposure [74, 75]. Looking at the observational studies is difficult given the high level of concomitant use of tobacco by many patients. One longitudinal study of 20 years looked at the association of cannabis and tobacco use with a variety of physical health indices [76]. The only major concern noted was periodontal disease. Interesting, in comparison to tobacco use, cannabis has a limited effect on pulmonary function, systemic inflammation, and metabolic health. One of the major concerns is the risk of dependence and addiction with cannabis. Comparative epidemiology of dependence showed that cannabis users had the lowest dependence (9%) compared to other drugs such as tobacco (32%), alcohol (15%), prescription drugs (9%), cocaine (17%), and heroin (23%) [77].
Cannabinoid Interaction with Opioids
Opioid use with cannabis has the potential to decrease alertness and cause cognitive effects. At the same time, suggested studies support a synergistic analgesic effect. The putative mechanism is the stimulation of beta-endorphins by THC and potential inhibition of opioid tolerance and withdrawal [78, 79]. One supportive clinical study shows that cannabinoids and opioids may act synergistically [63]. Twenty-one individuals with chronic pain on a regimen of morphine or oxycodone were treated with vaporized cannabis and noted a decrease in pain with no significant increase in plasma levels of oxycodone or morphine. At the same time, patients experienced increased quality and duration of high with inhalation of cannabis. The combination may allow opioid treatment at lower doses with fewer side effects. Opioid analgesic overdose mortality has risen, driven by increases in prescribing for chronic non-cancer pain. State medical cannabis laws in the USA have been associated with significantly lower opioid overdose mortality rates of 24.8% when compared to states without medical cannabis laws during the period of 1999 to 2010 [80]. A more specific retrospective survey of 244 medical cannabis patients found an associated 64% decrease in opioid use, decrease in side effects of medications and improved quality of life in 45% of patients [81]. More research is needed to validate these findings.
Conclusion
Medical cannabis has been used for centuries and recent evidence for pain and spasticity has lead to a resurgence of interest. The strongest clinical evidence in support of cannabinoids appears to be for cancer-related pain. Evidence has grown for use in neuropathic pain states such as HIV neuropathy and multiple sclerosis. Evidence for acute pain is mixed with no improvement to mild improvement. Chronic pain beyond neuropathic conditions has promise as seen with rheumatoid arthritis. The benefit appears to extend beyond analgesia, with notable improvements in sleep and quality of life. Headache and migraine clinical studies are lacking with anecdotal evidence and historical accounting of cannabis use for headache by Drs. Osler and OShaughnessy.
The study of cannabis as a whole plant versus selective cannabinoids such as THC is complex. The identification of multiple active ingredients which can interact in a synergistic or non-synergistic way may lead to variations in the effects as seen in studies. Cannabinoids such as cannabidiol and terpenes are agents that have analgesic benefit without the psychoactive effects of THC. Antioxidants such as flavonoids may play a role in mitigating tumor genesis from carcinogens released in the combustion of cannabis. The route of delivery of cannabis may also play a role in the analgesic effect and side effect profile. The strain of cannabis may also play a role as a wide variability is noted in the relative concentrations and types of cannabinoids and other substances. Ideal dosing for one patient to population of patients is unclear and may be difficult given a patient tolerance and pharmacokinetics may play a role in proper dose.
Further research is needed to evaluate the risks and benefits of medical cannabis. Policy and patients are forcing the US federal government to allow more medical research and alter the scheduling of marijuana from schedule I. Our current understanding is that the long-term effects of cannabis need to be addressed with rigorous analysis through the lens of the current opioid epidemic to ensure that medical use of cannabis today does not lead to an analogous situation in the futureWe should not simply replace one controlled substance with another without further analysis.
References:
Lamarine RJ. Marijuana: modern medical chimaera. J Drug Educ. 2012;42(1):111.
Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21(12):5218. Good review of cannabinoid system and landmark.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):837.
Howlett AC, Johnson MR, Melvin LS, Milne GM. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol. 1988;33(3):297302.
Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102(8):30938.
Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8(6):40321. Updated review of endocannabinoid system.
Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):53948.
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):51527. Review of cannabis plant benefits from scientists.
Banerjee SP, Snyder SH, Mechoulam R. Cannabinoids: influence on neurotransmitter uptake in rat brain synaptosomes. J Pharmacol Exp Ther. 1975;194(1):7481.
Evans AT, Formukong E, Evans FJ. Activation of phospholipase A2 by cannabinoids. Lack of correlation with CNS effects. FEBS Lett. 1987;211(2):11922.
McPartland JM, Pruitt PL. Side effects of pharmaceuticals not elicited by comparable herbal medicines: the case of tetrahydrocannabinol and marijuana. Altern Ther Health Med. 1999;5(4):5762.
Zuardi AW, Hallak JE, Crippa JA. Interaction between cannabidiol (CBD) and (9)-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berlin). 2012;219(1):2479.
Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290(13):175762.
Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6(4):71337. Updated review of basic clinical and scientific view of cannabinoids.
Walker JM, Hohmann AG, Martin WJ, Strangman NM, Huang SM, Tsou K. The neurobiology of cannabinoid analgesia. Life Sci. 1999;65(67):66573.
Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007;152(5):76577.
Strangman NM, Walker JM. Cannabinoid WIN 55,212-2 inhibits the activity-dependent facilitation of spinal nociceptive responses. J Neurophysiol. 1999;82(1):4727.
Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276(2):58593.
Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75(1):1119.
Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett. 2004;25(12):319.
Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, et al. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32(6):138490. Understanding the endocannabinoid system role in migraines.
Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124(3):30923.
Cupini LM, Costa C, Sarchielli P, Bari M, Battista N, Eusebi P, et al. Degradation of endocannabinoids in chronic migraine and medication overuse headache. Neurobiol Dis. 2008;30(2):1869.
Volfe Z, Dvilansky A, Nathan I. Cannabinoids block release of serotonin from platelets induced by plasma from migraine patients. Int J Clin Pharmacol Res. 1985;5(4):2436.
Akerman S, Holland PR, Goadsby PJ. Cannabinoid (CB1) receptor activation inhibits trigeminovascular neurons. J Pharmacol Exp Ther. 2007;320(1):6471.
Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126(1):2138. New concepts in the role of cannabinoids in gastrointestinal diseases.
Watson SJ, Benson Jr JA, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57(6):54752.
Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106(12):16972.
Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth. 2006;53(8):76975.
Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329(7460):253.
Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008;9(3):25464.
Wissel J, Haydn T, Muller J, Brenneis C, Berger T, Poewe W, et al. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain : a double-blind placebo-controlled cross-over trial. J Neurol. 2006;253(10):133741.
Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008;336(7637):199201.
Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9(2):16473.
Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110(2):60410.
Milstein SL, MacCannell K, Karr G, Clark S. Marijuana-produced changes in pain tolerance. Experienced and non-experienced subjects. Int Pharmacopsychiatry. 1975;10(3):17782.
Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology. 2013;38(10):198492.
Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59(3):26175.
Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, et al. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107(5):78596.
Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIVassociated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68(7):51521.
Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(3):67280.
Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694701.
Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):50621. One many studies support cannabis for neuropathic pain.
Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain. 2016;17(9):9821000.
Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14(2):13648.
Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005;76(12):16649. One of many studies supporting cannabis for multiple sclerosis.
Ernst G, Denke C, Reif M, Schnelle M, Hagmeister H. Standarized cannabis extract in the treatment of postherpetic neuralgia: a randomized, double blind, placebo-controlled cross-over study. Leiden: International Associatin for Cannabis as Medicine; 2005.
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):43441.
Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 N of 1 studies. Anaesthesia. 2004;59(5):44052.
Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112(3):299306.
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):8129.
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006;45(1):502.
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manag. 2010;39(2):16779. Support cannabis for cancer related pain.
Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):43849.
Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler. 2010;16(11):134959.
Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem Biodivers. 2007;4(8):172943.
Carter GT, Weydt P, Kyashna-Tocha M, Abrams DI. Medicinal cannabis: rational guidelines for dosing. IDrugs. 2004;7(5):46470. Background on developing dosing for cannabis in many routes.
Zuurman L, Ippel AE, Moin E, van Gerven JM. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 2009;67(1):521.
Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770804.
Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38(1):2143.
Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005;63(2):93100.
Hashibe M, Morgenstern H, Cui Y, Tashkin DP, Zhang ZF, Cozen W, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based casecontrol study. Cancer Epidemiol Biomarkers Prev. 2006;15(10):182934.
Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90(6):84451. Good explanation on where opioids and cannabinoids interact in a close relationship.
Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28(3):40916.
Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195209. Good review on cannabis side effects and complications.
Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J. Marijuana-laced brownies: behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol. 1988;12(4):16975.
Merrick J, Lane B, Sebree T, Yaksh T, ONeill C, Banks S. Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis Cannabinoid Res. 2016;1(1):10212.
Cooper ZD, Haney M. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend. 2016;167:11220.
Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;371(9):879. Up to date review of side effects and complication of cannabis.
Zullino DF, Delessert D, Eap CB, Preisig M, Baumann P. Tobacco and cannabis smoking cessation can lead to intoxication with clozapine or olanzapine. Int Clin Psychopharmacol. 2002;17(3):1413.
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):32760.
Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1(1):8198.
Rossato M, Pagano C, Vettor R. The cannabinoid system and male reproductive functions. J Neuroendocrinol. 2008;20 Suppl 1:903.
Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167(3):2218. Review on impact of cannabis on the pulmonary systems.
Mehra R, Moore BA, Crothers K, Tetrault J, Fiellin DA. The association between marijuana smoking and lung cancer: a systematic review. Arch Intern Med. 2006;166(13):135967.
Meier MH, Caspi A, Cerda M, Hancox RJ, Harrington H, Houts R, et al. Associations between cannabis use and physical health problems in early midlife: a longitudinal comparison of persistent cannabis vs tobacco users. JAMA Psychiat. 2016;73(7):73140.
Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence of tobacco, alcohol, controlled substances, and inhalants: basic finding from the national comorbidity survey. Exp Clin Psychopharmacol. 1994;2(3):24468.
Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res Mol Brain Res. 1998;55(1):12632.
Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: doseresponse analysis and receptor identification. J Pharmacol Exp Ther. 1999;289(2):85967.
Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 19992010. JAMA Intern Med. 2014;174(10):166873. Report of the impact on cannabis on the opioid epidemic.
Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17(6):73944.

Return to THE OPIOID EPIDEMIC
Return to SPINAL PAIN MANAGEMENT
Return to ALTERNATIVE HEADACHE TREATMENTS
Since 3-27-2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |