|
Magnetic Resonance Imaging of the Upper Cervical Spine
Detection of Atrophic Changes in Rectus Capitis Posterior Minor Muscles
Thanks to Rick Hallgren for the use of these articles!
We are currently using MRI to investigate the functional integrity
of the upper cervical spine. We started out looking for hypertonic
muscles in a population of patients who were suffering from chronic
head and neck pain. Our hypothesis was that head and neck pain
might be caused by increased muscle activity resulting in muscle
tightness. Since muscle tension can be detected by an osteopathic
physician, and increased metabolic activity can be quantified
using MRI data, we felt that we should be able to separate hypertonic
muscles from normal muscles at rest. We proposed to collect image
data from two groups of subjects: those free from both pain and
significant motion restrictions, and those having significant
motion restrictions and pain thought to be caused by abnormal,
neuromuscular conditions. We proposed to collect pixel intensity
data and then analyze these data to see if there were significant
statistical differences between the two groups. My first task
was to collect MRI data and to identify suboccipital muscles within
the MR images. After looking back and forth between the screen
of my computer terminal and an anatomy text for two days, it became
obvious that I needed some help. So I brought together a physician
and an anatomy professor to see if they could help me out. Their
comments were classic. The anatomy professor said, "The
reason you can't find those muscles is because they are not there."
The physician immediately responded by saying, "No wonder
these patients don't get any better." I had been using
images that were collected from a chronic pain patient, and it
was apparent that the rectus capitis posterior minor muscles were
missing. When we looked at images from a control subject it was
very easy to locate these muscles. At that point, the focus of
our research switched from looking for hypertonic muscles to comparing
muscle density between the control group and the chronic pain
group.
 Figure 1
Figure 1
 Figure 2
Figure 2
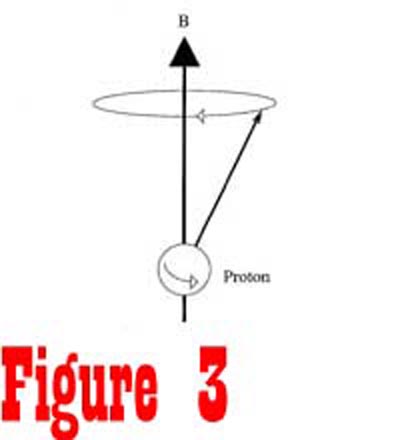 Figure 3
Figure 3
 Figure 4
Figure 4
 Figure 5
Figure 5
|
Biological tissues have an abundance of positively charged hydrogen
nuclei. We know from physics that any moving charged particle
will generate a magnetic field. Normally biological tissues do
not generate a net magnetic field because of the random orientation
of the nuclei (See Figure 1). However, if biological tissues are
placed into a magnetic field, such as exists in an MR imager,
the randomly oriented nuclei, spinning in three-dimensional space,
will align either parallel or anti-parallel to the direction of
the external magnetic field (See Figure 2). Even though the nuclei
are in a state of equilibrium with the external magnetic field,
the magnetic moment of protons contained in the tissue is not
in stable alignment with the external magnetic field. Since the
nucleus is spinning, it responds to the external magnetic forces
in the manner of a gyroscope, with its axis rotating around the
direction of the external magnetic field in a process called precession
(See Figure 3). The angular frequency of spin is known as the
Larmor frequency and is directly related to the strength of the
external magnetic field. Consequently, if the external magnetic
field increases linearly as a function of distance, the angular
frequency of nuclei would also increase linearly as a function
of distance. This characteristic is extremely important, allowing
the MR device to selectively examine points in three-dimensional
space.
Suppose we take a quick look at a simple device which could be
used to detect magnetic resonance of protons in a sample of extracellular
fluid (See Figure 4). The sample is placed between the pole pieces of a magnet that is used to generate a static magnetic field.
A radio frequency (RF) generator is used to supply a pulse of
energy to move the nuclei out of a state of equilibrium. The frequency
of the RF generator is set to be equal to the Larmor frequency
of the nuclei, which is determined by the magnitude of the static
magnetic field generated by the fixed magnet. The energy from the RF generator causes the nuclei to rotate away from the axis of the magnetic field. Figure 5 shows a spinning proton that has been given enough energy to rotate 90° away from the axis of the magnetic field. Once the RF generator is turned off, the receiver,
tuned to the Lamor frequency, can be turned to detect the signal
that is generated by the nuclei as they once again align with
the external magnetic field. At the instance that the RF generator
is turned off, the nuclei are processing in phase at a common
frequency. However, this is a transient condition since the net
magnetic moment of the sample is no longer in equilibrium with
the external magnetic field.
There are three characteristics that are primarily responsible for the intensity of the signal that is received from a sample:
1) The number of proton nuclei contained
within the sample;
2) The speed at which the magnetic moment returns to equilibrium with the external magnetic field (T1 or longitudunal relaxation);
3) The speed at which the nuclei lose phase (T2 or
transverse relaxation).
While the nuclei remain in phase and as
the net magnetic moment begins to swing back toward equilibrium,
the signal detected by the tuned receiver is relatively large.
But as the nuclei get out of phase with one another and as the
net magnetic moment approaches equilibrium, the magnitude of the
signal exponentially approaches zero. The magnitude of the signal,
along with the rate at which it decays, are determined by physical
characteristics of the sample.
Biological tissues have intrinsic characteristics that permit
reliable identification of tissue types from MR image data. In
T1-weighted images, gray scale intensity is inversely related
to the value of T1, meaning that tissues having short values for
T1 (fat and blood) produce bright images, while tissues having
long values for T1 (edema and tumors) produce dark images. In
T2-weighted images, gray scale intensity is directly related to
the value of T2, meaning that tissues having short values for
T2 (tendons, cartilage, and muscle) produce dark images, while
structures having long values for T2 (urine, edema, and fat) produce
bright images. By manipulating system parameters, the image intensity
of skeletal muscle (T1 = 900 msec, T2 = 30 msec) can be changed
relative to fatty tissues (T1 = 250 msec, T2 = 80 msec). It is
this ability to control image contrast that makes MRI so uniquely
qualified to noninvasively study musculoskeletal trauma and dysfunction.
 Figure 6
Figure 6
 Figure 7
Figure 7
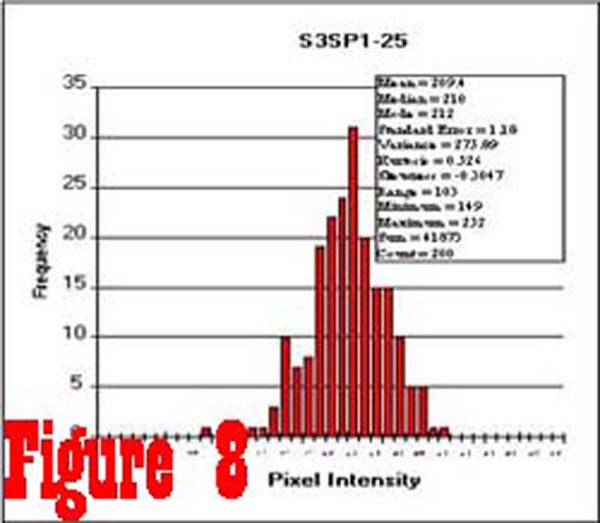 Figure 8
Figure 8
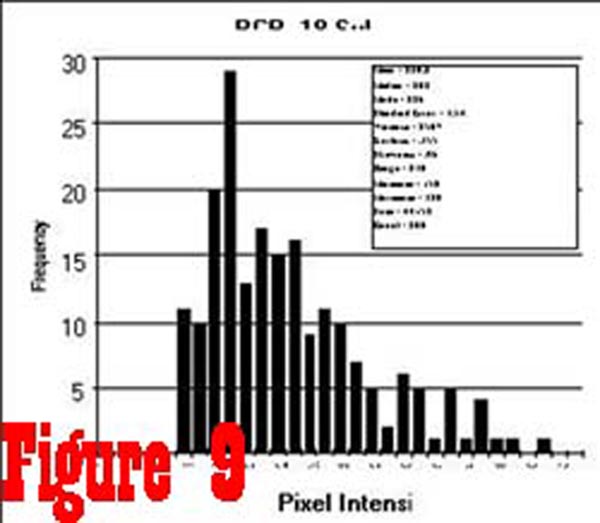 Figure 9
Figure 9
|
For all of our subjects, 4 mm thick contiguous slices of data
were collected inferior and superior to the posterior arch of
the atlas using a 1.5-Tesla superconducting magnet (SignaTM; General
Electric Medical Systems, Milwaukee, WI) with TR = 2000 msec,
TE = 25 msec, a 12 cm field of view, a 128 x 256 matrix, NEX =
2, and a total scan time approximately equal to 8.5 minutes.
Figure 6 shows image data from one subject that was considered to be
representative of the control group. The boundaries of muscles
are well defined and the muscles are of uniform intensity.
Figure 7 shows image data from one of the chronic pain subjects. There
is a significant difference between the appearance of rectus capitis
posterior major (RCPMA) muscles between the two groups. In general,
muscles from the chronic pain group have an appearance that is
characteristic of skeletal muscle that has died and been replaced
with fatty tissue. The intensity of tissue infiltrating the RCPMI
muscles in chronic pain patients in proton density-weighted (balanced)
and in T2-weighted images (TR = 2000 msec., TE = 80 msec.) follows
the intensity of structures that are known to be fat.\
Figure 8 shows a histogram of 200 samples taken from an image
of the spinal cord of a control subject. These data are typical
of spinal cord data from both control and chronic pain subjects,
and show that the distribution of pixel intensity values is, for
all practical purposes, Gaussian. The average intensity of a sample
of pixel values taken from within the spinal cord of each subject,
from eight consecutive slice levels, was used to calculate an
individual normalization factor that permitted the comparison
of muscle intensity values between subjects. Samples taken from
the RCPMI muscles of control subjects had the same Gaussian distribution
as the samples from the spinal cord. However, the histogram of
pixel intensity samples taken from the RCPMI muscles of chronic
pain subjects (See Figure 9) was found to be skewed towards
higher intensity values as a result of an increased percentage
of high intensity pixels (ie. fatty tissue).
The two most probable causes of this pathology are disuse atrophy
and neurogenic atrophy. While suboccipital muscles can be functionally
classified as extensors, their small size, relative to larger
muscles that act in parallel with them, minimizes their contribution
to motion. Consequently, if the atrophy were due to disuse then
we would expect to see atrophy of the larger, parrallel muscles
that are mainly responsible for motion. We have not seen atrophy
in any of the parallel muscles. Ruling out myopathic disease,
local trauma, and disuse atrophy, progressive wasting of skeletal
muscle, accompanied by increased excitability of its fibers, is
an indication that there is motor axonal loss consistant with
Wallerian degeneration. Disruption of the neural pathway can result
from mechanical trauma to the nerve such as occurs from a tractional
injury, or as a result of compression of the nerve at the point
that it penetrates a muscle.
The C1 dorsal ramus, arising from the C1 spinal nerve as it crosses
the superior aspect of the posterior arch of the atlas, branches
to innervate the suboccipital muscles. The C1 dorsal ramus passes
dorsolaterally through the suboccipital plexus of veins, describing
an upward arch to enter the suboccipital triangle where it divides
to form a branch for the RCPMI muscles. Most commonly the suboccipital
muscles are innervated on their dorsal surfaces, however the branch
to the RCPMI muscles may innervate its muscle ventrally or by
penetrating the RCPMA muscle. Anatomic variations such as this,
both intra- and interindividually, are commonly reported. At this
potential site of entrapment (See Figure 10), the nerve root to
the RCPMI muscles is vulnerable to traction during whiplash-type
neck distortions. The presence of abnormal spontaneous electrical activity (fibrillation
potentials and positive sharp waves), when a muscle is relaxed
and an EMG needle electrode is not being moved, is an indication
of abnormal nerve and/or muscle membrane stability that most commonly
accompanies lower motor neuron trauma that results in denervation
of skeletal muscle. As a rule, no abnormal spontaneous activity
develops in skeletal muscle weakened by disuse atrophy. Consequently,
abnormal spontaneous activity, if reproducible in at least two
muscle sites, indicates the existence of an abnormality that is
associated with denervated muscle and certain primary muscle diseases.
We have collected EMG data from three chronic pain subjects.
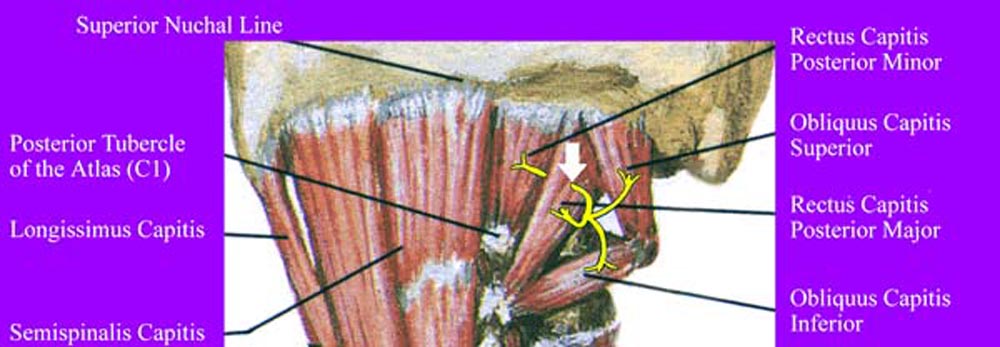
Figure 10. Innervation of suboccipital muscles by
C1 dorsal ramus. Note the course of the C1 nerve going through
the RCPMA muscle prior to innervation of the RCPMI muscle. The
white arrow shows the region of potential entrapment.
Positive sharp waves were seen in 4 areas of the RCPMI muscle
on the right side of one of the chronic pain subjects. There were
between 15-25 acceptable insertions observed on this person suggesting
that approximately 20% of all insertions would show abnormal spontaneous
activity. No fibrillation potentials were seen (See Figure 11).
On the left side of the same patient, no spontaneous activity
was seen. However, rapid repeated recruitment of the rectus capitis
posterior minor muscle with a firing rate between 25-50 hertz
in one motor unit was observed and documented (See Figure 12).
The amplitude of the motor units were a maximum of 1-1.5 k and
did not appear to be a long duration (approximately 10 msec).
The second chronic pain subject exhibited markedly reduced insertional
activity. The third chronic pain subject was not able to tolerate
the procedure.
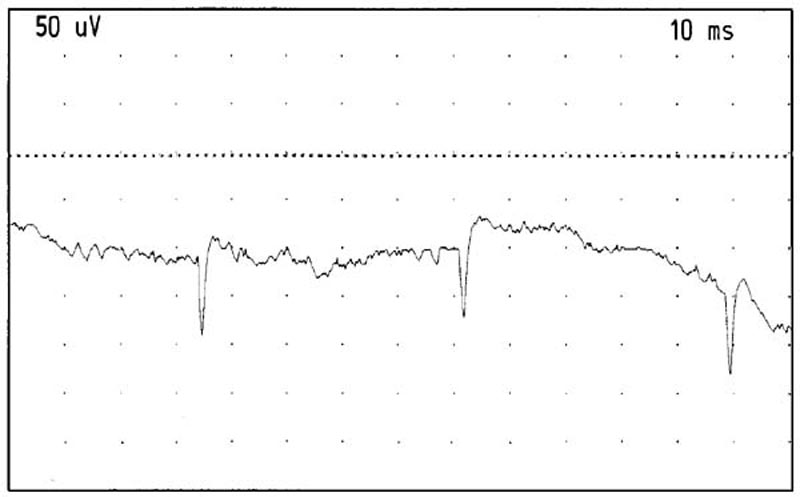
Figure 11. Positive sharp waves recorded from the
right RCPMI muscle. Gain: 50 microvolts/division. Sweep: 10 milliseconds/division.
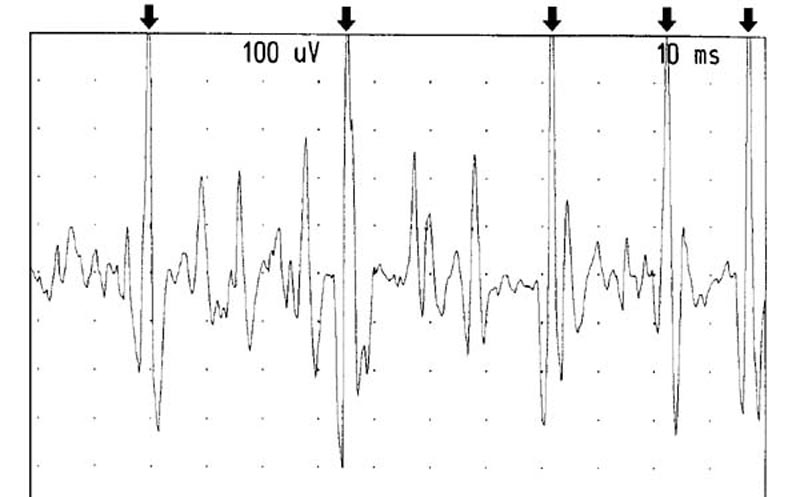
Figure 12. Recruitment from the left RCPMI muscle.
The arrows point out one motor unit firing at between 25-50 Hertz.
Gain: 100 microvolts/division. Sweep: 10 milliseconds/division.
Data were also collected from a 49 year old male control subject
with no history of whiplash or neck pain. The MRI of his rectus
capitis posterior minor muscles was normal. This subject did not
show any evidence of positive sharp waves, fibrillation potentials,
spontaneous activity or abnormal recruitment of the RCPMI muscles.
At least 20 areas of the right and left RCPMI muscles were explored
for abnormal insertional activity and spontaneous activity. Recruitment
did not show any evidence for rapid firing motor units. We were
able to isolate motor units that were approximately 1,000 mv in
size.
Detection of a Cervical Myodural Bridge
We have recently described a previously unreported connection
between the rectus capitis posterior minor (RCPMI) muscle and
the spinal dura (See Figure 13). Nowhere in Gray's Anatomy is any functional
relationship described between the RCPMI muscle and the dura mater.
The existence of a cervical myodural bridge is of special interest
to the osteopathic profession because it provides a direct physical
link between the musculoskeletal system and the dura mater.

Figure 13. Line drawing of a hemisected head showing
the region displayed in Figure 14.
A midline sagittal section was performed on a head and neck specimen
obtained from a fresh unembalmed human adult male cadaver. The RCPMI muscle was immediately visible arising from the
posterior arch of the atlas and ascending to be inserted into
the surface of the occipital bone from the inferior nuchal line
to the foramen magnum (See Figure 14). The influence of the RCPMI
muscle on the dura mater was artificially produced in the hemisected
specimen. Artificially functioning the muscle produced obvious
movement of the spinal dura between the occiput and the atlas,
and resultant fluid movement was observed to the level of the
pons and cerebellum (See Figures 15 and 16).

Figure 14. Photograph of fresh hemisected cadaveric
specimen showing:
1) posterior border of foramen magnum;
2) posterior arch of C1;
3) posterior atlanto-occipital (PAO) membrane-spinal dura complex;
4) connective tissue attaching the RCPMI muscle to the PAO membrane-spinal dura complex;
5) rectus capitis posterior minor (RCPMI) muscle.

Figure 15. Photograph of fresh hemisected cadaveric
specimen showing the spinal dura at rest with no tension on the RCPMI muscle. 1) posterior border of foramen magnum; 2) posterior
arch of C1.

Figure 16. Photograph of fresh hemisected cadaveric
specimen showing the effect upon the spinal dura when tension is applied to the RCPMI muscle.
1) posterior border of foramen
magnum; 2) posterior arch of C1.
It has been suggested that the function of the RCPMI muscle is
to provide static and dynamic proprioceptive feedback to the CNS,
monitoring movement of the head and influencing movement of the
surrounding musculature. We suggest that the RCPMI muscle may
also act to monitor and control movement (such as folding) and
tension of the spinal dura. The dura may tend to fold or pleat during extension of the cervical spine with the greatest amount of folding theoretically occurring
in the area capable of the greatest degree of extension, which
would be the atlanto-occipital joint. Consequently, the RCPMI may act to remove the dural folds thus protecting
cerebrospinal fluid hydrodynamics (flow) during head extension, and this mechanism
may be compromised when atrophy of the RCPMI muscle occurs. Hypertrophy of this connection
could result in excessive tension being placed upon the dura, resulting in pain. Pathology in a muscle having direct influence
on a pain sensitive structure suggests an alternative mechanism
for generation of cervical headache.
In a recent article in the JAOA, researchers describe the effects
of placing a physicians hands on the suboccipital region of the
cervical spine and performing a simple circular kneading to the
region. This soft tissue manipulative technique is similar to
the occipitoatlantal technique of Sutherland. The study found
that simply placing the physicians hands under the head caused
vasodilation to occur in the subject's finger. A larger increase
in pulse amplitude was observed when manipulation was applied.
Variations in digital pulse amplitude can be used as a relatively
direct and immediate index of vasomotor tone of the dermal arterioles.
The authors suggest that the this sympathetic response may occur
as a result of a perturbation of the cerebrospinal fluid resulting
from mechanical pressure.
Future Work
In order to more accurately quantify the extent of atrophy, we
will be collecting additional data using a contiguous slice, multi-echo,
multi-planar spin-echo protocol (TR=2500, TE=15/80, NEX=1.0, 192x256,
16mm FOV). We plan on reformatting the image set to obtain a new set of images that will be approximately perpendicular
to the long axis of a specified muscle. This will enable us to collect pixel intensity
data along the long axis of the muscle from a
region surrounding the very center of the muscle, thus reducing
the possibility of an operator specified region of interest that
might produce significant error due to using images that have
"cut" the muscle at an oblique angle. Figure 17 shows
a sequential series of MR images from a cervical spine data set
of a control subject, reformatted for analysis of the right, obliquus
capitis inferior muscle.
We also plan on collecting images to see if the spinal dura folds
doing extension of the cervical spine in individuals who have atrophy
of the RCPMI muscles.
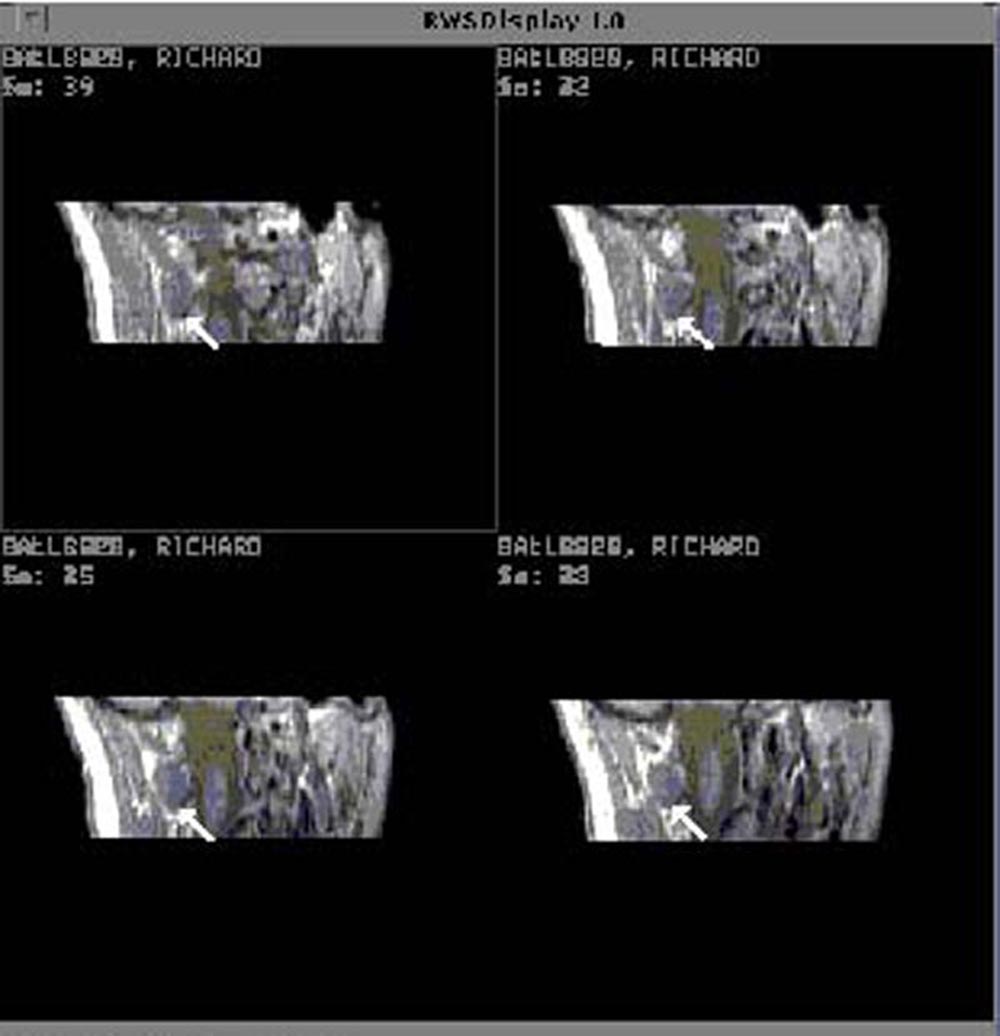
Figure 17. Sequential MR images from the cervical
spine of a control subject reformatted for analysis of the right, obliquus capitis inferior muscle.
Conclusions
Chronic pain syndromes are costly to both the individual and to
society. Even apart from the personal implications of spending
20-40 years in disabling pain, the financial burden of chronic
pain is estimated to cost our country over 60 billion dollars
annually, with most of this expense attributed to a small group
of patients who are considered to be disabled. Chronic pain syndromes
are often difficult to treat because a clear cause for the pain
is often absent. For this reason, detection of fatty infiltration
in suboccipital muscles of individuals being treated for chronic
head and neck pain is a significant finding. Equally important
is the discovery of a connection between the RCPMI muscle and
the spinal dura. The ability to identify atrophic and hypertrophic
changes in muscles of the upper cervical spine from MRI data at
an early point in the disease process could enhance a physician's
ability to initiate appropriate treatment that would prevent the
condition from progressing from the acute to the chronic stage.
Based upon preliminary data, we conclude that some individuals
suffering from chronic head and neck pain have significant infiltration
of a fatty type of tissue into suboccipital muscles, accompanied
by EMG abnormalities compatible with denervated muscles. The presence
of positive sharp waves in EMG recordings strongly suggests that
there are dennervated muscle cells in the RCPMI muscle. High frequency
recruitment in the contralateral muscle also suggests decreased
innervation of the RCPMI muscle. These findings give support to
the hypothesis that neck trauma can cause peripheral mononeuropathy
resulting in dennervation atrophy in skeletal muscle and associated
clinical symptoms of chronic pain. While the anatomic and physiologic
basis for the chronic pain is far from certain, we propose the
following possibilities:
During flexion/extension/rotation of the atlanto-axial-occipital motion segment as a consequence of whiplash-type neck distortions, there is stretching and/or contraction of the rectus capitis posterior major (RCPMA) muscles that causes traumatic constriction and/or entrapment of the Cl dorsal ramus
Damage to the Cl dorsal ramus may result in deafferentation pain. It has been reported that low back operations sometimes cause lesions to back muscle innervation, with corresponding dennervation atrophy, and that failure of paravertebral musculature has the potential to cause spinal instability that may result in low back pain.
Atrophy of the RCPMI muscles may result in decreased proprioceptive activity, resulting in dizziness and/or balance problems. Functional weakness of RCPMI muscles may also result in increased infolding of the spinal dura during movement of the head and neck which may result in pain.
Entrapment of the Cl dorsal ramus may result in ectopic pain generators that are hyper-sensitive to motion and touch. Abnormal impulses generated in peripheral nerve at the level of Cl are conducted to the trigeminocervical nucleus where they may be interpreted by the brain as painful stimuli originating from muscles, joints, and ligaments of the head and neck.

Return to HEADACHE
Return to ChiroZINE ARTICLES
|

















