

Relationship Between Early Prescription Dispensing Patterns
and Work Disability in a Cohort of Low Back Pain Workers'
Compensation Claimants: A Historical Cohort StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Occup Environ Med. 2019 (Aug); 76 (8): 573–581 ~ FULL TEXT
Nancy Carnide • Sheilah Hogg-Johnson • Mieke Koehoorn • Andrea D Furlan1 • Pierre Côté
Institute for Work and Health,
Toronto, Ontario, Canada.
OBJECTIVES: To examine and compare whether dispensing of prescription opioids, non-steroidal anti-inflammatory drugs (NSAIDs) and skeletal muscle relaxants (SMRs) within 8 weeks after a work-related low back pain (LBP) injury is associated with work disability.
METHODS: A historical cohort study of 55 571 workers' compensation claimants with LBP claims in British Columbia from 1998 to 2009 was conducted using linked compensation, dispensing and healthcare data. Four exposures were constructed to estimate the effect on receipt of benefits and days on benefits 1 year after injury: drug class(es) dispensed, days' supply, strength of opioids dispensed and average daily morphine-equivalent dose.
RESULTS: Compared with claimants receiving NSAIDs and/or SMRs, the incidence rate ratio (IRR) of days on benefits was 1.09 (95% CI 1.04 to 1.14) for claimants dispensed opioids only and 1.26 (95% CI 1.22 to 1.30) for claimants dispensed opioids with NSAIDs and/or SMRs. Compared with weak opioids only, the IRR for claimants dispensed strong opioids only or strong and weak opioids combined was 1.21 (95% CI 1.12 to 1.30) and 1.29 (95% CI 1.20 to 1.39), respectively. The incident rate of days on benefits associated with each 7-day increase in days supplied of opioids, NSAIDs and SMRs was 10%, 4% and 3%, respectively. Similar results were seen for receipt of benefits, though effect sizes were larger.
CONCLUSIONS: Findings suggest provision of early opioids leads to prolonged work disability compared with NSAIDs and SMRs, though longer supplies of all drug classes are also associated with work disability. Residual confounding likely partially explains the findings. Research is needed that accounts for prescriber, system and workplace factors.
KEYWORDS: administrative data; cohort study; low back pain; opioids; prescription dispensing; work disability; workers’ compensation
Key messages
What is already known about this subject?
Prior studies have found that early opioid provision after work-related low back injuries is associated with prolonged work disability among workers’ compensation claimants, but these studies have suffered from important biases.
What are the new findings?
Workers receiving early opioids are at a higher risk of work disability compared with workers receiving only non-steroidal anti-inflammatory drugs (NSAIDs) and/or skeletal muscle relaxants and workers receiving strong opioids also had a greater risk of work disability compared with those receiving only weak opioids.
Increasing days’ supply for all three drug classes was also associated with work disability.
How might this impact on policy or clinical practice in the foreseeable future?
While residual confounding may partially explain the findings, results suggest opioids confer no advantage over NSAIDs and muscle relaxants with respect to work disability and may in fact lead to poorer disability outcomes.
Given the risk of harms and consistent with guidelines, clinicians should avoid the use of opioids for workers in the early stages of low back pain injuries, while still ensuring adequate treatment of pain.
From the FULL TEXT Article:
Introduction
Prescription opioid use among injured workers in North America has been a significant source of concern for more than a decade. From approximately 2000 until 2010, opioid prescriptions provided to injured workers in North America rose steadily. [1, 2] While use among workers’ compensation claimants is on the decline, [1, 3, 4] opioids remain among the most commonly reimbursed prescriptions. [5] Specifically, early opioid use for work-related low back pain (LBP) has been broadly documented. [6–10]
Our previous systematic review demonstrated an association between early opioids and prolonged work disability for workers with work-related LBP. [11] However, studies were prone to exposure measurement bias (eg, incomplete prescription data, immortal time bias) and residual confounding, namely related to indication (eg, pain intensity) and preinjury and concomitant healthcare. Two subsequently published studies have been conflicting, with one finding a significant positive association [6] and the other no association. [9] Similar limitations, however, persist in these studies.
In our previous analyses, opioids, non-steroidal anti-inflammatory drugs (NSAIDs) and skeletal muscle relaxants (SMRs) were all commonly dispensed early after a work-related LBP injury, [12] a finding consistent with other LBP studies. [13, 14] These three drug classes have also been included in clinical guidelines on management of acute and subacute episodes of LBP. [15, 16] Research assessing how opioids impact work disability compared with these other clinically relevant medications has yet to be conducted, but can further provide new insights into the management of acute LBP injuries.

Figure 1 We aimed to determine and compare whether prescription opioids, NSAIDs and SMRs dispensed in the first 8 weeks following a compensated LBP injury are associated with work disability over 1 year. We hypothesised that several factors could influence both the choice of prescription and development of work disability (Figure 1). In the current analysis, we attempted to minimise the effect of residual confounding identified in previous studies by accounting for a variety of claimant-level factors. Specifically, we aimed to minimise confounding by indication due to injury severity by restricting the study sample to workers with at least one prescription and, hence, an indication for an LBP-relevant medication. Other study limitations identified in previous studies were addressed by capturing all prescriptions irrespective of payment source and using an event-based analysis to address temporality. [17]
Methods
Study design and setting
We conducted a historical cohort study (previously described12) of workers’ compensation claimants with new short-term disability claims for LBP injuries. Eligible claims were filed between 1998 and 2009 with WorkSafeBC, the provincial workers’ compensation organisation in British Columbia (BC), Canada. The portion of the workforce eligible for coverage during our study ranged from 92.5% to 94%. [18]
WorkSafeBC Claims and Firm Level Files [19] were linked with data from five administrative data sets by Population Data BC using deterministic and probabilistic matching techniques. PharmaNet is a province-wide system capturing all prescriptions dispensed from community and hospital outpatient pharmacies in BC. [20] The Medical Services Plan (MSP) Payment Information File contains data on all medically required outpatient services provided by fee-for-service practitioners. [21] Until 2001, a limited number of yearly visits to supplementary healthcare practitioners were partially reimbursed through MSP to all insured individuals. From 2002 onwards, these services were only insured in medically necessary cases for lower income individuals. The Discharge Abstract Database (DAD) contains hospital data for inpatients and day surgery patients from acute care hospitals. [22] The MSP Practitioner File contains demographic data on practitioners enrolled in MSP, [23] while the MSP Registration and Premium Billing file provides demographic information for individuals registered to receive health services in BC. [24] Data were linkable through claimant’s Personal Health Number, WorkSafeBC claim numbers and practitioner’s MSP registration numbers, and were provided at the individual level using anonymous study identifiers.
Study population
All claims were extracted for workers filing at least one new short-term disability claim between 1998 and 2009 and eligibility was first assessed at the claim level. Claims were eligible if they were accepted for a non-specific LBP disorder (online supplementary table 1), had at least 1 day of wage replacement benefits within 8 weeks following injury, were not consolidated claims (eg, duplicate claims) and had no LBP-related hospitalisation and/or serious outpatient service within 5 days after injury (online supplementary table 2). Minimum eligible age was 18 years and claimants had to be BC residents continuously eligible for health services 2 years before through 1 year after injury. Finally, injury date had to equal or precede claim registration date
From this group of eligible claims, one index claim per claimant was selected as the earliest claim where, in the year after injury, there were no other allowed claims and total benefits paid exceeded zero. Among eligible claimants, we excluded those with at least one hospitalisation and/or two outpatient billings for cancer 2 years before through 1 year after injury, as well as claimants with no prescriptions for opioids, NSAIDs or SMRs in the first 8 weeks.
Exposures
Exposure variables were constructed using dispensing data for opioids (American Hospital Formulary Service (AHFS) codes [25] 28:08.08, 28:08.12), NSAIDs (28:08.04.08, 28:08.04.24, 28:08.04.92) and SMRs (12.20.04, 12.20.08, 12.20.12). We used data from the first 8 weeks after injury as WorkSafeBC policy during the study period limited reimbursement of opioids to the first 8 weeks after injury or surgery for most claims. [26]
Four prescription dispensing exposures were constructed:Drug class(es) dispensed were categorised as NSAID(s) and/or SMR(s) (reference), opioid(s) only, and opioid(s) with NSAID(s) and/or SMR(s).
Cumulative days’ supply was calculated by summing days’ supply across all prescriptions for a given drug class (among claimants with at least 1 day’s supply). Seven days was chosen as the unit of analysis because opioids and SMRs are recommended as short course treatments and based on median days’ supply for all drug classes in this sample (8–17 days). [12]
Strength of opioid(s) dispensed (among claimants receiving at least one opioid) was categorised as weak opioids only (reference), strong opioids only, and weak and strong opioids, with strength determined relative to morphine. [27–31]
Average daily morphine-equivalent dose (MED): Daily opioid dose was converted into an MED using published ratios. [27–31] Average daily MED was calculated as the sum of the daily MED over total days supplied with opioids. This was done for oral and transdermal formulations only, as dispensed quantity for other routes (0.6% of records) was unclear. The chosen unit of analysis was 30 mg/day, as low doses of opioids are typically recommended for initial prescriptions and average daily MED in the first 8 weeks was 30 mg/day. [12]Outcomes
Days on short-term disability benefits (count variable) was calculated as the total number of days receiving short-term disability benefits from WorkSafeBC after the 8-week exposure window and up to 52 weeks after injury. Receipt of at least 1 day of short-term disability benefits (yes/no) in this same outcome window was also constructed.
Potential confoundersSociodemographic, work-related and injury-related factors Data on sex, regional health authority (Fraser Health, Vancouver Coastal Health, Vancouver Island Health, Northern Health, and Interior Health), age at injury (18–24, 25–34, 35–44, 45–54, ≥55 years) and neighbourhood income quintile were obtained from the MSP Registration File. Claimant occupation, obtained from the WorkSafeBC claim, was linked to the National Occupational Classification Career Handbook [32] to categorise occupations as having heavy physical strength requirements (ie, handling loads greater than 20 kg) and/or use of equipment/machinery/instruments (yes/no). Injury year, International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) diagnosis code (recategorised to the first three digits: 722, 724, 846, 847) and number of prior workers’ compensation claims (0, 1, ≥2) were also obtained from the WorkSafeBC Claims File.
Comorbidities and healthcare utilisation Pre-existing health conditions, including pain-related conditions, mental health and substance use disorders, and other chronic conditions, were identified where one DAD and/or two MSP records within 2 years before injury had a relevant diagnostic code (online supplementary table 3).
Dichotomous variables for preinjury spinal X-rays (year before) and surgeries (2 years before), as well as concurrent X-rays and surgery in the exposure window were defined as at least one MSP or DAD record with a relevant procedure code (online supplementary table 4). Hospitalisation in the year before injury and concurrent hospitalisation were defined as at least one DAD record with any diagnosis (yes/no).
Using MSP data, the number of outpatient physician visits in the year before injury was determined for general practitioners (GP), medical specialists commonly seen for pain-related complaints (eg, physiatry, neurology, orthopaedics, rheumatology) and other medical specialists. A dichotomous variable describing the presence of at least one pain-related specialist visit in the exposure window was also constructed.
Separate variables for the number of outpatient visits to physiotherapists, chiropractors and massage therapists in the year before injury (0, 1–5, ≥6) were also constructed, along with separate dichotomous variables describing the presence of at least one physiotherapist, chiropractor or massage therapist visit in the exposure window. This was done for claimants with injury years 1998–2001 when MSP provided limited insured benefits.
Using PharmaNet data, cumulative days’ supply of opioid, NSAID and SMR prescriptions in the year before injury was calculated by summing days’ supply across all prescriptions for a given drug class. This was also done for antidepressants (AHFS 28:16.04), anticonvulsants (28:12) and sedative hypnotics/anxiolytics (28:24), as they may be used as analgesic adjuvants in management of LBP. Separate dichotomous variables describing the presence of at least one dispense for these latter drug classes in the exposure window were also constructed.Statistical analyses
A zero-inflated negative binomial model was used to generate crude and adjusted incidence rate ratios (IRR) and corresponding 95% CIs for the association between each exposure and number of days on benefits, as well as ORs for receipt of at least 1 day of benefits. Each model was adjusted for age, sex, neighbourhood income and injury year. Confounding by other variables (previously described) was assessed using the change-in-estimate criterion, [33] using 10% as the cut-off. Specific to the exposure strength of opioid dispensed, the potential confounder of cumulative days of opioids in the prior year was separated based on opioid strength, as it was hypothesised strength of opioids received previously could influence opioids received after injury. For average daily MED, confounding by concurrent NSAID and SMR dispenses (yes/no) was considered. Finally, for cumulative days’ supply, confounding by concurrent cumulative days’ supply of the other two drug classes was considered.
In sensitivity analyses, we examined the impact of prior and concurrent supplementary healthcare as potential confounders for claimants with injury years 1998 through 2001. Data analyses were conducted using SAS software V.9.3 (SAS Institute).
Results
Study sample
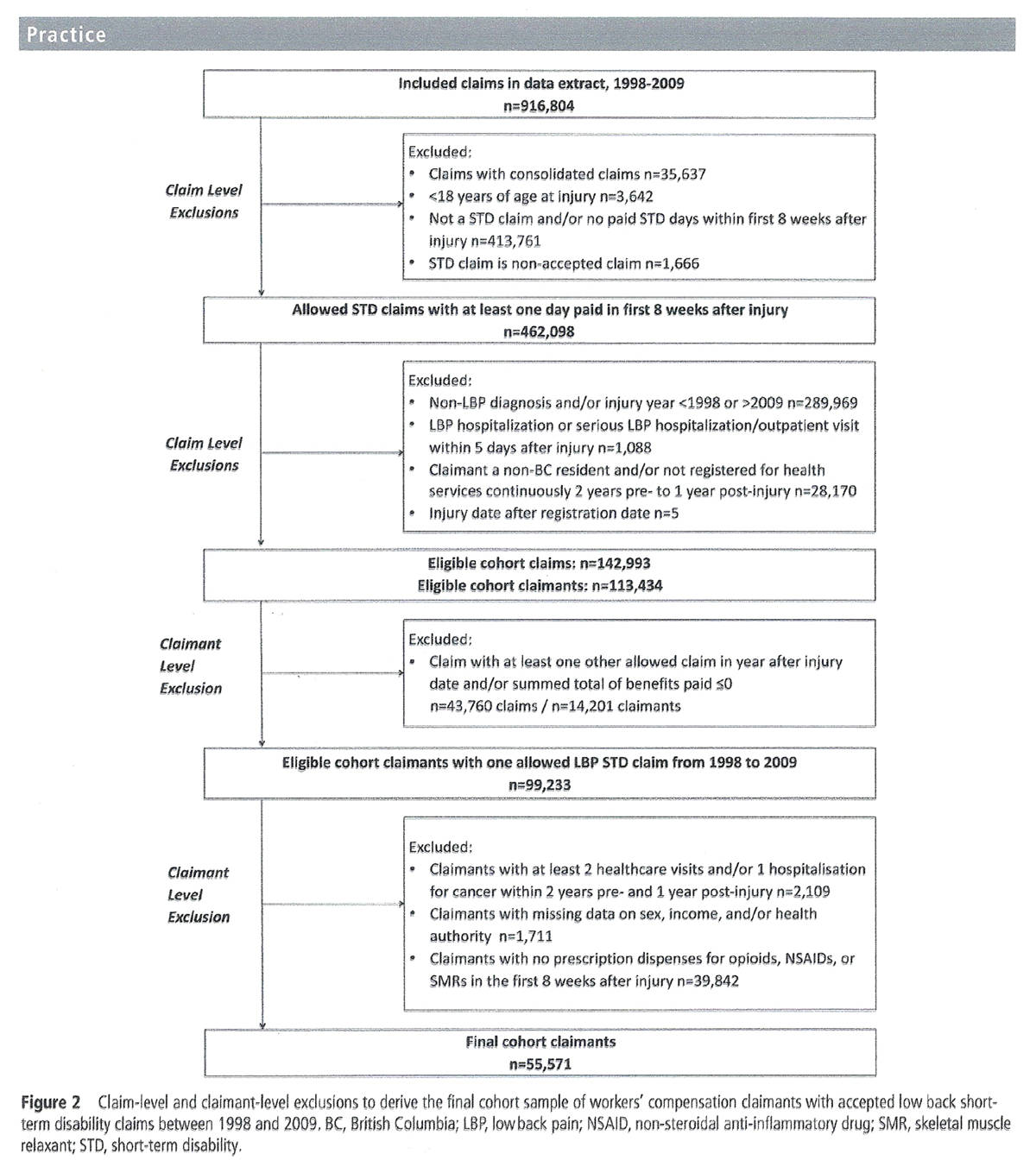
Figure 2

Table 1 Among 916,804 claims, a total of 142,993 claims were eligible, linked to 113,434 claimants (Figure 2). Selection of one index claim per claimant resulted in 99,233 claimants, from which 2,109 were excluded due to a history of cancer and 1,711 due to missing data. The sample was limited to claimants with at least one dispense within 8 weeks after injury, for a final sample of 55,571.
Most claimants were men (63.1%) between the ages of 35 and 54 years (59.2%) (Table 1 and online supplementary table 5) and 86.6% had sprains and strains. Prior workers’ compensation claims were common (32.1%) and just over half worked at a job involving heavy physical strength requirements and/or the use of equipment or machinery (57.5%). Pre-existing comorbidities and preinjury physician visits (namely GP visits) were prevalent. Spine surgeries and hospitalisations before or early after injury were uncommon, while 25.8% of claimants received spine X-rays in the first 8 weeks.
About a quarter of claimants had at least 1 day’s supply of opioids or NSAIDs, while 7.9% received an SMR in the year before injury. The proportion of claimants receiving benefits after the first 8 weeks up to 1 year after injury was 42.7%, while the median (IQR) number of benefit days during this period was 0 (IQR 0–24) days.
Days on benefits after 8 weeks

Table 2

Table 3 Compared with claimants receiving NSAIDs and/or SMRs in the first 8 weeks, the adjusted IRR for days on benefits for claimants dispensed opioids only or opioids with SMRs and/or NSAIDs in the first 8 weeks was 1.09 (95% CI 1.04 to 1.14) and 1.26 (95% CI 1.22 to 1.30), respectively (Table 2). The adjusted IRR of days on benefits after 8 weeks comparing claimants dispensed strong opioids only to those dispensed weak opioids only in the first 8 weeks (IRR 1.21, 95% CI 1.12 to 1.30) was similar to that seen for claimants dispensed a combination of weak and strong opioids (IRR 1.29, 95% CI 1.20 to 1.39).
With each 7-day increase in cumulative days supplied for opioids within 8 weeks after injury, there was a 10% increase (95% CI 1.09 to 1.11) in the number of days on benefits after the first 8 weeks. The increase was smaller for NSAIDs (IRR 1.04, 95% CI 1.03 to 1.05) and SMRs (IRR 1.03, 95% CI 1.01 to 1.04) (Table 3). For every 30 mg/day increase in daily average MED, the number of days on benefits after this period increased by 4% (95% CI 1.02 to 1.07).
Receipt of benefits after 8 weeks
There was no significant difference in the odds of receiving at least 1 day of benefits between claimants receiving opioids only and claimants receiving NSAIDs and/or SMRs in the first 8 weeks (OR 0.99, 95% CI 0.93 to 1.05), but claimants receiving opioids with NSAIDs and/or SMRs had 61% higher odds (95% CI 1.54 to 1.69) (table 2). Claimants dispensed strong opioids only (OR 1.18, 95% CI 1.05 to 1.32) or weak and strong opioids (OR 2.27, 95% CI 2.00 to 2.63) also had higher odds of receiving benefits after 8 weeks than claimants dispensed weak opioids only.
Every 7-day increase in days supplied with opioids in the first 8 weeks resulted in a 35% increase in the odds of being on benefits after that period (95% CI 1.33 to 1.39), while for NSAIDs and SMRs, the increase was 25% (95% CI 1.23 to 1.28) and 23% (95% CI 1.20 to 1.27), respectively (table 3). The relationship between average daily MED and receipt of benefits was not significant (OR 1.02, 95% CI 0.99 to 1.04).
Sensitivity analyses
Among claimants with injury years 1998 through 2001, including preinjury and concurrent supplementary care visits in the models did not have any impact on the findings (details available on request).
Discussion
Our results suggest workers with a compensated work-related LBP injury receiving early opioids are at a higher risk of work disability compared with workers receiving NSAIDs and/or SMRs, particularly those receiving opioids with NSAIDs and/or SMRs. Claimants receiving strong opioids, especially when combined with weak opioids, also had a greater risk of work disability than those receiving only weak opioids. Increasing days’ supply for all three drug classes was also associated with work disability, while increasing opioid dose in the early weeks was not.
Our findings are generally consistent with prior studies that found an increase in work disability associated with insurer-reimbursed opioid prescriptions early after compensated LBP injuries. [6, 11] However, our effect size estimates are generally smaller, particularly for days on benefits, which may be due to differences in our methodology, including our attempts to minimise the effects of confounding. We also observed some evidence that the relationship with work disability was greater for claimants dispensed opioids in combination with NSAIDs and/or SMRs and those receiving stronger opioids together with weak opioids. Claimants receiving various drug combinations may be a subgroup struggling the most with their injuries and physicians may be attempting various medications and formulations to manage their pain. Further, we also observed an association between increasing cumulative days’ supply for all drug classes and disability. Regardless of medication type, those receiving longer supplies may be experiencing greater difficulty in their recovery. Although the current study accounted for various sources of confounding that could not be measured in previous studies, the pattern of results overall suggests residual confounding by indication may partially account for the findings in this study.
Prior studies have found an increase in the risk of disability associated with increasing total MED, comparing claimants with and without opioids. [7, 10] In contrast, we examined average daily MED among claimants with at least 1 day’s supply of opioids. Our results suggest each 30 mg/day increase in dose is associated with a small, but statistically significant increased risk of prolonged disability. It should be noted that 90% of claimants had an average daily MED of 56 mg/day or less. Due to a lack of exposure variability, we may have been unable to detect a stronger relationship.
Our study has a number of strengths. In particular, we ensured separation of exposure and outcome windows to avoid immortal time bias and our prescription data were comprehensive. Our sample was restricted to claimants with at least one dispensed prescription to minimise the potential for confounding by indication and severity and we conducted comparative analyses between claimants receiving opioids and an active reference group receiving other drugs commonly prescribed for LBP. We also accounted for a variety of potential confounders that could not be addressed in previous studies. A number of factors frequently acted as confounders, including ICD-9 diagnosis, preinjury and co-occurring medications, early visits to pain specialists and early spine X-rays, which may represent markers for severity or indication.
However, as mentioned, we cannot rule out residual confounding because we were unable to control for clinical variables that could be associated with both the choice of prescription and development of disability. Therefore, we cannot rule out that injury severity, pain intensity, functional status and psychosocial factors (eg, recovery expectations) biased our results. [34, 35] The use of high-dimensional propensity scores to adjust for claimant-level confounding may be considered in future studies. [36] This methodology can be used to mine administrative health data and potentially identify confounding unknown to the investigator. In a sensitivity analysis, we built a model investigating the relationship between opioids with and without NSAIDs/SMRs versus NSAIDs/SMRs on receipt of benefits, adjusting for the confounders identified in our analyses, along with a high-dimensional propensity score. Additional adjustment for the propensity score resulted in regression estimates similar in size and direction of effect to those obtained using only traditional confounding adjustment (details available on request).
We lacked data on system and workplace factors that could influence both prescribing and work disability, such as psychosocial working conditions, availability of work accommodations after injury, insurer policies and access to non-pharmacological care. We also could not account for the impact of the physician managing the worker’s compensation claim. Prescriber comfort, satisfaction and prior experience with opioid prescribing and their perceptions about risks have been shown to influence opioid prescribing behaviour. [37, 38] Physicians also often play the role of healthcare gatekeeper in a worker’s compensation context, providing return to work recommendations, and likely vary in how they manage workers’ compensation patients and how cautious they are in their recommendations. Importantly, evidence from several studies also demonstrates physicians’ lack of adherence to LBP clinical guidelines, which may be an important contributing factor to LBP-related disability. [13, 39, 40] Future research in this area would greatly benefit from examining the role of the physicians involved in the prescribing and management of compensated injuries in influencing the work disability trajectory of claimants.
We were able to assess the influence of supplementary healthcare (ie, chiropractic, massage, physiotherapy) on the relationship between early dispensing and work disability. However, this analysis was limited to a subset of our cohort and some degree of non-differential misclassification is likely, as we were unable to capture visits paid through other means.
Despite the potential for residual confounding, it is important to consider that accounting for these factors would be unlikely to change the direction of the relationship and, instead, would likely lead to a null association. To date, no study has demonstrated any benefits of early provision of opioids on work disability outcomes after an LBP injury, including our study.
This study has additional limitations. We only had information on the number of benefit days paid by month and year that may have led to non-differential misclassification of the outcomes. We do not know whether prescriptions were actually consumed as dispensed and lacked data on over-the-counter medications. Dispensing records also lacked information on indication and prescriptions could not be attributed to the injury. However, it is still important to identify drugs claimants are exposed to as part of their disability trajectory and recovery. While this study was also conducted using data from 1998 to 2009 and current dispensing patterns may differ, this is unlikely to have affected the internal validity of the relationships examined. Finally, generalisability may be limited with respect to non-claimants or workers with no initial lost time due to injury.
Findings suggest opioids confer no advantage over NSAIDs and SMRs with respect to work disability. Residual confounding may partially explain our results and future research should consider the influence of prescriber, system and workplace factors. However, we cannot exclude the possibility that early opioid exposure after an LBP injury, in particular strong opioids, is causally related to work disability and future research is needed that elucidates the mechanism by which opioids may contribute to prolonged disability. Given the potential for serious harms and consistent with recent LBP guidelines,16 our study supports the notion that clinicians should avoid early use of opioids among injured workers with LBP injuries, while ensuring timely access to alternative pain relief measures and adequate treatment of pain.
Acknowledgments
We thank Hyunmi Lee for her indispensable assistance with data cleaning, management and analysis. The BC Ministry of Health, WorkSafeBC, PharmaNet, and the College of Physicians and Surgeons of BC approved access to and use of the data for this study facilitated by Population Data BC.
References:
Hayes S, Swedlow A.
Trends in the Use of Opioids in California’s Workers' Compensation System.
California: California Workers' Compensation Institute, 2016. Available:
https://www.cwci.org/document.php?file=2957.pdf (Accessed 23 Mar 2018)Laws C.
Narcotics in Workers Compensation.
Florida: National Council on Compensation Insurance (NCCI), 2012. Available:
https://www.ncci.com/Articles/Documents/II_narcotics-wc.pdf (Accessed 23 Mar 2018)Franklin GM, Mai J, Turner J, et al.
Bending the prescription opioid dosing and mortality curves: impact of the Washington State
opioid dosing guideline.
Am J Ind Med2012;55:325–31.doi:10.1002/ajim.21998Garg RK, Fulton-Kehoe D, Turner JA, et al.
Changes in opioid prescribing for Washington workers' compensation claimants after implementation of
an opioid dosing guideline for chronic noncancer pain: 2004 to 2010.
J Pain2013;14:1620–8.doi:10.1016/j.jpain.2013.08.001Lipton B, Colon D.
Workers compensation and prescription drugs: 2016 update.
Florida: National Council on Compensation Insurance, 2016. Available:
https://www.ncci.com/Articles/Documents/II_ResearchBrief_WC_Prescription_Drugs.pdfBusse JW, Ebrahim S, Heels-Ansdell D, et al.
Association of Worker Characteristics and Early Reimbursement for
Physical Therapy, Chiropractic and Opioid Prescriptions With
Workers' Compensation Claim Duration, For Cases of Acute
Low Back Pain: An Observational Cohort Study
BMJ Open. 2015 (Aug 26); 5 (8): e007836Franklin GM, Stover BD, Turner JA, et al.
Early opioid prescription and subsequent disability among workers with back injuries:
the Disability Risk Identification Study Cohort.
Spine2008;33:199–204.doi:10.1097/BRS.0b013e318160455cGross DP, Stephens B, Bhambhani Y, et al.
Opioid prescriptions in canadian workers' compensation claimants: prescription trends and associations
between early prescription and future recovery.
Spine2009;34:525–31.doi:10.1097/BRS.0b013e3181971Lee SS, Choi Y, Pransky GS.
Extent and impact of opioid prescribing for acute occupational low back pain in the emergency department.
J Emerg Med2016;50:376–84.doi:10.1016/j.jemermed.2015.10.015Webster BS, Verma SK, Gatchel RJ.
Relationship between early opioid prescribing for acute occupational low back pain and disability duration,
medical costs, subsequent surgery and late opioid use.
Spine2007;32:2127–32.doi:10.1097/BRS.0b013e318145a731Carnide N, Hogg-Johnson S, Côté P, et al.
Early prescription opioid use for musculoskeletal disorders and work outcomes:
a systematic review of the literature.
Clin J Pain2017;33:647–58.doi:10.1097/AJP.0000000000000452Carnide N, Hogg-Johnson S, Furlan AD, et al.
Prescription dispensing patterns before and after a workers' compensation claim: an historical cohort study
of workers with low back pain injuries in British Columbia.
J Occup Environ Med2018;60:644–55.doi:10.1097/JOM.0000000000001311Ivanova JI, Birnbaum HG, Schiller M, et al.
Real-world practice patterns, health-care utilization, and costs in patients with low back pain:
the long road to guideline-concordant care.
Spine J2011;11:622–32.doi:10.1016/j.spinee.2011.03.017Ritzwoller DP, Crounse L, Shetterly S, et al.
The association of comorbidities, utilization and costs for patients identified with low back pain.
BMC Musculoskelet Disord2006;7:72.doi:10.1186/1471-2474-7-72Wong JJ, Cote P, Sutton DA, et al.
Clinical Practice Guidelines for the Noninvasive Management of Low Back Pain: A Systematic Review
by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration
European J Pain 2017 (Feb); 21 (2): 201–216Schreijenberg M, Koes BW, Lin CC.
Guideline Recommendations on the Pharmacological Management of Non-specific
Low Back Pain in Primary Care – Is There a Need to Change?
Expert Rev Clin Pharmacol. 2019 (Feb); 12 (2): 145–157Suissa S.
Immortal time bias in pharmaco-epidemiology.
Am J Epidemiol2008;167:492–9.doi:10.1093/aje/kwm324Association of Workers’ Compensation Boards of Canada (AWCBC). 2013.
Customized KSM Report. Percentage of Workforce Covered [%]. British Columbia. 2000-2009 [data file]. Available:
http://awcbc.org/?page_id=9755WorkSafeBC [creator] (2012):
WorkSafeBC Claims and Firm Level Files. V2. Population Data BC [publisher].
Linked Data Set. WorkSafeBC. 2013British Columbia Ministry of Health [creator] (2012):
PharmaNet. V2. British Columbia Ministry of Health [publisher]. Data Extract.
Data Stewardship Committee. 2013British Columbia Ministry of Health [creator] (2013):
Medical Services Plan (MSP) Payment Information File. V2.
Population Data BC [publisher]. Data Extract. MOH. 2013Canadian Institute for Health Information [creator] (2013):
Discharge Abstract Database (Hospital Separations). V2.
Population Data BC [publisher]. Data Extract. CIHI. 2013British Columbia Ministry of Health [creator] (2013):
Medical Services Plan (MSP) Practitioner File. V2. Population Data BC [publisher].
Data Extract. College of Physicians and Surgeons of British Columbia (CPSBC). 2013British Columbia Ministry of Health [creator] (2013):
Consolidation File (MSP Registration & Premium Billing). V2.
Population Data BC [publisher]. Data Extract. MOH. 2013American Society of Hospital Pharmacists.
AHFS drug information. Bethesda, MD:
Published by authority of the Board of Directors of the American Society of Hospital Pharmacists, 2011WorkSafeBC Compensation Practice & Quality Department.
Practice Directive #C10-1. Claims with opioids prescribed.
Richmond, BC: WorkSafeBC, 2009Equianalgesic Dosing of Opioids for Pain Management.
PL detail document. Stockton, CA: Therapeutic Research Center, 2015Washington State Agency Medical Directors’ Group (AMDG).
Interagency guideline on prescribing opioids for pain. 2015. Available:
http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf (Accessed 2 Sep 2015)Compendium of pharmaceuticals and specialties.
online version (e-CPS) [Internet].www.pharmacists.ca/products-services/compendium-of-pharmaceuticals-and-specialties/
(Accessed 12 Mar 2015)Svendsen K, Borchgrevink P, Fredheim O, et al.
Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption
is a useful addition to defined daily doses.
Palliat Med2011;25:725–32.doi:10.1177/0269216311398300Von Korff M, Korff MV, Saunders K, et al.
De facto long-term opioid therapy for noncancer pain.
Clin J Pain2008;24:521–7.doi:10.1097/AJP.0b013e318169d03bHuman Resources & Development Canada.
National occupational classification career handbook.
Ottawa, ON: Government of Canada, 2011Rothman KJ, Greenland S, Lash TL.
Modern epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins, 2008Cancelliere C, Donovan J, Stochkendahl MJ, et al.
Factors Affecting Return To Work After Injury Or Illness: Best Evidence Synthesis of Systematic Reviews
Chiropractic & Manual Therapies 2016 (Sep 8); 24 (1): 32Steenstra I, Irvin E, Mahood Q, et al.
Systematic review of prognostic factors for workers’ time away from work due to acute low-back pain:
an update of a systematic review. Final report to workers compensation board of Manitoba.
Toronto, ON: Institute for Work & Health, 2011Schneeweiss S, Rassen JA, Glynn RJ, et al.
High-dimensional propensity score adjustment in studies of treatment effects using health care claims data.
Epidemiology2009;20:512–22.doi:10.1097/EDE.0b013e3181a663ccWenghofer EF, Wilson L, Kahan M, et al.
Survey of Ontario primary care physicians' experiences with opioid prescribing.
Can Fam Physician2011;57:324–32Dobscha SK, Corson K, Flores JA, et al.
Veterans affairs primary care clinicians' attitudes toward chronic pain and correlates of
opioid prescribing rates.
Pain Med2008;9:564–71.doi:10.1111/j.1526-4637.2007.00330.xSomerville S, Hay E, Lewis M, et al..
Content and outcome of usual primary care for back pain:
a systematic review
Br J Gen Pract 2008 (Nov); 58 (556): 790-797Mafi, J. N., McCarthy, E. P., Davis, R. B. & Landon, B. E. (2013)
Worsening Trends in the Management and Treatment of Back Pain
JAMA Internal Medicine 2013 (Sep 23); 173 (17): 1573–1581
Return to LOW BACK PAIN
Return to WORKERS' COMPENSATION
Since 5-21-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |
