A Risk-benefit Assessment Strategy to Exclude Cervical Artery Dissection
in Spinal Manual-therapy: A Comprehensive ReviewThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Annals of Medicine 2019 (Mar 19): 51 (2): 118–127 ~ FULL TEXT
OPEN ACCESS Aleksander Chaibi & Michael Bjørn Russell
Head and Neck Research Group,
Research Centre, Akershus University Hospital,
Oslo, Norway.Cervical artery dissection refers to a tear in the internal carotid or the vertebral artery that results in an intramural haematoma and/or an aneurysmal dilatation. Although cervical artery dissection is thought to occur spontaneously, physical trauma to the neck, especially hyperextension and rotation, has been reported as a trigger. Headache and/or neck pain is the most common initial symptom of cervical artery dissection. Other symptoms include Horner's syndrome and lower cranial nerve palsy. Both headache and/or neck pain are common symptoms and leading causes of disability, while cervical artery dissection is rare. Patients often consult their general practitioner for headache and/or neck pain, and because manual-therapy interventions can alleviate headache and/or neck pain, many patients seek manual therapists, such as chiropractors and physiotherapists. Cervical mobilization and manipulation are two interventions that manual therapists use. Both interventions have been suspected of being able to trigger cervical artery dissection as an adverse event. The aim of this review is to provide an updated step-by-step risk-benefit assessment strategy regarding manual therapy and to provide tools for clinicians to exclude cervical artery dissection.
Key messages
Cervical mobilization and/or manipulation have been suspected to be able to trigger cervical artery dissection (CAD). However, these assumptions are based on case studies which are unable to established direct causality.
The concern relates to the chicken and the egg discussion, i.e. whether the CAD symptoms lead the patient to seek cervical manual-therapy or whether the cervical manual-therapy provoked CAD along with the non-CAD presenting complaint.
Thus, instead of proving a nearly impossible causality hypothesis, this study provide clinicians with an updated step-by-step risk–benefit assessment strategy tool to
(a) facilitate clinicians understanding of CAD,
(b) appraise the risk and applicability of cervical manual-therapy, and
(c) provide clinicians with adequate tools to better detect and exclude CAD in clinical settings.
There are more articles like this @ our:
STROKE AND CHIROPRACTIC PageKEYWORDS: Cervical artery dissection; carotid artery dissection; manipulation; manual-therapy; stroke; vertebral artery dissection
From the FULL TEXT Article:
Introduction
Cervical artery dissection (CAD) refers to a tear in the internal carotid artery (ICA) or the vertebral artery (VA), resulting in an intramural haematoma and/or an aneurysmal dilatation, which can ultimately be detrimental to the individual. The pathophysiology of CAD is not fully understood, but multiple coexisting pathological processes leading to a predisposing weakness of the arterial wall, namely, a large aortic root diameter, increased stiffness of the carotid wall, material and circumferential wall stress, hypertension, endothelial dysfunction, and arterial redundancies, have been proposed to increase the risk of CAD. [1]
Fortunately, the incidence rate of CAD is relatively low, estimated at 2.9/100,000 individuals per year in the general population. [2] Internal carotid artery dissections (ICADs) occur approximately 3–5 times more frequently than vertebral artery dissections (VADs). [3, 4] This corresponds to 9476 new American CAD cases and 21,537 new European CAD cases per year. CAD usually only occurs once.
Ischaemic stroke is the most common clinical symptom in CAD, and the risk of subarachnoid haemorrhage (SAH) is greater in intracranial artery dissection than in CAD. [5] However, there is also a selection bias towards those with CAD without subarachnoid haemorrhage being recruited through neurology departments, whereas recruitment through departments of neurosurgery or interventional neuroradiology is biased towards CAD with SAH [5]. A French prospectively neurological department study in tertiary health care found clinical symptoms to occur in 156 (92%) of 169 patients with spontaneous VAD, i.e. stroke in 114 (67%), TIA in 17 (10%), and occipital head and/or neck pain alone in 21 (12%) patients, SAH without ischaemia were found in three (2%) patients, and sensorimotor cervical radiculopathy C5/ C6 in one patient (1%). Three of the patients with ischemic stroke also showed signs of SAH on brain imaging. [6] A Swiss prospectively neurological department study in tertiary health care found clinical symptoms to occur in 290 (97%) of 298 patients with spontaneous ICAD, i.e. stroke in 165 (55%), TIA in 37 (12%), amaurosis fugax in 8 (3%), local symptoms, and signs (headache, neck pain, Horner’s syndrome and nerve palsy) in 80 (27%), while eight (3%) were asymptomatic. [7]
The prognosis of disability after CAD at 3 months is good for 89% of cases, with 91% for ICAD and 88% for VAD, according to the modified Rankin score (mRS 0–2): (0) no symptoms, (1) no significant disability despite symptoms and able to carry out all usual duties and activities, and (2) slight disability, unable to carry out all previous activities, but able to look after own affairs without assistance. [2, 8] The mortality rate is less than 4% [1, 4]: mean 5.1% (SD 7.2) for ICAD [2, 9–21] and mean 1.3% (SD 2.3) for VAD [2, 14, 16, 18, 20–22], calculated by the authors. The mean age of CAD patients is 44 years (46 years for men and 41 years for women [23], with a slight male preponderance (55%). [24] CAD is extremely rare in children and adults beyond the age of 65 years. [25]
Headache and/or neck pain are the most common initial symptoms of CAD. [26] Other symptoms are Horner’s syndrome and lower cranial nerve palsy. [4] The headache is often new and unilateral, exhibits sudden onset, and may resemble a migraine or cluster headache. [27] The time from initial symptoms to ischaemic stroke varies from minutes to few weeks. [28]
With headaches and/or neck pain as common initial physical symptoms, many patients seek manual therapy to alleviate the pain. [29–34] Headaches and neck pain have also been among the leading global causes of disability within the last decade. [35] Although CAD is thought to occur spontaneously, physical trauma to the neck, especially traumas involving hyperextension and rotation, has been suspected to trigger CAD. [4, 36, 37] Some case reports have suggested that CAD may be an adverse event (AE) following manual therapy that includes cervical mobilization and/or manipulation intervention. [38–45]
However, although some have argued that the presenting symptoms of headaches and/or neck pain in manual-therapy settings are symptoms that are caused by a CAD in progress, questions remain as to whether the manual technique is directly responsible for CAD or can worsen a CAD in progress and, importantly, whether the diagnosis of CAD is overlooked prior to the manual intervention.
It is paramount that all clinicians, especially manual therapists who utilize cervical mobilization and/or manipulation techniques, are well informed of the possible red flags and are capable of referring patients to essential medical examinations and treatments (anticoagulants or antiplatelet drugs) before initiating manual-therapy interventions, as recommended by the American Heart and Stroke Association. [46]
The objective of this review is to present an updated step-by-step risk–benefit assessment strategy tool to (a) provide clinicians with knowledge and adequate tools to better exclude CAD and (b) appraise the risk and applicability of cervical manual therapy.
Manual therapy does not result in an increased risk of CAD
The World Health Organization regards manual mobilization and/or spinal manipulative treatment conducted by chiropractors to be a safe and effective treatment with few, mild, transient AEs [47], such as local soft tissue tenderness and tiredness on the treatment day. [48–55] A few case studies have reported serious AEs following cervical spinal manipulative therapy (SMT) [56–64], but whether there is a causal relationship between cervical SMT and CAD has not been determined because of the methodological design, low level of evidence and low prevalence. [40, 42, 43]
The lack of established causality relates to the chicken and egg discussion, i.e. whether the CAD symptoms lead the patient to seek cervical SMT or whether the cervical SMT provokes CAD along with the non-CAD presenting headache and/or neck complaint. The infrequent reporting of serious AEs in prospective manual-therapy randomized controlled trials might be a true figure or an underestimation. [39, 42, 44, 48–55, 65–67] The rarity of CAD also makes the provision of epidemiological evidence challenging. However, several extensive cohort studies and metaanalyses have found no excess risk of CAD resulting in secondary ischaemic stroke for chiropractic SMT compared to primary care follow-up. [39, 44, 68] Similarly, retrospective cohort studies have reported no association with traumatic injury to the head or neck after SMT for neuromusculoskeletal pain. [69] Invasive studies have further disproven any misconception as to whether VA strains during head movements, including SMT, exceed failure strains. [70, 71] No changes in blood flow or velocity in the VA of healthy young male adults were found in various head positions and during a cervical SMT. [72] Thus, these studies support the evidence of spontaneous causality or minimally suggest a very low risk for serious AEs following SMT. [41, 73, 74]
Thus, there is no strong evidence in the literature that manual therapy provokes CAD. However, to provide evidence-based knowledge on AEs, all health personnel must record AEs observed in all their patients in relation to the treatments they provide.
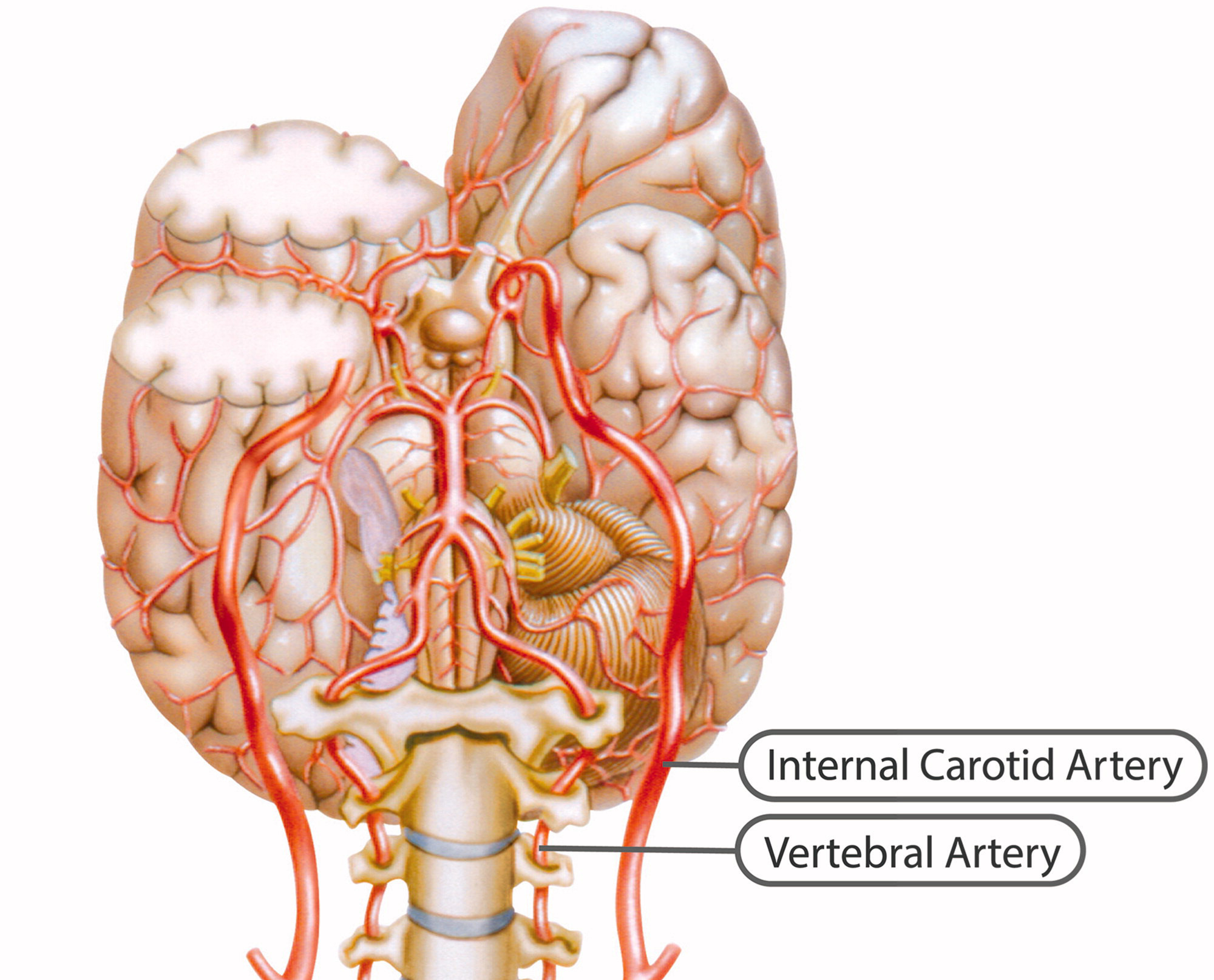
Internal carotid artery

Figure 1

Figure 2 The ICA is a terminal branch of the common carotid artery and arises around the level of the fourth cervical vertebra, where it continues intracranially to join the circle of Willis (Figure 1). The ICA moves freely within the cervical pathway and enters the carotid canal first above the first cervical vertebrae, where it becomes fixed. [75] The ICA provides the most significant proportion of blood to the brain, specifically the anterior portion that includes the retina, while the external carotid artery supplies the face, scalp, skull, and meninges. Any disruption to the anterior circulation can cause retinal and/or cerebral ischaemic symptoms, namely, hemiparesis, hemisensory loss, neglect, aphasia, gaze deviation, dysarthria, and monocular visual loss. The location of pain symptoms from an ICAD can vary, but neck pain commonly involves the periorbital, frontal, or upper anterior cervical region and is unilateral and ipsilateral to the affected cervical artery (Figure 2). The headache can resemble migraine or cluster headaches with its unilateral pain location. [25]
However, neither a migraine nor cluster headache are characterized by pain in the upper cervical region, with migraines often presenting with accompanying symptoms such as nausea, vomiting, and photo- and phonophobia, while cluster headaches have one or more associated symptoms such as ipsilateral tearing, injection, rhinorrhoea, nasal stenosis, miosis, and/or ptosis. [27] Symptoms of ICAD can also be mild and, thus, may only be detected by coincidence, while asymptomatic cases are not diagnosed. When local symptoms are due to compression of adjacent structures, such as by aneurysm formation, peripheral cranial nerve involvement becomes apparent, commonly as N. hypoglossus and less commonly as N. accessories, N. vagus, or N. glossopharyngeus, as these cranial nerves are in close proximity to the carotid artery in the cervical region. An intracranial ICA rupture can cause subarachnoid haemorrhage, which causes a sudden onset of a severe headache. Thus, based on the ICA location, it is highly unlikely that mobilization and manipulative techniques that tend to be specific with minimal force and amplitude affect the ICAs.
Vertebral artery
The VAs supply blood to the posterior portion of the brain. Once the VAs project into the skull, the two VAs merge to form the basilar artery, which in turn feeds into the circle of Willis as the a. cerebri posteriors (Figure 1). Any disruption to the posterior circulation might, therefore, produce brainstem ischaemic symptoms, namely, ipsilateral loss of pain and contralateral temperature sensation in the body, ipsilateral hemiparesis, nausea, vomiting, vertigo, nystagmus, diplopia, dysphagia, dysarthria, dysphonia, and/or cerebellar ischaemic symptoms, such as ataxia, vertigo, and/or nystagmus. The location of pain symptoms in VAD varies. However, neck pain commonly involves the unilateral suboccipital region and is ipsilateral to the affected artery (Figure 2), while the character of the new and sudden onset headache is similar to that of ICAD but with a unilateral and suboccipital location. [25, 27]
As the VA runs through the intervertebral foramen of the six cervical vertebrae (C1–C6), the VA is more vulnerable to mechanical stress than the ICA. The VA is thought to be most susceptible to injury due to extreme rotatory head movements, especially in the transverse foramen in the first cervical vertebrae, as the VA abruptly transitions from a vertical path to a horizontal orientation (Figure 1). [76] Previous neck trauma has also been more commonly reported in VAD than in ICAD, which probably also relates to the vulnerable location of the artery. However, from a pragmatic view, all people execute several different head and neck movements every day, including side-to-side neck rotations that consequently stretch the VA. Fortunately, this usually does not trigger CAD.
Neck pain and CAD
Neck pain is often not specified in the CAD literature. By definition, neck pain is a subjective and personal experience, and in contrast to CAD, the first experience with neck pain often occurs early in life. [77] The 1–year prevalence of neck pain is up to 50% in the adult population, and up to 40% of the general population report having experienced neck pain in the past month. [78] Usually, neck pain presents in episodes during a lifetime with variable degrees of recovery between episodes. [77] However, when coexisting CAD is responsible for neck pain, the pain is often sudden, sharp, severe, steady and different from previously experienced neck pain. [79, 80] In general, pain due to a vascular condition tends to present as throbbing, pounding, pulsing, and/or beating, while musculoskeletal pain conditions usually have an aching, sore, heavy, hurting, deep, cramping, and/or dull character. [81]
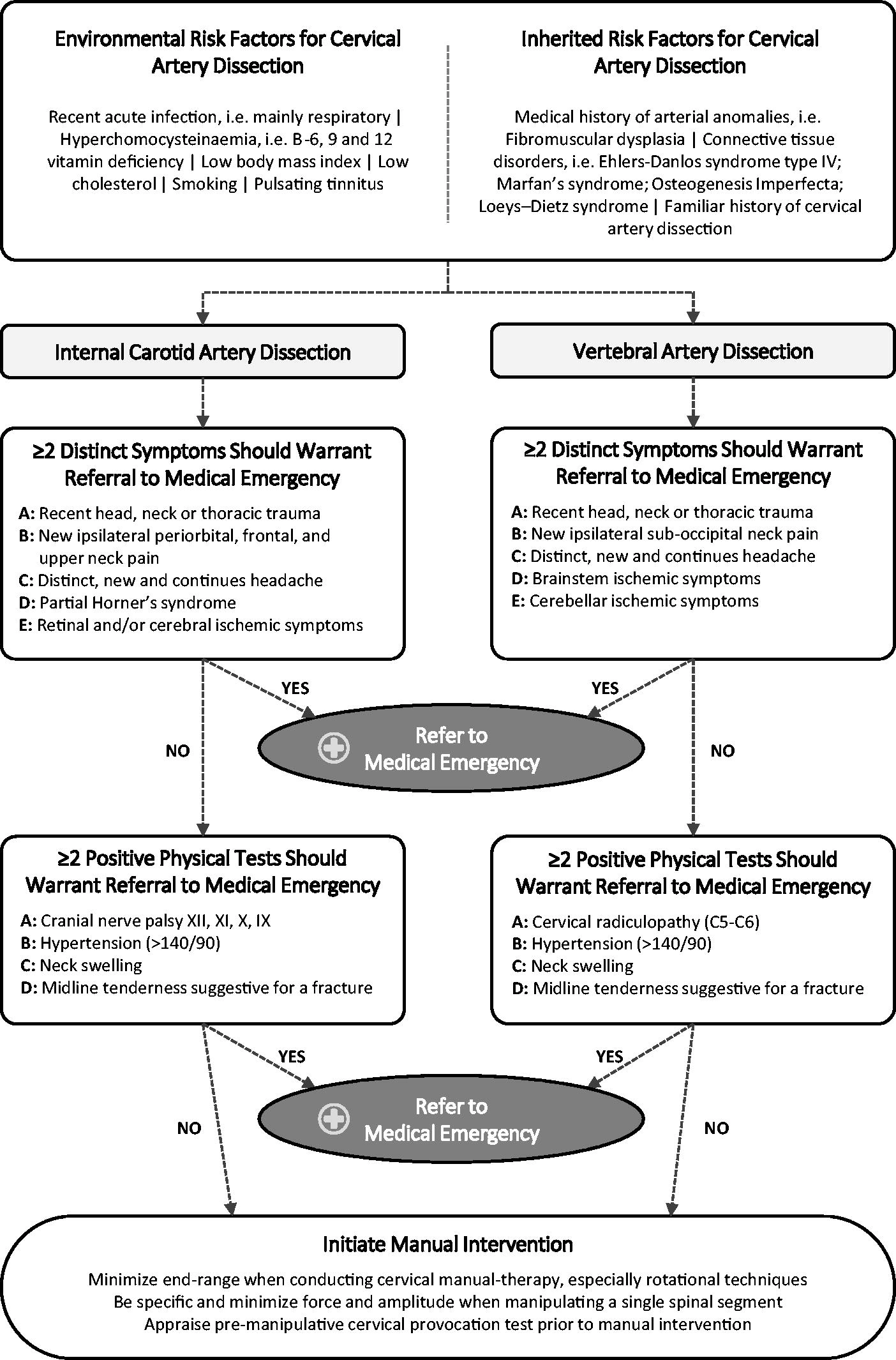
Figure 3 The Bone and Joint Decade 2000–2010 Task Force on neck pain presented evidence-based guidelines for primary care clinicians to inform their assessments of neck pain [78]: For grade I, complaints of neck pain may be associated with stiffness or tenderness but no significant neurological complaints; for grade II, neck pain interferes with daily activities, but no signs or symptoms are evident to suggest major structural pathology or significant nerve root compression; for grade III, complaints of neck pain are associated with significant neurological signs; and for grade IV, neck pain includes complaints of neck pain and/or its associated disorders, and the examining clinician detects signs or symptoms suggestive of major structural pathology. A similar classification was proposed by the Quebec Task Force on whiplash-associated disorders but with a grading system from 0 to 4: For grade 0, no complaint of neck problems and no physical sign(s); for grade 1, neck pain, stiffness, or tenderness, with no physical sign(s); for grade 2, neck pain, stiffness, or tenderness, with musculoskeletal sign(s); for grade 3, neck pain, stiffness, or tenderness, with neurological signs; and for grade 4, neck fracture or dislocation. [82] Thus, for both classifications, grades 3 and 4 should warrant alertness by the clinician regarding possible CAD, and a careful history and thorough examination for other neurological signs should be obtained and performed as outlined in Figure 3.
Unfortunately, most clinical examinations have little or no proven usefulness, unless radiculopathy or serious structural disease is strongly suspected. [83] Musculoskeletal pain is often reproducible, namely, the pain can be provoked by active or passive provocative movements and relieved by certain movements that diminish the muscle tension, which is not the case if the pain is of vascular origin. Furthermore, vascular pain is not typically relieved by analgesics, but in VAD, the pain progresses around its known location, which is often occipital and medially along the nucheal line (Figure 2). [84] Musculoskeletal neck stiffness often presents with a reduced rotational and/ or lateral flexion range of motion with sharp, stabbing and/or stinging pain characteristics. A recent study of patients with acute neck pain investigated 1,235 distinct cervical SMTs conducted by chiropractors and found that 74% of the cervical SMTs were conducted in the lower cervical spine. [85] Thus, although the literature is sparse on specific locations of mechanical musculoskeletal pain, mechanical neck pain appears to usually present in the lower cervical spine.
Thus, with common symptoms such as neck pain, stiffness, and/or tenderness, without a change in response to mechanical provocation manoeuvres, the clinician should be alerted to a possible red flag. In situations where neurological deficits are also present along with the history of the musculoskeletal complaint, CAD should be suspected until proven otherwise (Figure 3).
Risk–benefit identification strategy assessment tool
The initial signs of CAD can be very difficult to detect. Therefore, a multifactorial approach must be considered when evaluating the risk–benefit ratio of any manual intervention, including mobilization and/or manipulation, with specific emphasis on history taking and the physical examination, in relation to a possible latent CAD. As there are known important risk factors for CAD that have been shown to increase the incidence rate, clinicians and, especially, manual therapists should specifically be aware of the importance of history taking (Figure 3).
That is, environmental risk factors such as recent acute respiratory infection [1, 4]; hyperhomocysteinaemia, namely, B-6, -9, and 12 vitamin deficiency [1, 4]; a low body mass index and low cholesterol [1, 4]; smoking [1]; and pulsating tinnitus. [4] While inherited risk factors include medical and/or family history of arterial anomalies and/or CAD, respectively [1, 4], and connective tissue disorders, i.e. Ehlers–Danlos syndrome type IV, Marfan’s syndrome, Osteogenesis Imperfecta, or Loeys–Dietz syndrome (Figure 3). [1, 4]
A significant amount of information is available to clinicians to help them understand and evaluate the risk–benefit ratio for any cervical manual intervention and the exclusion of CAD. Thus, Figure 3 outlines a risk–benefit assessment strategy that should provide additional knowledge and improve the vigilance of all clinicians to enable them to exclude CAD, refer patients with suspected CAD to appropriate care, and consequently prevent CAD from progressing. One report has argued that most patients present with at least two physical symptoms. [86]
The clinical characteristics and recommendations in Figure 3 follow this assumption. This figure is intended to function as a knowledge base that should be implemented in preliminary screening and be part of good clinical practice. This knowledge base will likely contribute to sharpening the attention of the clinician and alert him or her as to whether the presenting complaint, combined with a collection of warning signs listed in Figure 3, deviates from what he or she considers to be a usual musculoskeletal presentation.
Discussion
This review outlines a systematic approach to exclude a serious and potential life-threatening disorder, such as CAD, within primary care and, especially, the manualtherapy clinical settings. The risk–benefit analysis tool is based on the most recent evidence and should benefit clinicians to help them better understand the many warning signs, especially if the presenting complaint deviates from what they consider to be the usual musculoskeletal presentation. Thus, this approach should help clinicians refer patients to medical examinations and treatment immediately upon a suspicion of CAD.
Considering the rarity of CAD, which reportedly constitutes as few as 1 per 8.1 million chiropractic office visits and 1 per 5.9 million cervical manipulations by practising chiropractors in Canada [38], conducting sufficiently powered clinical manual-therapy randomized controlled trials to evaluate causality is nearly impossible. Naturally, one can suggest that to scientifically establish the prevalence of CAD as a direct trigger of cervical mobilization and/or manipulation intervention, a prospective study would need to include 1000 manual therapists treating the cervical spine 100 times per week for 52 weeks. As a consequence, most manual-therapy randomized controlled trials have abundant type II errors.
The assumption that the cervical manual-therapy intervention triggers CAD in rare cases has been dominated by single-case reports and retrospective case series or surveys from neurologists who naturally lack substantial methodological quality to establish definitive causality. [87] These neurological case reports have probably contributed to an over-reporting of serious and catastrophic AEs compared to minor and moderate AEs, which are likely to occur more frequently. [55, 88]
In light of the evidence provided in this comprehensive review, the reality is(a) that there is no firm scientific basis for direct causality between cervical SMT and CAD;
(b) that the ICA moves freely within the cervical pathway, while 74% of cervical SMTs are conducted in the lower cervical spine where the VA also moves freely;
(c) that active daily life consists of multiple cervical movements including rotations that do not trigger CAD, as is true for a range of physical activities; and
(d) that a cervical manipulation and/or grade C cervical mobilization goes beyond the physiological limit but remains within the anatomical range, which theoretically means that the artery should not exceed failure strain.These factors underscore the fact that no serious AE was reported in a large prospective national survey conducted in the UK that assessed all AEs in 28,807 chiropractic treatment consultations, which included 50,276 cervical spine manipulations. [50]
While no mechanical event is reported in one-third of patients, spontaneous CAD has been reported to be caused by minor traumas to the neck from various activities, namely, skating, tennis, basketball, volleyball, swimming, scuba diving, weight lifting, dancing, yoga, jumping on a trampoline, roller coaster rides, sexual intercourse, coughing or sneezing and dental procedures, while traumatic CAD, a major trauma is present, namely, neck fractures, spinal cord injuries, and muscular tears. [36, 37, 89] Minor traumas together with a recent infection may contribute to the hypothesized direct injury or weakness in the vessel wall, respectively, while increased systolic blood pressure can contribute to arterial stiffness. [90] Thus, these factors must be incorporated in the risk–benefit assessment and in the evaluation of the true musculoskeletal problem presentation versus a latent CAD.
An intervention with a proven effect for a range of musculoskeletal disorders cannot be without risk. However, instead of proving a nearly impossible causality hypothesis that today is based on insufficient and poorly descriptive case studies, focus should be directed to the early detection and exclusion of CAD, and questions should be raised on how to minimize the risk.
History taking, especially regarding the time of symptom onset, is the single most important factor for detecting subtle symptoms of CAD; thus, primary care clinicians and, especially, manual therapists should dedicate enough time during the first consultation to allow for thorough history taking and physical examination. During history taking, follow-up of the patient’s answers in relation to his or her (new) neck pain and/or headache is extremely important to obtain sufficient knowledge and understanding, and one must not accept a simple yes or no answer. In cases with suspicion of high-risk CAD, which contain a combination of several warning signs, there should be an immediate referral to the medical emergency department. If time allows, this referral can include a short description of the clinician’s report of his or her findings. In time, this vigilant act by a professional may contribute to closer cooperation and mutual respect between physicians and manual therapists, which is highly important for improving the efficiency of today’s healthcare and ultimately benefits patients.
There is no sufficient evidence to support cervical VA tests to identify patients with a higher risk, and the validity and reliability of these tests are low. [91] A recent guideline by the Australian Physiotherapy Association suggests that sustained cervical rotation might be an alternative test to assess for blood flow insufficiency in a controlled way. [92] However, the validity and reliability of this new test conforms to the same uncertainties as previous tests for vascular insufficiency. [91] Thus, in the absence of a physical vascular screening test, manual therapists would need to rely on a thorough history and clinical reasoning to follow the principles of do-no-harm.
Although the chiropractic profession evolved in the early nineteen hundreds as an art, philosophy, and science, neck manipulation should not resemble a martial art. Thus, when cervical manipulation techniques are being conducted, one must be specific when manipulating a single spinal segment, minimizing the end range in cervical techniques, especially rotational techniques, and minimizing force, all of which have been recommended to reduce the risk of serious AEs. [93] We propose that considering the risk–benefit assessment tool outlined in Figure 3, clinicians will consequently enhance decision-making with respect to differential diagnoses and enable the detection of a larger number of latent and possible progressive CADs, regardless of the actual manual intervention.
Disclosure statement
The authors have completed the ICMJE uniform disclosure form and declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The first and lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies in the study as originally planned have been explained.
REFERENCES:
Debette S.
Pathophysiology and risk factors of cervical artery dissection:
what have we learnt from large hospital-based cohorts?
Curr Opin Neurol. 2014;27:20–28.Bejot Y, Daubail B, Debette S, et al.
Incidence and outcome of cerebrovascular events related to cervical artery dissection:
the Dijon Stroke Registry.
Int J Stroke. 2014;9:879–882.Hart RG, Easton JD.
Dissections of cervical and cerebral arteries.
Neurol Clin. 1983;1:155–182.Debette S, Leys D.
Cervical-artery dissections: predisposing factors, diagnosis, and outcome.
Lancet Neurol. 2009;8:668–678.Debette S, Compter A, Labeyrie MA, et al.
Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection.
Lancet Neurol. 2015;14:640–654.Arnold M, Bousser MG, Fahrni G, et al.
Vertebral artery dissection: presenting findings and predictors of outcome. Stroke;
J Cerebral Circ. 2006;37:2499–2503.Georgiadis D, Arnold M, von Buedingen HC, et al.
Aspirin vs anticoagulation in carotid artery dissection: a study of 298 patients.
Neurology. 2009;72: 1810–1815.Banks JL, Marotta CA.
Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials:
a literature review and synthesis.
Stroke. 2007;38:1091–1096.Mokri B, Sundt TM Jr, Houser OW, et al.
Spontaneous dissection of the cervical internal carotid artery.
Ann Neurol. 1986;19:126–138.Bogousslavsky J, Despland PA, Regli F.
Spontaneous carotid dissection with acute stroke.
Arch Neurol. 1987;44:137–140.d’Anglejan Chatillon J, Ribeiro V, Mas JL, et al.
[Dissection of the extracranial internal carotid artery. 62 cases].
Presse Medicale (Paris, France: 1983). 1990; 19:661–667.Pozzati E, Giuliani G, Acciarri N, et al.
Long-term follow-up of occlusive cervical carotid dissection.
Stroke. 1990;21:528–531.Ast G, Woimant F, Georges B, et al.
Spontaneous dissection of the internal carotid artery in 68 patients.
Eur J Med. 1993;2:466–472.Schievink WI, Mokri B, O’Fallon WM.
Recurrent spontaneous cervical-artery dissection.
N Engl J Med. 1994; 330:393–397.Treiman GS, Treiman RL, Foran RF, et al.
Spontaneous dissection of the internal carotid artery: a nineteenyear clinical experience.
J Vasc Surg. 1996;24:597–605. Discussion 605–7.Bassetti C, Carruzzo A, Sturzenegger M, et al.
Recurrence of cervical artery dissection. A prospective study of 81 patients. Stroke;
J Cerebral Circ. 1996;27: 1804–1807.Engelter ST, Lyrer PA, Kirsch EC, et al.
Long-term follow-up after extracranial internal carotid artery dissection.
Eur Neurol. 2000;44:199–204.Touze E, Gauvrit JY, Moulin T, et al.
Risk of stroke and recurrent dissection after a cervical artery dissection:
a multicenter study.
Neurology. 2003;61:1347–1351.Kremer C, Mosso M, Georgiadis D, et al.
Carotid dissection with permanent and transient occlusion or severe stenosis: long-term outcome.
Neurology. 2003; 60:271–275.Arauz A, Hoyos L, Espinoza C, et al.
Dissection of cervical arteries: long-term follow-up study of 130 consecutive cases.
Cerebrovasc Dis. 2006;22:150–154.de Bray JM, Marc G, Pautot V, et al.
Fibromuscular dysplasia may herald symptomatic recurrence of cervical artery dissection.
Cerebrovasc Dis. 2007;23: 448–452.Mokri B, Houser OW, Sandok BA, et al.
Spontaneous dissections of the vertebral arteries.
Neurology. 1988; 38:880–885.Metso AJ, Metso TM, Debette S, et al.
Gender and cervical artery dissection.
Eur J Neurol. 2012;19:594–602.Biller J, Sacco RL, Albuquerque FC, et al.:
Cervical Arterial Dissections and Association With Cervical
Manipulative Therapy A Statement for Healthcare Professionals
From the American Heart Association/ American Stroke Association
Stroke. 2014 (Oct); 45 (10): 3155–3174Blum CA, Yaghi S.
Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome.
Arch Neurosci. 2015;2:e26670.Olesen J, Goadsby PJ, Ramadan NM, et al.
The headache. 3rd ed. USA:
Lippincott Williams & Wilkins; 2006.ICHD.
Headache Classification Committee of the International Headache Society (IHS)
The International Classification of Headache Disorders, 3rd edition.
Cephalalgia. 2018;38:1–211.Cadena R.
Cervical artery dissection: early recognition and stroke prevention.
Emerg Med Pract. 2016;18: 1–24.Chalbi, A, Tuchin, PJ, and Russell, MB.
Manual Therapies for Migraine: A Systematic Review
J Headache Pain. 2011 (Apr); 12 (2): 127–133Chaibi A, Russell MB.
Manual Therapies for Cervicogenic Headache: A Systematic Review
Journal of Headache and Pain 2012 (Jul); 13 (5): 351–359Chaibi and Russell, 2014
Manual Therapies for Primary Chronic Headaches:
A Systematic Review of Randomized Controlled Trials
J Headache Pain. 2014 (Oct 2); 15: 67Gross A, Langevin P, Burnie SJ, et al.
Manipulation and mobilisation for neck pain contrasted against an
inactive control or another active treatment.
Cochrane Database Syst Rev. 2015;9:Cd004249.Chaibi A, Benth JS, Tuchin P, et al.
Chiropractic Spinal Manipulative Therapy For Migraine: A Three-Armed, Single-Blinded, Placebo,
Randomized Controlled Trial
European Journal of Neurology 2017 (Jan); 24 (1): 143–153Chaibi A, Knackstedt H, Tuchin PJ, et al.
Chiropractic Spinal Manipulative Therapy for Cervicogenic Headache:
A Single-blinded, Placebo, Randomized Controlled Trial
BMC Res Notes. 2017 (Jul 24); 10 (1): 310GBD 2015 Disease and Injury Incidence and Prevalence Collaborators.
Global, regional, and national incidence, prevalence, and years lived with disability for 310
diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015.
Lancet. 2016;388:1545–1602.Kim YK, Schulman S.
Cervical artery dissection: pathology, epidemiology and management.
Thromb Res. 2009;123:810–821.Engelter ST, Grond-Ginsbach C, Metso TM, et al.
Cervical artery dissection: trauma and other potential mechanical trigger events.
Neurology. 2013;80: 1950–1957.Haldeman S, Carey P, Townsend M, Papadopoulos C.
Arterial Dissections Following Cervical Manipulation: The Chiropractic Experience
Canadian Medical Association Journal (CMAJ) 2001 2001 (Oct 2); 165: 905–906Cassidy JD, Boyle E, Cote P, et al.
Risk of Vertebrobasilar Stroke and Chiropractic Care:
Results of a Population-based Case-control
and Case-crossover Study
Spine (Phila Pa 1976) 2008 (Feb 15); 33 (4 Suppl): S176–183Rubinstein, S.M.
Adverse Events Following Chiropractic Care for Subjects With Neck or Low-Back Pain:
Do The Benefits Outweigh the Risks?
J. Manip. Physiol. Ther. 2008, 31, 461–464.Tuchin P.
A Replication of the Study “Adverse Effects of Spinal Manipulation: A Systematic Review”
Chiropr Man Ther. 2012;20:30.Wynd S., Westaway M., Vohra S., Kawchuk G.
The Quality of Reports on Cervical Arterial Dissection
Following Cervical Spinal Manipulation
PLoS ONE 2013 (Mar 20); 8 (3): e59170Chung CL, Cote P, Stern P, et al.
The association between cervical spine manipulation and carotid artery dissection:
a systematic review of the literature.
J Manipulative Physiol Ther. 2015;38:672–676.J.D. Cassidy, E. Boyle, P. Cote, S. Hogg-Johnson, S.J. Bondy, S. Haldeman
Risk of Carotid Stroke after Chiropractic Care: A Population-Based Case-Crossover Study
J Stroke Cerebrovasc Dis. 2017 (Apr); 26 (4): 842–850Kranenburg HA, Schmitt MA, Puentedura EJ, et al.
Adverse events associated with the use of cervical spine manipulation or mobilization and patient characteristics:
a systematic review.
Musculoskelet Sci Pract. 2017;28:32–38.Jauch EC, Saver JL, Adams HP Jr, et al.
Guidelines for the early management of patients with acute ischemic stroke:
a guideline for healthcare professionals from the American Heart Association/
American Stroke Association.
Stroke. 2013;44:870–947.World Health Organization (WHO)
WHO Guidelines on Basic Training and Safety in Chiropractic
Geneva, Switzerland: (November 2005)Cagnie B, Vinck E, Beernaert A, et al.
How Common Are Side Effects of Spinal Manipulation And Can These Side Effects Be Predicted?
Manual Therapy 2004 (Aug); 9 (3): 151–156Hurwitz EL, Morgenstern H, Vassilaki M, et al.
Adverse reactions to chiropractic treatment and their effects on satisfaction and clinical
outcomes among patients enrolled in the UCLA Neck Pain Study.
J Manipulative Physiol Ther. 2004;27:16–25.Thiel HW, Bolton JE, Docherty S, Portlock JC:
Safety of Chiropractic Manipulation of the Cervical Spine: A Prospective National Survey
Spine (Phila Pa 1976). 2007 (Oct 1); 32 (21): 2375–2378Rubinstein SM, Leboeuf-Yde C, Knol DL, de Koekkoek TE,
Pfeifle CE, van Tulder MW.
The Benefits Outweigh the Risks for Patients Undergoing Chiropractic
Care for Neck Pain A Prospective, Multicenter, Cohort Study
J Manipulative Physiol Ther 2007 (Jul); 30 (6): 408–418Eriksen K, Rochester RP, Hurwitz EL.
Symptomatic Reactions, Clinical Outcomes and Patient Satisfaction Associated with Upper Cervical
Chiropractic Care: A Prospective, Multicenter, Cohort Study
BMC Musculoskelet Disord. 2011 (Oct 5); 12: 219Walker, BF, Hebert, JJ, Stomski, NJ et al.
Outcomes of Usual Chiropractic.
The OUCH Randomized Controlled Trial of Adverse Events
Spine (Phila Pa 1976). 2013 (Sep 15); 38 (20): 1723–1729Maiers et al., 2015
M. Maiers, R. Evans, J. Hartvigsen, C. Schulz, G. Bronfort
Adverse Events Among Seniors Receiving Spinal Manipulation and Exercise
in a Randomized Clinical Trial
Manual Therapy 2015 (Apr); 20 (2): 335–341Chaibi A, Benth JS, Tuchin P, et al.
Adverse events in a chiropractic spinal manipulative therapy singleblinded, placebo, randomized
controlled trial for migraineurs.
Musculoskelet Sci Pract. 2017;29:66–71.Parenti G, Orlandi G, Bianchi M, et al.
Vertebral and carotid artery dissection following chiropractic cervical manipulation.
Neurosurgical Rev. 1999;22:127–129.Siegel D, Neiders T.
Vertebral artery dissection and pontine infarct after chiropractic manipulation.
Am J Emerg Med. 2001;19:171–172.Parwar BL, Fawzi AA, Arnold AC, et al.
Horner’s syndrome and dissection of the internal carotid artery after chiropractic manipulation of the neck.
Am J Ophthalmol. 2001;131:523–524.Stevinson C, Honan W, Cooke B, et al.
Neurological complications of cervical spine manipulation.
J R Soc Med. 2001;94:107–110.Jeret JS, Bluth M.
Stroke following chiropractic manipulation.
Cerebrovasc Dis. 2002;13:210–213.Nadgir RN, Loevner LA, Ahmed T, et al.
Simultaneous bilateral internal carotid and vertebral artery dissection following chiropractic manipulation:
case report and review of the literature.
Neuroradiology. 2003;45: 311–314.Jay WM, Shah MI, Schneck MJ.
Bilateral occipital-parietal hemorrhagic infarctions following chiropractic cervical manipulation.
Semin Ophthalmol. 2003;18: 205–209.Chen WL, Chern CH, Wu YL, et al.
Vertebral artery dissection and cerebellar infarction following chiropractic manipulation.
Emerg Med J: EMJ. 2006;23:e1.Mikkelsen R, Dalby RB, Hjort N, et al.
Endovascular treatment of basilar artery thrombosis secondary to bilateral vertebral artery
dissection with symptom onset following cervical spine manipulation therapy.
Am J Case Rep. 2015;16:868–871.Moher D, Hopewell S, Schulz KF, et al.
CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials.
BMJ. 2010; 340:c869.Gross A, Miller J, D’Sylva J, Burnie SJ, Goldsmith CH, Graham N, et al.
Manipulation or Mobilisation For Neck Pain: A Cochrane Review
Manual Therapy 2010 (Aug); 15 (4): 315–333Gorrell LM, Engel RM, Brown B, et al.
The reporting of adverse events following spinal manipulation in randomized clinical trials –
a systematic review.
Spine J. 2016;16:1143–1151.Church EW, Sieg EP, Zalatimo O, Hussain NS, Glantz M, Harbaugh RE.
Systematic Review and Meta-analysis of Chiropractic Care and
Cervical Artery Dissection: No Evidence for Causation
Cureus 2016 (Feb 16); 8 (2): e498Whedon, JM, Mackenzie, TA, Phillips, RB, and Lurie, JD.
Risk of Traumatic Injury Associated with Chiropractic Spinal Manipulation
in Medicare Part B Beneficiaries Aged 66-99
Spine (Phila Pa 1976) 2015 (Feb 15); 40 (4): 264–270Herzog, W., Leonard, T. R., Symons, B., Tang, C., & Wuest, S.
Vertebral Artery Strains During High-speed,
Low amplitude Cervical Spinal Manipulation
J Electromyography and Kinesiology 2012 (Oct); 22 (5): 740–746Piper SL, Howarth SJ, Triano J, et al.
Quantifying strain in the vertebral artery with simultaneous motion analysis of the head and neck:
a preliminary investigation.
Clin Biomech (Bristol, Avon). 2014;29:1099–1107.Quesnele JJ, Triano JJ, Noseworthy MD, Wells GD.
Changes in Vertebral Artery Blood Flow Following Various Head Positions and Cervical Spine Manipulation
J Manipulative Physiol Ther. 2014 (Jan); 37 (1): 22–31Gouveia LO, Castanho P, Ferreira JJ.
Safety of chiropractic interventions: a systematic review.
Spine (Phila, PA, 1976). 2009;34:E405–E413.Whedon, JM, Song, Y, Mackenzie, TA, Phillips, RB, Lukovits, TG, and Lurie, JD.
Risk of Stroke After Chiropractic Spinal Manipulation in Medicare B Beneficiaries
Aged 66 to 99 Years With Neck Pain
J Manipulative Physiol Ther. 2015 (Feb); 38 (2): 93–101Ringel SP, Harrison SH, Norenberg MD, et al.
Fibromuscular dysplasia: multiple "spontaneous" dissecting aneurysms of the major cervical arteries.
Ann Neurol. 1977;1:301–304.Reddy M, Reddy B, Schoggl A, et al.
The complexity of trauma to the cranio-cervical junction: correlation of clinical presentation
with Doppler flow velocities in the V3-segment of the vertebral arteries.
Acta Neurochir (Wien). 2002;144:575–580. discussion 580.Guzman J, Hurwitz EL, Carroll LJ, Haldeman S, Cote P, Carragee EJ, et al.
A New Conceptual Model Of Neck Pain: Linking Onset, Course, And Care
Results of the Bone and Joint Decade 2000–2010 Task Force on
Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S14–23Guzman J, Haldeman S, Carroll LJ, et al.
Clinical Practice Implications of the Bone and Joint Decade 2000-2010
Task Force on Neck Pain and Its Associated Disorders:
From Concepts and Findings to Recommendations
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S199–S212Silbert PL, Mokri B, Schievink WI.
Headache and neck pain in spontaneous internal carotid and vertebral artery dissections.
Neurology. 1995;45:1517–1522.Arnold M, Bousser MG.
Clinical manifestations of vertebral artery dissection.
Front Neurol Neurosci. 2005; 20:77–86.Melzack R.
The McGill Pain Questionnaire: major properties and scoring methods.
Pain. 1975;1:277–299.Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E.
Scientific Monograph of the Quebec Task Force on Whiplash-Associated Disorders
Redefining Whiplash and its Management
Spine (Phila Pa 1976). 1995 (Apr 15); 20 (8 Suppl): S1-S73Nordin M, Carragee EJ, Hogg-Johnson S, Weiner SS, Hurwitz EL, Peloso PM, et al.
Assessment of Neck Pain and Its Associated Disorders:
Results of the Bone and Joint Decade 2000–2010 Task Force on
Neck Pain and Its Associated Disorders
Spine (Phila Pa 1976). 2008 (Feb 15); 33 (4 Suppl): S101–S122Campos CR, Calderaro M, Scaff M, et al.
Primary headaches and painful spontaneous cervical artery dissection.
J Headache Pain. 2007;8:180–184.Haneline MT, Cooperstein R.
Chiropractic care for patients with acute neck pain: results of a pragmatic practice-based feasibility study.
J Chiropr Med. 2009;8: 143–155.Schievink WI.
Spontaneous dissection of the carotid and vertebral arteries.
N Engl J Med. 2001;344: 898–906.Lee KP, Cslini WG, McCormick GF, et al.
Neurologic complications following chiropractic manipulation: a survey of California neurologists.
Neurology. 1995;45: 1213–1215.Carnes D, Mars TS, Mullinger B, et al.
Adverse events and manual therapy: a systematic review.
Man Ther. 2010;15:355–363.Thomas LC, Rivett DA, Attia JR, et al.
Risk factors and clinical presentation of cervical arterial dissection:
preliminary results of a prospective case–control study.
J Orthop Sports Phys Ther. 2015;45:503–511.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al.
Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study.
Circulation. 2006;113:657–663.Kerry R, Taylor AJ, Mitchell J, et al.
Manual therapy and cervical arterial dysfunction, directions for the future: a clinical perspective.
J Man Manip Ther. 2008; 16:39–48.APA.
Clinical Guidelines for Assessing Vertebrobasilar Insufficiency in the Management of
Cervical Spine Disorders
Australia: APA; 2006.Rushton A, Rivett D, Carlesso L, et al.
International framework for examination of the cervical region for potential of Cervical Arterial Dysfunction
prior to Orthopaedic Manual Therapy intervention.
Man Ther. 2014;19:222–228.
Return to STROKE
Since 5-22-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |