

Changes in H-reflex and V-waves
Following Spinal ManipulationThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Exp Brain Res. 2015 (Apr); 233 (4): 1165–1173 ~ FULL TEXT
Niazi IK1, Türker KS, Flavel S, Kinget M, Duehr J, Haavik H.
Centre for Chiropractic Research,
New Zealand College of Chiropractic,
6 Harrison Rd Mt Wellington,
Newmarket, PO Box 113-044,
Auckland, New Zealand.
This study investigates whether spinal manipulation leads to neural plastic changes involving cortical drive and the H-reflex pathway. Soleus evoked V-wave, H-reflex, and M-wave recruitment curves and maximum voluntary contraction (MVC) in surface electromyography (SEMG) signals of the plantar flexors were recorded from ten subjects before and after manipulation or control intervention. Dependent measures were compared with 2-way ANOVA and Tukey's HSD as post hoc test, p was set at 0.05. Spinal manipulation resulted in increased MVC (measured with SEMG) by 59.5 ± 103.4 % (p = 0.03) and force by 16.05 ± 6.16 4 % (p = 0.0002), increased V/M max ratio by 44.97 ± 36.02 % (p = 0.006), and reduced H-reflex threshold (p = 0.018). Following the control intervention, there was a decrease in MVC (measured with SEMG) by 13.31 ± 7.27 % (p = 0.001) and force by 11.35 ± 9.99 % (p = 0.030), decreased V/M max ratio (23.45 ± 17.65 %; p = 0.03) and a decrease in the median frequency of the power spectrum (p = 0.04) of the SEMG during MVC.
The H-reflex pathway is involved in the neural plastic changes that occur following spinal manipulation. The improvements in MVC following spinal manipulation are likely attributed to increased descending drive and/or modulation in afferents. Spinal manipulation appears to prevent fatigue developed during maximal contractions. Spinal manipulation appears to alter the net excitability of the low-threshold motor units, increase cortical drive, and prevent fatigue.
KEYWORDS: H-reflex · V-wave · Spinal manipulation · Maximal voluntary contraction · Evoked potentials · Neural adaptations
From the Full-Text Article:
Introduction
Over the past 10 years, several research groups have demonstrated that spinal manipulation can change various aspects of nervous system function, including muscle reflexes, cognitive processing, reaction time, and the speed at which the brain processes information (Murphy et al. 1995; Herzog et al. 1999; Suter et al. 1999, 2000; Kelly et al. 2000; Haavik Taylor and Murphy 2007a, b, 2008, 2010a)+. One group has hypothesized that the articular dysfunction component of the chiropractic clinical construct, the vertebral subluxation, results in altered afferent input to the central nervous system that modifies the way in which the CNS processes and integrates all subsequent sensory input (Haavik Taylor and Murphy 2007a; Haavik Taylor et al. 2010). This processing (i.e., sensorimotor integration) is a central nervous system (CNS) function that appears most vulnerable to altered inputs.
Over recent years, a series of experiments have been conducted to further investigate this potential relationship between the putative vertebral subluxation and altered CNS function (for recent review see Haavik and Murphy 2012). Multiple studies have indicated neural plastic changes occur when spinal manipulation of such dysfunctional spinal segments is performed. The neural adaptations include altered sensorimotor integration and altered motor control following spinal manipulation (Haavik Taylor and Murphy 2007a, b, 2008, 2010a, b). The level of CNS involvement and the exact mechanisms underlying these neural adaptations following spinal manipulation remain unclear. This study sought to investigate possible neural plastic changes with spinal manipulation by measuring reflex responses such as the H-reflex and V-wave. Although these evoked responses are affected by common neural mechanisms, it has been shown in previous studies that these methods can differentiate between altered presynaptic inhibition and motoneuron excitability as measured with the H-reflex (Hultborn et al. 1987; Pierrot-Deseilligny and Mazevet 2000; Nordlund et al. 2002; Misiaszek 2003; Ekblom 2010), and changes in supraspinal input to the motor neuron pool as measured with the V-wave (Upton et al. 1971; Aagaard et al. 2002; Duclay and Martin 2005; Vila-Chã et al. 2012). Therefore, combining measures of the H-reflex and V-wave may provide a better understanding of the neural plastic changes that occur with spinal manipulation.
A few previous studies have looked at the effects of spinal manipulation on the H-reflex recorded from the medial aspect of the triceps surae of lumbar disk herniation patients (Floman et al. 1997), and of healthy asymptomatic individuals (Dishman and Burke 2003), and from the soleus in both asymptomatic subjects (Murphy et al. 1995; Suter et al. 2005) and low back pain patients (Suter et al. 2005). Several of these studies showed a decrease in the H-reflex indicating a transient attenuation of motoneuronal activity of the lumbosacral spine in asymptomatic subjects (Murphy et al. 1995; Dishman and Burke 2003) and in low back pain patients (Suter et al. 2005).
However, there have been advances in the both data collection and data analysis methodology since the above cited studies (for example see Tucker et al. 2005; Brinkworth et al. 2007). There is, for example, a known natural variation in the H-reflex response, which is why it is necessary to record and average multiple responses (Tucker et al. 2005). It is therefore not common practice to analyze single H-reflex responses as has been done in the past (Dishman and Burke 2003). The purpose of the current project therefore is to take advantage of the recent discoveries and understanding about the standardized data collection and analysis methodologies (Tucker et al. 2005; Brinkworth et al. 2007) related to the H-reflex and V-waves and explore what effect, if any, spinal manipulation of vertebral subluxations will have on them.
Methods
Subjects
A total of 18 men took part in the study. Study one included ten volunteers aged 27.6 ± 5.4 years, and study two included six volunteers aged 32.6 ± 9.3 years. All subjects were required to be aged 18–40, have evidence of spinal dysfunction but have no known contraindications to spinal manipulation such as recent history of trauma, known conditions such as metabolic disorders, inflammatory or infectious arthropathies, or bone malignancies. All subjects were required to have a self-reported history of subclinical spinal pain, i.e., recurring, intermittent low-grade spinal pain, ache, or tension. Participants were excluded if they reported current pain anywhere in the body (to remove the confounding effect of current pain), diagnosed degenerative joint disease, or any medical condition affecting the sensorimotor system. In keeping with the definition of subclinical spinal pain, participants were excluded if they had sought previous treatment for their intermittent problem. All participants gave their informed written consent before inclusion in the study. The study was approved by The Northern Y Regional Ethics Committee approved this study in accordance with the Declaration of Helsinki.
InstrumentationSurface EMG Bipolar surface electrodes (20 mm Blue Sensor Ag/AgCl, AMBU A/S, Denmark) were used to record the surface electromyographic (SEMG) activity of the soleus muscle (SOL) of the right leg for all aspects of the experiments. Surface electrodes were placed 2 cm distal to the lateral gastrocnemius muscle and 2 cm apart, and a ground electrode was placed over the right malleolie at the ankle. SEMG signals were amplified in custom-built EMG amplifier, and were recorded with CED Power 1401 MK 2 data acquisition board at 5 kHz and band-pass filtered at 20–1,000 Hz.
Electrical stimulation The H-, M-, and V-waves of the SOL muscle were elicited by stimulation of the tibial nerve. The electrical stimulus was provided by an isolated stimulator (Digitimer DS7AH, UK). Stimulating electrodes (32 mm, PALS® Platinum, Patented Conductive Neurostimulation Electrodes, Axelgaard Manufacturing Co., Ltd. USA), a custom-built silver ball with 10-mm diameter was placed on the tibial nerve (cathode) located in the popliteal fossa of the right leg, and the other stimulating electrode (PALs platinum rectangular electrode, 75 × 100 mm; Axelgaard Man) was placed proximal to the right patella (anode). The position of the cathode and the intensity of the stimulus were manipulated until the greatest response with the minimum stimulus intensity was achieved.Force measurement
The force recordings were performed using a strain gauge (10 mV/Nm) attached to a custom-made ankle brace that signal was recorded by a CED Power 1401 MK 2 Data Acquisition Board at a sample rate of 1 kHz. The force measures were recorded, while the subject performed maximum voluntary ankle dorsiflexion contractions. Three recordings were made, and the maximum force produced during these contractions was used for normalization.Experimental procedures During Study one, the ten subjects attended two sessions each, the control and the experimental (spinal manipulation) session. The order of these two sessions was randomized and at least 1 week separated the two sessions. All experiments for study one were performed on the right leg, while the volunteers were comfortably lying prone on massage table with their right leg firmly strapped to the table with Velcro. The subjects maintained their hips and legs straight and with the ankle at 90° of plantar flexion. The right foot was firmly attached to the leg of the table. Particular care was taken to monitor the posture of the subjects and ensure their posture and position remained unchanged. During both the experimental (spinal manipulation) and the control sessions, the following measures were collected pre and post the interventions: SEMG signals during MVC; Hand M-recruitment curves; H-reflex area under curve normalized to Mmax (Harea/Mmax), H-reflex threshold, V-wave normalized to Mmax (V/Mmax), M-wave slope, H-reflex slope and the mean power frequency (MPF) of a fast Fourier transform (FFT) of the SEMG during MVC. In Study two, an additional group of eight participants attended two more sessions each, one control and one experimental (spinal manipulation) session, where only force was measured.
Maximal voluntary contractions (MVC) The subjects performed three progressive MVCs of the plantar flexors of 5 s of duration, separated by 2-min rest. Subjects were verbally encouraged to produce maximal contraction. The highest plantar flexor SEMG activity during MVC in each experimental or control session was used for analysis and to compute the submaximal target contraction levels for H- and M-recruitment curve recordings. In study two, only the force was measured.
H- and M-recruitment curve recordings During the H- and M- recruitment curve recordings, the subject was asked to plantar flex his right leg at around 10 % of his own MVC (rectified and 0.5 Hz low-pass smoothed SEMG as feedback). Subject was provided with online feedback of the contraction level exerted on a computer monitor placed under the table which can be clearly seen by the subject. While the subject was performing this low-level tonic contraction, the direct motor response (M-wave) and the H-reflex of the SOL muscle were elicited via electrical stimulation of the tibial nerve. To get the maximum peak-to-peak amplitude of the M-wave, subjects were stimulated progressively by increasing the current intensity in 5-mA increments. A total of three trials at each current intensity were recorded.
Then, at each current intensity, the preceding M-wave peak-to-peak amplitude was compared with the new M-wave peak-to-peak amplitude. Once the preceding M-wave peak-to-peak amplitude and new M-wave peak-to-peak amplitude had reached a plateau over the three trials, the current intensity of the previous stimulation was considered the maximum current intensity. To construct the M- and H-recruitment curves, the maximum intensity was divided into 16 segments that were equally separated. For each randomly chosen current intensity, a total of five stimuli were delivered at varying time intervals between 2 and 3 s. To avoid fatigue and mental distraction of the participants, rest periods of 2 min were given every 80 stimuli. Moreover, the subjects were given the possibility to pause the experiment at any time if they reported fatigue.
V-wave recordings The subjects were asked to perform 7–9 MVCs of 5 s duration, with 2 min of rest in between prior to and postspinal manipulation and control session. During each of the MVC attempts, five supramaximal stimuli (110 % of the current needed to evoke maximal M-wave; 1-ms square pulse) were applied to the tibial nerve at the times when the SEMG was above 90 % of the level achieved during MVC.
MPF measure The development of fatigue in a muscle can be observed by amplitude and spectral analysis of SEMG recordings (Hagberg 1981). The time-dependent shift in mean power frequency (MPF) of SEMG signals to lower frequencies during the fatigue process is a well-established phenomenon. For this purpose, SEMG recorded during the MVC preand post-manipulation session or control intervention was used. MVC data segments epoched offline and processed in MATLAB using purpose written scripts. A fast Fourier transform (FFT) was performed, and the mean power frequency (MPF) was calculated as the frequency (Hz) center of the spectrum.Interventions
Spinal manipulation The entire spine and sacroiliac joints were assessed for segmental dysfunction (also known as vertebral subluxation by the chiropractic profession), and adjusted where deemed necessary by a registered chiropractor with at least 10-year clinical experience. The clinical indicators that were used to assess the function of the spine prior to and after each spinal manipulation intervention included assessing for tenderness to palpation of the relevant joints, manually palpating for restricted intersegmental range of motion, assessing for palpable asymmetric intervertebral muscle tension, and any abnormal or blocked joint play and end-feel of the joints. All of these biomechanical characteristics are known clinical indicators of spinal dysfunction (Jull et al. 1988; Hubka and Phelan 1994; Strender et al. 1997; Hestboek and Leboeuf-Yde 2000; Fryer et al. 2004; Cooperstein et al. 2010, 2013).
All of the spinal manipulations carried out in this study were high-velocity, low-amplitude thrusts to the spine or pelvic joints. This is a standard manipulation technique used by chiropractors. The mechanical properties of this type of CNS perturbation have been investigated; and although the actual force applied to the subject’s spine depends on the therapist, the patient, and the spinal location of the manipulation, the general shape of the force–time history of spinal manipulations is very consistent (Hessell et al. 1990) and the duration of the thrust is always <200 ms (for review see Herzog 1996). The high-velocity type of manipulation was chosen specifically because previous research (Herzog et al. 1995) has shown that reflex SEMG activation observed after manipulations only occurred after high-velocity, low-amplitude manipulations (as compared with lower-velocity mobilizations). This manipulation technique has also been previously used in studies that have investigated neurophysiological effects of spinal manipulation (for review see Haavik and Murphy 2012).
Control intervention The control intervention consisted of passive and active movements of the subject’s head, spine, and body that are carried out by the same chiropractor who pre-checks the subjects for vertebral subluxations and who performs the spinal manipulations in the experimental intervention session. This control intervention involved the subjects being moved into the manipulation setup positions where the chiropractor would normally apply a thrust to the spine to achieve the manipulations. However, the experimenter was particularly careful not to put pressure on any individual spinal segments. Loading a joint, as is done prior to spinal manipulation, has been shown to alter paraspinal proprioceptive firing in anesthetized cats (Pickar and Wheeler 2001) and therefore was carefully avoided by ending the movement prior to endrange- of-motion when passively moving the subjects.
No spinal manipulation was performed during any control intervention. This control intervention is not intended to act as a sham manipulation but to act as a physiological control for possible changes occurring due to the cutaneous, muscular or vestibular input that would occur with the type of passive and active movements involved in preparing a subject/patient for a manipulation. It also acts as a control for the effects of the stimulation necessary to collect the dependent measures of the study and acts as a control for the time required to carry out the manipulation intervention.Data analysis
For the evoked potentials, peak-to-peak amplitude of the H-reflex, M-wave, and V-wave were computed offline from the unrectified SEMG signals. To reduce inter-subject variability, H-, M-, and V-waves were normalized to the corresponding maximal M-wave (Mmax), thus the (Hmax)/Mmax and V/Mmax ratios were computed. For each recruitment curve, the current intensity at Hmax and Mmax was identified. Since the size of the M-wave is affected by contraction intensity (Pensini and Martin 2004), the Mmax wave elicited concomitantly either with H-reflex or with V-wave was used for the respective normalization. The ascending part of recruitment curve was fit by a general least squares model, as described by (Brinkworth et al. 2007). From the curve fit analysis, the following parameters were analyzed: current intensity at H-reflex threshold (Hthresh); current intensity at 50 % of the Hmax (50 %Hmax); and the slope of the ascending limb of the recruitment curve at 50 % of the Hmax (Hslope). The dependant measures were: H- and M-recruitment curves; H-reflex area under curve normalized to Mmax (Harea/Mmax), H-reflex threshold, V-wave normalized to Mmax (V/Mmax), M-wave slope, H-reflex slope and the mean power frequency (MPF) of a fast Fourier transform (FFT) of the SEMG during MVC and absolute force measures.
Surface EMG background level
Average rectified value (ARV) of the SOL SEMG was estimated from each sweep for an epoch of 500 ms before the stimulation and then averaged. The ARV values were normalized with respect to the ARV computed from the highest MVC SEMG and expressed as a percentage.
Statistical analysis
All pre- to post-intervention changes were evaluated using two-way ANOVA with factors intervention (spinal manipulation and control) and time (Pre and Post). Post hoc pairwise comparison was done using Tukey’s HSD tests if required. Statistical significance was set at p < 0.05 for all comparisons.
Results

Figure 1

Figure 2
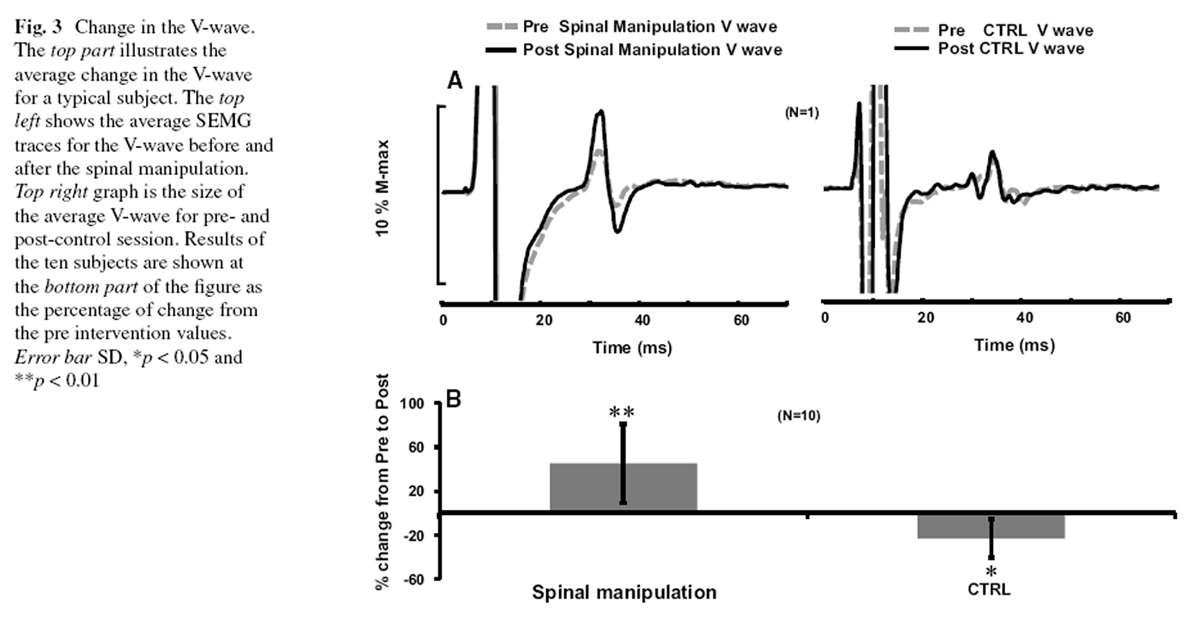
Figure 3 In ten subjects using the two procedures, we obtained 20 sets of results. To be able to fully characterize the possible changes in the motoneuron excitability and the synaptic efficacy, the M-wave and H-reflex curves were established. For that, 16 levels of stimuli were utilized. The stimuli were adjusted to the stimulus level that induced the 110 % of the maximal M response (this level was labeled as level 16; see the “Methods” for details). The curves are shown in Figure 1, where the M-waves were fitted with the curve so that preand post-M-wave curves could be superimposed on top of each other to allow any genuine changes in the H-reflex curve to be highlighted. As can be seen in the figure, this made not only the ordinate of the figures normalized (to the M Max) but the abscissa as well (to the stimulus intensity that generates the M Max).
Figure 2 illustrates the changes in the H-reflex values, the maximal voluntary contraction and the fatigue as a result of spinal and control manipulations. As can be seen, there are some significant affects from the manipulations. There was no significant difference between the spinal manipulation and control group in baseline data (premeasures). The asterisks highlight the significant changes as a result of the manipulation or control intervention for each of the parameters tested against the pre-manipulation values. The threshold to elicit the H-reflex significantly decreased by 8.5 % (p = 0.01) as a result of spinal manipulation and + did not change after the control session (1.5 % change). The subjects’ SEMGs indicated a significant level of decrease in the power spectrum as indicated by the median frequency of the power spectrum (139–124 Hz; p = 0.04) only after the control session (–9.4 % change), but fatigue did not develop after the spinal manipulation.
The value of the maximal voluntary contraction as determined by the SEMG increased significantly by 59.5 ± 103.4 % (p = 0.03) after the spinal manipulation but not after the control. In fact, the SEMG during MVC decreased significantly after the control session by 13.31 ± 7.27 % (p = 0.001). In the follow-up study with eight subjects, absolute force during MVC measure increased by 16.05 ± 6.16 4 % (p = 0.0002) for intervention session and following the control intervention there was a decrease in force by 11.35 ± 9.99 % (p = 0.030).
The V-wave amplitude (V/Mmax ratio) also changed dramatically as a result of the interventions in this study as shown in Figure 3. The change was a significant increase reaching around 44.97 ± 36.02 % (p = 0.006) after the spinal manipulation and a significant decrease 23 ± 17.65 % (p = 0.03) after the control.
Discussion
This study discovered three original findings: Firstly, that, the H-reflex pathways can be significantly affected by the spinal manipulation; and, secondly, that, the cortical drive as expressed by the size of the V-wave and the SEMG and force measure during MVC is significantly increased by the spinal manipulation. Thirdly, the spinal manipulation intervention appears to have prevented fatigue from occurring in the SOL, as indicated by a significant decrease in median frequency in the power spectrum from the control subjects’ SEMGs only.
H-reflex pathway
H-reflex pathway for the soleus muscle involves the spindle primary afferent fibers (Ia) originating from the soleus muscle, single synapses in the spinal cord, and the motoneurons that innervate the soleus muscle (Pierrot-Deseilligny and Burke 2005). The excitability of the motoneuron and the efficacy of the synaptic terminal of the spindle primary afferents are also affected by the supraspinal and spinal inputs. The methodology we have used avoids any possible changes in the position of the stimulating electrodes relative to the nerve as it not only normalizes the amplitude of the H-reflex to the M Max but also to the stimuli that induces the maximal M-wave curve in each trial [“Methods”; for details refer to (Brinkworth et al. 2007)].
Our results of this study indicate that the H-reflex pathway has been affected as a result of spinal manipulation. Since spinal manipulation has lowered the recruitment threshold of motoneurons to Ia afferent input, it is suggested that either the low-threshold motoneurons have become more excitable or the synapses of the Ia primary afferents became more efficient since a lower stimulus intensity can now recruit the same motoneuron. Spinal manipulation therefore appears to alter the net excitability for the low-threshold motoneurons and/or the efficacy of the Ia synapse.
Three previous studies have indicated that spinal manipulation decreases motoneuron excitability in asymptomatic subjects (Murphy et al. 1995; Dishman and Burke 2003) and in low back pain patients (Suter et al. 2005). Our results on the other hand indicate an increase in the excitability of low-threshold motoneurons. None of the previous studies, however, investigated the H-reflex threshold as was done in the current study. Murphy et al. (1995) found a decrease in the peak of the H-reflex curve. We observed a similar decrease in the peak of the curve (see Fig. 3). However, according to the currently published standardized data analysis methodologies (Brinkworth et al. 2007), the entire area under the curve should be analyzed, not just the maximum H-reflex. In the current study, there was a trend toward a decreased area, but this did not reach significance.
Dishman and Burke’s findings (2003) may reflect the apparent decrease in peak of the H-reflex, as found by Murphy et al. (1995) and seen in this study, or their finding may have merely reflected the known natural variation in the H-reflex response, as they recorded single H-reflexes only. It has been shown that to ensure robust findings, it is necessary to record and average multiple responses (Tucker et al. 2005). Suter et al. (2005) study was conducted in a low back pain population. It is therefore possible that low back pain patients have alterations involving the H-reflex pathway that spinal manipulation effects in a different manner to that of the healthy young male volunteers that took part in the current study.
Maximal voluntary contraction
The current results demonstrate that the spinal manipulation increases maximum voluntary contraction in SEMG signals of the SOL, which may indicate an increase in drive to this muscle that lasted for at least 30 min. This improvement in SEMG signals and absolute measure of force during MVC following spinal manipulation are likely to be attributed to an increase in the descending drive and/or modulation in afferents. This is further evidenced by the significant increase in the V-wave measurements as a result of the spinal manipulation. V-wave increase also depends upon the density of action potentials sent down from the supraspinal centers that block of the antidromic action potentials caused by the supramaximal stimulation of the tibial nerve. Interestingly, these findings following a single session of spinal manipulation are similar to what has been observed following strength training (Vila-Chã et al. 2012) who found that 3 weeks of strength training significantly increased the V-wave amplitude (as measured by V/Mmax ratio) by just over 55 %, SEMG during MVC increased by 14.4 %, and the H-reflex threshold was significantly decreased by 4.7 %. For comparison, our results from a single session of chiropractic adjustments demonstrated significant V-wave amplitude (V/Mmax ratio) increase of 45 %, an average increase in SEMG during MVC by almost 60 % and absolute force during MVC by 16 % and a significant decrease in the H-reflex threshold by 8.5 %.
This is the first study to discover that the spinal manipulation changes the H-reflex circuitry by increasing the excitability of the low-threshold motoneurons of the SOL and/or increasing the efficacy of the synaptic input to lowthreshold motoneurons from Ia primary afferents originating from the SOL. Recently, Pavlovian conditioning has been used to increase in the H-reflex for improving muscles that have lost their tonus (Chen et al. 2010). Therefore, our results suggest that chiropractic treatment may also be useful in such conditions instead of lengthy training of subjects. However, this study only used a single treatment, and we are at present studying the effects of long-term treatment on the H-reflex circuitry. If that induces long-lasting changes on the H-reflex, it would then be very useful for the treatment to strengthen muscles that have lost their tonus due to a variety of reasons.
Changes in fatigue
Although MVC force significantly increased after spinal manipulation, it decreased significantly after the control session. Supporting the occurrence of fatigue after control but not after the spinal intervention as the MPF of the surface SEMG records fell only after the control manipulation (Lowery et al. 2002). That is, there was a significant change in the MPF only in the control condition. This suggests that spinal manipulation can prevent fatigue in the SOL lasting for at least 30 min. This may be of relevance for sports performers. However, this should be interpreted with caution, as this study was not carried out in a sports population, but average healthy young male subjects.
Limitations
Maximal voluntary contraction is a subjective measure, and unless it is backed up using twitch interpolation studies, it may be misleading as it is completely depends upon subject’s participation (Gandevia 2001). Maximal voluntary contraction can depend not only on the drive that one can physically achieve, but also the training that he/ she needs to learn to utilize the activation of the muscles’ potential. Therefore, any improvement of the SEMG and force during MVC can be initially due to the confidence the subject gains on the procedure and applies more of the capacity that he/she has. The fact that our subjects increased their MVCs as a consequence of the spinal manipulation but not as a result of the control manipulation suggests that the treatment actually gave our subjects more confidence and possibly even opened up some of the neuronal pathways that allow the person to apply more of the force. These issues have to be further confirmed using twitch interpolation techniques as well to make a more concrete conclusion.
Clinical relevance
This study is the first to indicate that the chiropractic adjustments of the spine can actually induce significant changes in the net excitability for the low-threshold motor units, and/or alters the synaptic efficacy of the Ia synapse with these low-threshold homonymous motoneurons. The study also indicates that spinal manipulation can improve the confidence of the subject to activate his/her muscle as evidence with the increase in the SEMG signals and force during MVC, and/or alters motor neuron recruitment patters. The results suggest that the improvements in MVC following spinal manipulation are likely attributed to the increased descending drive and/or modulation in afferents. They also indicate that spinal manipulation prevents fatigue. Spinal manipulation may therefore be indicated as part of the medical treatment for the patients who have lost tonus of their muscle and or are recovering from muscle degrading dysfunctions such as stroke or orthopedic operations. These results may also be of interest to sports performers. We suggest these findings should be followed up in the relevant populations.
Acknowledgments
The authors would like to acknowledge the following organizations for support and funding Australian Spinal Research Foundation, Hamblin Chiropractic Research Fund Trust, New Zealand College of Chiropractic and Koç University. KST is a Fellow of the Turkish Academy of Sciences Association.
References:
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002)
Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses.
J Appl Physiol 92:2309–2318Brinkworth RSA, Tuncer M, Tucker KJ, Jaberzadeh S, Türker KS (2007)
Standardization of H-reflex analyses.
J Neurosci Methods 162:1–7.
doi:10.1016/j.jneumeth.2006.11.020Chen XY, Chen Y, Wang Y, Thompson A, Carp JS, Segal RL, Wolpaw JR (2010)
Reflex conditioning: a new strategy for improving motor function after spinal cord injury.
Ann N Y Acad Sci 1198:E12–E21Cooperstein R, Haneline M, Young M (2010)
Interexaminer Reliability of Thoracic Motion Palpation Using
Confidence Ratings and Continuous Analysis
J Chiropractic Medicine 2010 (Sep); 9 (3): 99–106Cooperstein R, Young M, Haneline M (2013)
Interexaminer Reliability of Cervical Motion Palpation Using Continuous Measures
and Rater Confidence Levels
J Can Chiropr Assoc. 2013 (Jun); 57 (2): 156–164Dishman JD, Burke J (2003)
Spinal reflex excitability changes after cervical and lumbar spinal manipulation: a comparative study.
Spine J 3:204–212Duclay J, Martin A (2005)
Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction.
J Neurophysiol 94:3555–3562Ekblom MM (2010)
Improvements in dynamic plantar flexor strength after resistance training are associated with
increased voluntary activation and V-to-M ratio.
J Appl Physiol 109:19–26Floman Y, Liram N, Gilai A (1997)
Spinal manipulation results in immediate H-reflex changes in patients with unilateral disc herniation.
Eur Spine J 6:398–401Fryer G, Morris T, Gibbons P (2004)
Paraspinal muscles and intervertebral dysfunction: part one.
J Manipulative Physiol Ther 27:267–274Gandevia SC (2001)
Spinal and supraspinal factors in human muscle fatigue.
Psychol Rev 81:1725–1789Haavik, H and Murphy, B.
The Role of Spinal Manipulation in Addressing Disordered Sensorimotor Integration and
Altered Motor Control
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 768–776Haavik-Taylor H, Murphy B.
Cervical Spine Manipulation Alters Sensorimotor Integration:
A Somatosensory Evoked Potential Study
Clin Neurophysiol. 2007 (Feb); 118 (2): 391–402Haavik-Taylor H, Murphy B (2007b)
Transient modulation of intracortical inhibition following spinal manipulation.
J Aust Chiropr Assoc 37:106–116Haavik-Taylor H, Murphy B (2008)
Altered sensorimotor integration with cervical spine manipulation.
J Manipulative Physiol Ther 31:115–126Haavik-Taylor H, Murphy B.
Altered Central Integration of Dual Somatosensory Input After Cervical Spine Manipulation
J Manipulative Physiol Ther. 2010 (Mar); 33 (3): 178–188Haavik-Taylor H, Murphy B.
The Effects of Spinal Manipulation on Central Integration of Dual Somatosensory Input
Observed After Motor Training: A Crossover Study
J Manipulative Physiol Ther. 2010 (May); 33 (4): 261–272Haavik-Taylor H, Holt K, Murphy B.
Exploring the Neuromodulatory Effects of the Vertebral Subluxation and Chiropractic Care
Chiropractic Journal of Australia 2010 (Mar); 40 (1): 37–44Hagberg M (1981)
Muscular endurance and surface electromyogram in isometric and dynamic exercise.
J Appl Physiol Respir Environ Exerc Physiol 51:1–7Herzog W (1996)
Mechanical, physiologic, and neuromuscular considerations of chiropractic treatment.
In: Lawrence DJ, Cassidy JD, McGregor M, Meeker WC, Vernon HT (eds)
Advances in chiropractic.
Mosby-Year Book, New York, pp 269–285Herzog W, Conway PJ, Zhang YT, Gail J, Guimaraes ACS (1995)
Reflex responses associated with manipulative treatments on the thoracic spine: a pilot study.
J Manipulative Physiol Ther 18:233–234Herzog W, Scheele D, Conway PJ (1999)
Electromyographic responses of back and limb muscles associated with spinal manipulative therapy.
Spine 24:146–153Hessell BW, Herzog W, Conway PJ, McEwen MC (1990)
Experimental measurement of the force exerted during spinal manipulation using the Thompson technique.
J Manipulative Physiol Ther 13:448–453Hestboek L, Leboeuf-Yde C (2000)
Are chiropractic tests for the lumbo-pelvic spine reliable and valid? A systematic critical literature review.
J Manipulative Physiol Ther 23:258–275Hubka MJ, Phelan SP (1994)
Interexaminer reliability of palpation for cervical spine tenderness.
J Manipulative Physiol Ther 17:591–595Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M (1987)
Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man.
J Physiol 389:757–772Jull, G, Bogduk, N, and Marsland, A.
The Accuracy of Manual Diagnosis for Cervical
Zygapophysial Joint Pain Syndromes
Med J Aust 1988 (Mar 7); 148 (5): 233–236Kelly DD, Murphy BA, Backhouse DP (2000)
Use of a mental rotation reaction-time paradigm to measure the effects of upper cervical adjustments
on cortical processing: a pilot study.
J Manipulative Physiol Ther 23:246–251Lowery M, Nolan P, O’Malley M (2002)
Electromyogram median frequency, spectral compression and muscle fibre conduction velocity
during sustained sub-maximal contraction of the brachioradialis muscle.
J Electromyogr Kinesiol 12:111–118Misiaszek JE (2003)
The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function.
Muscle Nerve 28:144–160Murphy B, Dawson N, Slack J (1995)
Sacroiliac joint manipulation decreases the H-reflex.
Electromyogr Clin Neurophysiol 35:87–94Nordlund MM, Thorstensson A, Cresswell AG (2002)
Variations in the soleus H-reflex as a function of activation during controlled
lengthening and shortening actions.
Brain Res 952:301–307Pensini M, Martin A (2004)
Effect of voluntary contraction intensity on the H-reflex and V-wave responses.
Neurosci Lett 367:369–374Pickar, JG and Wheeler, JD.
Response of Muscle Proprioceptors to Spinal Manipulative-like Loads in the Anesthetized Cat
J Manipulative Physiol Ther. 2001 (Jan); 24 (1): 2–11Pierrot-Deseilligny E, Burke D (2005)
The circuitry of the human spinal cord. Its role in motor control and movement disorders.
Cambridge University Press, CambridgePierrot-Deseilligny E, Mazevet D (2000)
The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits.
Neurophysiol Clin 30:67–80Strender L, Sjoblom A, Sundell K, Ludwig R, Taube A (1997)
Interexaminer reliability in physical examination of patients with low back pain.
Spine 22:814–820Suter E, McMorland G, Herzog W, Bray R (1999)
Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain.
J Manipulative Physiol Ther 22:149–153Suter E, McMorland G, Herzog G, Bray R (2000)
Conservative lower back treatment reduces inhibition in knee-extensor muscles:
a randomized controlled trial.
J Manipulative Physiol Ther 23:76–80Suter E, McMorland G, Herzog W (2005)
short term effects of spinal manipulation on H-reflex amplitude in healthy and symptomatic subjects.
J Manipulative Physiol Ther 28:667–672Tucker KJ, Tuncer M, Türker KS (2005)
A review of the H-reflex and M-wave in the human triceps surae.
Hum Mov Sci 24:667–688Upton AR, McComas AJ, Sica RE (1971)
Potentiation of “late” responses evoked in muscles during effort.
J Neurol Neurosurg Psychiatr 34:699–711Vila-Chã C, Falla D, Correia MV, Farina D (2012)
Changes in H reflex and V-wave following short-term endurance and strength training.
J Appl Physiol 112:54–63
Return to SUBLUXATION
Since 1-18-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |