The Role of Spinal Manipulation in Addressing Disordered
Sensorimotor Integration and Altered Motor ControlThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 768–776 ~ FULL TEXT
Heidi Haavik, Bernadette Murphy
New Zealand College of Chiropractic,
Auckland, New Zealand.
This review provides an overview of some of the growing body of research on the effects of spinal manipulation on sensory processing, motor output, functional performance and sensorimotor integration. It describes a body of work using somatosensory evoked potentials (SEPs), transcranial magnetic nerve stimulation, and electromyographic techniques to demonstrate neurophysiological changes following spinal manipulation. This work contributes to the understanding of how an initial episode(s) of back or neck pain may lead to ongoing changes in input from the spine which over time lead to altered sensorimotor integration of input from the spine and limbs.
From the Full-Text Article:
Introduction
Over the past 15 years our research group has conducted a variety of human experiments that have added to our understanding of the central neural plastic effects of manual spinal manipulation (Haavik and Murphy, 2011; Haavik-Taylor and Murphy, 2007a,b, 2008, 2010c; Haavik-Taylor et al., 2010; Marshall and Murphy, 2006). Spinal manipulation is used therapeutically by a number of health professionals, all of whom have different terminology for the ‘‘entity’’ that they manipulate. This ‘‘entity’’ which generally describes areas of muscle tightness, tenderness and restricted movement may be called a ‘‘vertebral (spinal) lesion’’ by physical medicine specialists or physiotherapists, ‘‘somatic dysfunction’’ or ‘‘spinal lesion’’ by osteopaths, and ‘‘vertebral subluxation’’ or ‘‘spinal fixation’’ by chiropractors (Leach, 1986). For the purposes of this article, the ‘‘manipulable lesion’’ will be referred to as an area of spinal dysfunction or joint dysfunction. Joint dysfunction as discussed in the literature ranges from experimentally induced joint effusion (Shakespeare et al., 1985), pathological joint disease such as osteoarthritis (O’Connor et al., 1993) as well as the more subtle functional alterations that are commonly treated by manipulative therapists (Suter et al., 1999, 2000).

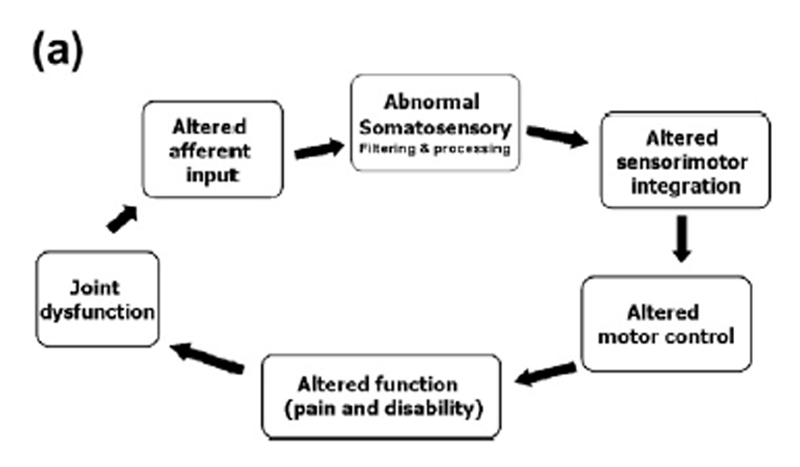
Figure 1 Based on our research findings we have proposed that areas of spinal dysfunction, represent a state of altered afferent input which may be responsible for ongoing central plastic changes (Haavik-Taylor et al., 2010; Haavik-Taylor and Murphy, 2007c). Furthermorewe have proposed a potential mechanism which could explain how high-velocity, low-amplitude spinal manipulation, also known as spinal adjustments, improve function and reduce symptoms. We have proposed that altered afferent feedback from an area of spinal dysfunction alters the afferent ‘‘milieu’’ into which subsequent afferent feedback from the spine and limbs is received and processed, thus leading to altered sensorimotor integration (SMI) of the afferent input, which is then normalized by highvelocity, low-amplitude manipulation (Haavik-Taylor et al., 2010; Haavik-Taylor and Murphy, 2007c). For a pictorial depiction of this hypothesis, see Figure 1.
This article will provide an overview of some of the growing body of research on the effects of spinal manipulation on sensory processing, motor output, functional performance and sensorimotor integration. This body of work contributes to our understanding of how an initial episode(s) of back or neck pain may lead to ongoing changes in input from the spine which over time lead to altered sensorimotor integration of input from the spine and limbs. Increasing this understanding may provide a neurophysiological explanation for some of the beneficial clinical effects reported by chiropractors and other manipulative therapists in day to day practice. Chronic musculoskeletal pain affects the lives of millions of individuals and places a great burden on health care systems in the western world. Some of the research discussed in this review has the potential to identify objective neurophysiological markers which may be able to predict which patients will respond best to spinal manipulative treatment and/or whether a patient has had a sufficient amount of treatment to normalize neurophysiological markers of disordered sensorimotor integration. The relationship between the development and maintenance of chronic musculoskeletal pain and neural markers of altered function is a promising area in need of further research.
Altered sensorimotor processing following spinal manipulation
Several studies utilizing somatosensory evoked potentials (SEPs) (Haavik-Taylor and Murphy, 2007c, 2010c; Haavik-Taylor et al., 2010) have shown that manipulation of areas of joint dysfunction in the cervical spine can alter somatosensory processing and early sensorimotor integration of input from the upper limb. These studies have shown alterations in the amplitude of the cortical SEP peaks N20 (Haavik-Taylor and Murphy, 2007c) and N30 (Haavik-Taylor and Murphy, 2010c) following high-velocity, lowamplitude cervical manipulation. The N20 SEP peak represents the arrival of the afferent volley at the primary somatosensory cortex (Desmedt and Cheron, 1980; Mauguiere, 1999; Nuwer et al., 1994). Later SEP peaks are thought to be generated by the processing of this somatosensory input (Cheron and Borenstein, 1991, 1992; Desmedt and Cheron, 1981; Desmedt et al., 1983; Kanovsky´ et al., 2003; Mauguiere et al., 1983; Rossini et al., 1989, 1987; Waberski et al., 1999) and are therefore thought to reflect early sensorimotor integration (Rossi et al., 2003).
One of these peaks, the N30 SEP component is thought to have multiple generators. Some authors suggest this peak is generated in the post-central cortical regions (i.e. S1) (Allison et al., 1989a,b, 1991), while evidence also suggests that this peak is related to a complex cortical and subcortical loop linking the basal ganglia, thalamus, pre-motor areas, and primary motor cortex (Cheron and Borenstein, 1991, 1992; Desmedt and Cheron, 1981; Desmedt et al., 1983; Kanovsky´ et al., 2003; Mauguiere et al., 1983; Rossini et al., 1989, 1987; Waberski et al., 1999). Hence the N30 peak in particular is thought to reflect early sensorimotor integration (Rossi et al., 2003). More recently, Cebolla et al. have used swLORETA (standardized weighted Low Resolution Brain Electromagnetic Tomography) taking into account both phasic and oscillatory generators to determine the neural generators of the N30 (Cebolla et al., 2011). They have determined that the N30 is generated by network activity in the motor, premotor and prefrontal cortex, adding further weight to its role as a marker of neural processing relevant to sensorimotor integration.
The results of these studies (Haavik-Taylor and Murphy, 2007c, 2010c; Haavik-Taylor et al., 2010) suggest that manipulation of dysfunctional cervical segments can alter early sensory processing and SMI of information from the upper limb. SMI is the process by which the nervous system coordinates incoming sensory (afferent) information from different parts of the body and integrates with the motor system to control movement.
Over the past three decades it has become well established that the human central nervous system (CNS) retains its ability to adapt to its ever-changing environment, and that both increased (hyperafferentation) and decreased (deafferentation) afferent input leads to changes in CNS functioning (Bertolasi et al., 1998; Brasil-Neto et al., 1993; Tinazzi et al., 1997). What has also become apparent is that these plastic changes may occur in a manner that is subjectively positive for the individual, such as with motor learning to enable complex finger movement (e.g. playing the piano). This is known as adaptive neuroplasticity. However, studies are also showing that these plastic changes may occur in a manner that has decidedly negative subjective outcomes for the individual, known as maladaptive neural plastic changes.
There is a growing body of literature that demonstrates maladaptive plastic changes are present in a variety of pain conditions/syndromes and musculoskeletal dysfunction (Falla, 2004; van Vliet and Heneghan, 2006), and that such adaptive changes can occur remarkably fast following an injury (Wall et al., 2002). This has lead various authors to hypothesize that such maladaptive neuroplastic changes present in long-term pain conditions rather than the actual pain itself is responsible for the individual sufferer’s symptoms and functional disturbances (Brumagne et al., 2000; Michaelson et al., 2003; Paulus and Brumagne, 2008). In particular, changes in the way the CNS processes proprioceptive information have been suggested as the most important factor responsible for the clinical presentation of neck pain sufferers (Paulus and Brumagne, 2008).
Numerous activities of daily living are dependent on appropriate SMI. Interactions between sensory and motor systems allow us to engage with our environment. It allows us to reach for and grasp objects, detect and turn towards an auditory stimuli or respond to perturbations from the environment in order to maintain postural stability, balance and locomotion (Chen et al., 2009). SMI involves strong feedback connections between different CNS structures that are associated with numerous, and perhaps all, neuroanatomical subsystems. These subsystems interconnect to form a dynamic, multimodal, sensorimotor integrative system. This system receives all afferent information from the environment. The CNS utilizes all of these peripheral signals continuously to build and maintain an internal reference frame (Lackner and DiZio, 2005; Sainsburg and Kalakanis, 1999).
This information is continuously processed in relation to information it receives regarding the voluntary intent of further movements, previous known estimations about such movements and internal sensory feedback from the actual movements when they take place. Continuous comparisons and error adjustments take place, and over time so does motor learning of frequent movements or actions. A breakdown anywhere in these multimodal sensorimotor feedback loops has the potential to greatly affect other interconnected neuroanatomical subsystems, in either an adaptive or maladaptive manner.
Motor commands or motor intention (also known as ‘‘efference copies’’) are for example known to interact with afferent signals to generate sensation, and are known to contribute to joint position sense (Smith et al., 2009). Under normal circumstances there is an integration of intention, action and sensory feedback. Furthermore, in a healthy state there is congruence between motor intention and sensory experience (both proprioceptive and visual) when we for example move a limb through space. Thus goal-directed action requires ongoing monitoring of sensorimotor inputs to ensure that motor outputs are congruent with current intentions as well as the proprioceptive feedback from the actual movement.
This monitoring is automatic but can become conscious if there is a mismatch between expected and realized sensorimotor states. A recent study has demonstrated that providing a sensorimotor conflict, i.e. providing unexpected visual feedback when moving a limb (via hiding a moving limb and/or distorting visual feedback of the movement of that limb) is sufficient to produce additional somaesthetic disturbances, and exacerbation of pre-existing symptoms in a group of fibromyalgia patients (McCabe et al., 2007). This suggests that a conflict between our expected and realized sensorimotor states can in some individuals produce or worsen pain sensations. It is therefore possible that a mechanism by which spinal manipulation relieves pain in patients is due to a central effect by improving somatosensory integration processes. This theory is however based on several assumptions that need to be verified in future studies, such as whether a mis-match in expected and actual sensory information can cause pain in healthy populations, whether spinal dysfunction causes somaesthetic disturbances and/or incongruence between motor intention and sensory experience (both proprioceptive and/or visual), and whether this is improved with spinal manipulation.
Discrepancies between expected and actual sensory information can cause pain in healthy populations and this has been explored in the laboratory setting (McCabe et al., 2005). Healthy pain-free volunteers were asked to perform a series of bilateral upper and lower limb movements whilst viewing these movements in a mirror that created varied degrees of sensory–motor conflict during the movements. They found that 66% of their healthy pain-free volunteers reported at least one anomalous sensory symptom at some stage in the protocol despite no peripheral nociceptive input. Several of these volunteers reported parasthesia sensations and mild aches or pain (McCabe et al., 2005), lending support to the hypothesis that motor–sensory conflict can induce pain and sensory disturbances in some normal individuals.
The importance of proprioception
Accurate proprioception is therefore an important component of sensorimotor integration in the CNS. Proprioception includes both joint position sense (JPS) and kinaesthesia (the sense of limb movement in the absence of visual cues) (for review see Gilman, 2002). The main source of afferent information for JPS arises from muscle spindles, however mechanoreceptors in joint capsules and cutaneous tactile receptors may also contribute (for review see Gilman, 2002).
Joint position sense has been extensively studied in the ankle, knee and hip joints (Adachi et al., 2007; Bennell et al., 2005; Beynnon et al., 2002; Hazneci et al., 2005; Hopper et al., 2003; Ishii et al., 1997; Karanjia and Ferguson, 1983; Larsen et al., 2005; Marks, 1996; Okuda et al., 2006; Reider et al., 2003; Ribeiro et al., 2007; Tsauo and Cheng, 2008),
particularly to investigate the effects ofreconstructive surgery (Adachi et al., 2007; Hopper et al., 2003; Reider et al., 2003),
osteoarthritis (Barrett et al., 1991; Bennell et al., 2003; Marks, 1996),
joint bracing (for review see Beynnon et al., 2002), and
various exercise or re-training programs (Friemert et al., 2006; Hazneci et al., 2005;
Ribeiro et al., 2007; Tsauo and Cheng, 2008).Recently there has also been an increased focus in the literature on spinal JPS (Allison, 2003; Jull et al., 2007; Learman et al., 2009; Strimpakos et al., 2006; Swinkels and Dolan, 1998), however, much less research has looked at the effect of the spine on limb JPS (Knox et al., 2006a,b; Knox and Hodges, 2005).
Improved head repositioning accuracy has been demonstrated by Palmgren et al. (2006) following chiropractic care, suggesting that spinal manipulation can improve spinal proprioception. The effects of improving spinal function on upper limb proprioception has also recently been investigated in a group of 25 participants with subclinical neck pain (SCNP) (i.e. reoccurring neck dysfunction such as neck pain, ache and/or stiffness with or without a history of known neck trauma) and 18 control participants (Haavik and Murphy, 2011). This study demonstrated that the SCNP group had reduced elbow joint position sense compared with those who had no history of any neck complaints.
Furthermore, the study showed that cervical spine manipulation of dysfunctional segments improved the accuracy of the SCNP groups’ elbow joint position sense (Haavik and Murphy, 2011). This suggests that cervical dysfunction can impair the way that proprioceptive information from the upper limb is processed. It also suggests that improving spinal function with manipulative treatment leads to more appropriate and accurate processing and integration of such proprioceptive input. However, it is worth noting that this study does not provide conclusive evidence that improving spinal ‘dysfunction’ was the precise cause of these observed effects.
In all of the cited studies by our group, spinal dysfunction was ‘quantified’ to some degree prior to and after each spinal manipulation intervention by assessing for tenderness to palpation of the relevant joints, manually palpating for restricted intersegmental range of motion, assessing for palpable asymmetric intervertebral muscle tension, and any abnormal or blocked joint play and end-feel of a joint. All of these biomechanical characteristics are known clinical indicators of spinal dysfunction (Fryer et al., 2004; Hestboek and Leboeuf-Yde, 2000). These findings were documented pre and post each spinal manipulation intervention. Improvements in segmental function following spinal manipulation were also recorded for each subject.
An important area for future research is to document more precise biomechanical data pre and post spinal manipulation to explore whether particular biomechanical characteristics of spinal dysfunction are associated with poor proprioception. Similarly it is necessary to explore whether particular biomechanical characteristics of improved spinal function correlate with improved proprioceptive processing. Furthermore, more precise reporting of biomechanical characteristics of the manipulation may reveal correlations between certain features of the manipulation and improved proprioceptive processing. This should be explored in future research.
In light of the above findings it is possible that the changes we previously observed in the cortical N20 and N30 SEP peaks following cervical spine manipulation (Haavik-Taylor and Murphy, 2007c, 2010c; Haavik-Taylor et al., 2010) reflect changes in the way the research participant’s CNS was perceiving and processing proprioceptive information from their stimulated upper limb. The low intensity stimuli applied during SEP recordings which are just above motor threshold, stimulate mainly large myelinated sensory afferents such as 1a muscle afferents (Gandevia and Burke, 1988; Gandevia et al., 1984).
There are numerous other studies that also implicate cervical spine impairment in maladaptive sensorimotor integration, for example affecting postural control and/or reduced JPS. This has been observed with chronic neck pain (Falla, 2004; Michaelson et al., 2003), neck muscle fatigue (Stapley et al., 2006), cervicobrachial pain syndrome (Karlberg et al., 1995), cervical root compression (Takayama et al., 2005a,b), and following whiplash injury (Stapley et al., 2006; Sterling et al., 2003). Therefore, there appears to be a considerable link between cervical function and accurate proprioceptive processing. Although most of these previous studies related to significant cervical injury or severe cervical symptoms, one study has demonstrated that changes in head and neck position in a group of participants without any history of neck pain or injury led to reduced accuracy of elbow joint position sense (Knox and Hodges, 2005). The authors of this study discussed how accurate execution of movement depends on the ability of the CNS to integrate somatosensory, vestibular, and visual information regarding the position of the body (Knox and Hodges, 2005). They argued that placing their subjects’ heads in full flexion and rotation could have led to an overload of the computational capacity of the CNS, thus resulting in increased JPS error (Knox and Hodges, 2005).
Altered somatosensory integration following spinal manipulation
A key component of typical SMI is early sensory integration of afferent input. The interaction and integration of afferent inputs from adjacent nerves at spinal, brainstem and cortical levels of the somatosensory system can be evaluated with a particular SEP protocol, i.e. by comparing spinal, brainstem and cortical SEP amplitudes obtained after stimulating two peripheral nerves simultaneously with the arithmetic sum of the SEP amplitudes obtained after stimulating the same nerves individually. In healthy subjects the dual input produces smaller SEP amplitudes compared with SEP amplitudes that were produced from the same nerves stimulated individually and arithmetically added together (Tinazzi et al., 2000). This type of sensory filtering is the ability of an individual’s CNS to suppress or attenuate the processing of multiple afferent peripheral, mainly proprioceptive, inputs. It is thought to reflect a type of ‘‘surround-like’’ inhibition, which in healthy individuals, allows for the contrast between stimuli to remain high by suppressing the processing of input from surrounding areas. In the somatosensory system, such inhibition allows for the body to perceive stimuli as separate and process them accordingly (Tinazzi et al., 2000a,b).

Figure 2 This filtering process has been found to be altered in individuals with neck pain, after repetitive muscular activities such as typing as well as other musculoskeletal disorders such as dystonia (Haavik- Taylor and Murphy, 2007a, 2010a,b, 2007c; Tinazzi et al., 2000). Two studies, utilizing this particular dual peripheral nerve stimulation SEP protocol both demonstrated that manipulating dysfunctional cervical segments can increase the surround-like inhibition of proprioceptive afferent input (Haavik-Taylor et al., 2010; Haavik-Taylor and Murphy, 2010c). Figure 2 is reprinted from Haavik- Taylor et al. (2010). It depicts a significant decrease in the N30 SEP peak when the median and ulnar nerve are stimulated simultaneously, as compared with the arithmetic sum of the SEP amplitudes obtained following stimulation of the two nerves individually.
Earlier work by Tinazzi et al. (2000) found that dystonia patients have a reduced ability to suppress the dual peripheral nerve input. They argued that this reduced ability to suppress the dual peripheral nerve input was evidence of inefficient integration, and that this could give rise to abnormal motor output, which might therefore contribute to the motor impairments present in dystonia. The decreased SEP ratios following cervical spine manipulation of dysfunctional spinal segments (Haavik-Taylor et al., 2010; Haavik-Taylor and Murphy, 2010c), indicative of an enhanced ability to filter sensory information, may therefore reflect an improvement in early sensory integration of afferent input. However, this assumes that a reduced ability to suppress dual peripheral nerve input is always a negative finding, and that increasing it reflects a positive or adaptive neuroplastic process, neither of which is firmly established.
The ability of the CNS to gate sensory information is thought to be important to maintain the internal representation of its current posture or activity and to avoid undesirable reactions to external or internal perturbations (Ivanenko et al., 2000; Paulus and Brumagne, 2008). As Tinazzi et al. demonstrated that gating of sensory information is distorted in patients with focal hand dystonia (Tinazzi et al., 2000). Other groups have also demonstrated that there is a shift in the gain of the sensory signals, i.e. a central re-weighting of proprioceptive input, in patients with spasmodic torticollis (Anastasopoulos et al., 2003) and low back pain patients (Brumagne et al., 2004).
Altered motor control following spinal manipulation

Figure 3

Figure 4 Several studies utilizing transcranial magnetic stimulation (TMS) (Haavik-Taylor and Murphy, 2007c, 2008) have also shown that manipulating dysfunctional segments in the cervical spine can alter sensorimotor integration of input from the upper limb. The TMS experimental measures utilized in these studies, such as shortinterval- intracortical-inhibition (SICI), short-interval-intracorticalfacilitation (SICF) and the cortical silent period (CSP), are measures of sensorimotor integration that are believed to reflect processing at the level of the cortex (Cantello et al., 1992; Chen et al., 1999, 1998; Di Lazzaro et al., 1998, 1999; Fisher et al., 2002; Hanajima et al., 2002; Inghilleri et al., 1993; Kujirai et al., 1993; Kukowski and Haug, 1992; Tokimura et al., 1996; Ziemann et al., 1998).
These motor control changes appear to occur in a muscle specific manner (Haavik- Taylor and Murphy, 2008). Figures. 3 and 4 are reprinted from Haavik-Taylor and Murphy (2008). Fig. 3 depicts a decrease in SICI for the abductor pollicis brevis (APB) muscle following spinal manipulation. No change was noted after the control intervention. Fig. 4 depicts an increase in SICF for the APB muscle and a decrease in SICF for the extensor indices proprios (EIP) muscle after the spinal manipulation session. Again no change was observed after the control intervention. The functional implications of these changes to upper limb function need further exploration.
Improved neuromuscular performance with spinal manipulation
When performing bodily movements, like throwing a ball for example, the central nervous system will activate a variety of postural trunk muscles prior to any movement of the arm in order to main postural stability during the throwing action. This process is known as feed-forward activation (FFA). Individuals with chronic low back pain are known to have delays in feedforward activation (Hodges and Richardson, 1999; Hodges et al., 1996), which is thought to influence postural stability. Early work by Murphy et al. (1995) had demonstrated the ability of sacroiliac joint (SIJ) manipulation to influence reflex excitability and her group sought to determine if SIJ manipulation might also influence feedforward activation. A study involving 90 healthy young males evaluated the participants for delays in FFA in the transversus abdominis muscle and internal obliques when undertaking rapid movements of the upper limb (Marshall and Murphy, 2006).
Seventeen subjects had a delay in FFA which was reproducible when retested 6 months later. These subjects were examined by a chiropractor and were all found to have dysfunction of the sacroiliac joint on the side of delayed FFA. Following a single chiropractic manipulation of the dysfunctional sacroiliac joint, the feed forward activation latency was reduced by an average of 38% (Marshall and Murphy, 2006). This study demonstrated an improvement in central nervous system activation times of muscles associated with the stability of a specific joint due to spinal manipulation. What is not known is whether the improvement in FFA persisted beyond the time of treatment. However, subsequent work by Marshall and Murphy (2008) demonstrated that chronic low back pain participants treated with manipulation and/or exercise, there was a continued improvement in delayed FFA times at 1 year follow-up.
Evidence for the association between delayed muscle activation times and impaired motor control is provided in a study by Radebold et al. (2001). They measured the ‘‘on’’ and ‘‘off’’ times of 12 agonist and antagonist trunk muscles during sudden trunk release movements in different directions, as well as postural sway in individuals with chronic back pain and controls. They found that chronic low back pain patients have delayed muscle ‘‘on’’ and ‘‘off’’ times and that these delays correlated significantly with impaired balance performance with eyes closed. FFA times have also been shown to correlate strongly with a patient’s self-rated disability (Marshall and Murphy, 2010) indicating that these neuromuscular measures may be useful markers of both treatment effects and potential for chronicity in neck and back pain patients.
Motor impairments are present in chronic neck pain patients. Impairment of deep cervical neck flexor activation and significant postural disturbances during walking and standing have been demonstrated in both insidious-onset and trauma-induced chronic neck pain conditions (Alund et al., 1993; Branstrom et al., 2001; Jull et al., 2004; Karlberg et al., 1995; Michaelson et al., 2003; Persson et al., 1996; Rubin et al., 1995). Altered sensitivity of proprioceptors within the neck muscles has been suggested to be related to the postural (i.e. motor control) disturbances seen in these patients (Michaelson et al., 2003; Persson et al., 1996). It has also been argued that the degree to which proprioceptive input to the central nervous system is disturbed, and even more importantly how the CNS processes, interprets and transforms this afferent information into motor commands, that determines the degree to which subjects can successfully execute more challenging balance tasks (Michaelson et al., 2003; Paulus and Brumagne, 2008).
It is therefore possible that spinal manipulation in patients with sub-clinical or more chronic neck pain is able to improve the central processing of proprioceptive information, and that this is part of the mechanism by which high-velocity, low-amplitude spinal manipulation improve function and reduce chronicity and reoccurrence in these patient populations. It is possible that the changes in cortical somatosensory processing (Haavik-Taylor and Murphy, 2007c; Zhu et al., 2000, 1993), sensorimotor integration (Haavik-Taylor and Murphy, 2007c, 2008) and motor control (Haavik-Taylor and Murphy, 2007b, 2008; Marshall and Murphy, 2006; Suter and McMorland, 2002; Suter et al., 1999) that have been previously documented following high-velocity, low-amplitude spinal manipulation reflect changes in central processing of proprioceptive afferent input.
Limitations
It is worth noting that the SEP recording protocol utilized in the cited studies (Haavik-Taylor and Murphy, 2007c, 2010c; Haavik- Taylor et al., 2010) cannot rule out spinal cord and/or brainstem or subcortical changes. For example, more than 500 stimuli need to be averaged for reliable far-field brainstem and subcortical SEP peaks. SEP recordings also took several minutes to record and the recordings did not commence until the electrode impedance was re-checked after the cervical manipulations had been performed. Short lived spinal and/or brainstem changes can therefore not be ruled out either.
It is also worth noting that the cited studies (Haavik and Murphy, 2011, 2007c, 2008, 2010a,c; Haavik-Taylor et al., 2010; Marshall and Murphy, 2006) do not provide conclusive evidence that the observed neuroplastic changes following spinal manipulation are changes due to the correction of joint dysfunction. Clinicians practicing manipulation assess, via palpation and observation, that the area of spinal dysfunction has had its appropriate range of movement restored following manipulation. It is tempting to attribute the documented neurophysiological changes to this restoration of appropriate movement (as we have proposed).
However, the observed neuroplastic changes could also merely be due to the afferent barrage associated with the manipulative thrust. Future studies should endeavour to address this question. We have however cited one longer term study which showed that the FFA time continued to improve up to one year after a 3 month period of treatment with exercise and/or manipulation in a group of chronic low back pain patients (Marshall and Murphy, 2008). This suggests that some of these neurophysiological markers do indeed have the capacity to improve following a period of successful care even in a chronic back pain group.
Conclusion
Many of the studies discussed in this paper show that spinal manipulation results in plastic changes in sensorimotor integration within the central nervous system in human participants. Collectively these studies provide evidence to support a central mechanism of action for high-velocity, low-amplitude spinal manipulation. What is not yet clear is the degree to which these changes correlate with beneficial clinical outcomes. It is also not clear whether these changes are due to the correction of spinal dysfunction, therefore normalizing aberrant afferent input to the CNS, or whether they are merely due to an afferent barrage associated with the manipulative thrust. These questions remain to be answered and are the focus of our ongoing research efforts.
Acknowledgments
The authors would like to acknowledge the following organizations for support and funding over the past 15 years: Australian Spinal Research Foundation, Hamblin Chiropractic Research Fund Trust, University of Auckland Vice Chancellors Fund, New Zealand Tertiary Education Commission Top Achievers Doctoral Scholarship, Foundation for Chiropractic Education and Research, Natural Science and Engineering Research Council of Canada, Canada Foundation for Innovation, the University of Ontario Institute of Technology and the New Zealand College of Chiropractic.
References:
Adachi N, Ochi M, Uchio Y, Iwasa J, Ishikawa M, Shinomiya R.
Temporal change of joint position sense after posterior cruciate ligament reconstruction using multi-stranded hamstring tendons.
Knee Surg Sports Traumatol Arthrosc 2007;15(1):2–8.Allison GT.
Trunk muscle onset detection technique for EMG signals with ECG artefact.
J Electromyogr Kinesiol 2003;13:209–16.Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD.
Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating short-latency activity.
J Neurophysiol 1989a;62(3):694–710.Allison T, McCarthy G, Wood CC, Jones SJ.
Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings.
Brain 1991;114(Pt 6):2465–503.Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD.
Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity.
J Neurophysiol 1989b;62(3):711–22.Alund M, Ledin T, Odkvist L. S.E. L.
Dynamic posturography among patients with common neck disorders.
J Vestib Res 1993;3:383–9.Anastasopoulos D, Nasios G, Mergner T, Maurer C.
Idiopathic spasmodic torticollis is not associated with abnormal kinesthetic perception from neck proprioceptive and vestibular afferences.
J Neurol 2003;250:546–55.Barrett DS, Cobb AG, Bentley G.
Joint proprioception in normal, osteoarthritic and replaced knees.
J Bone Joint Surg Br 1991;73(1):53–6.Bennell K, Wee E, Crossley K, Stillman B, Hodges P.
Effects of experimentallyinduced anterior knee pain on knee joint position sense in healthy individuals.
J Orthop Res 2005;23(1):46–53.Bennell KL, Hinman RS, Metcalf BR, Crossley KM, Buchbinder R, Smith M, et al.
Relationship of knee joint proprioception to pain and disability in individuals with knee osteoarthritis.
J Orthop Res 2003;21(5):792–7.Bertolasi L, Priori A, Tinazzi M, Bertasi V, Rothwell JC.
Inhibitory action of forearm flexor muscle afferents on corticospinal outputs to antagonist muscles in humans.
J Physiol 1998;511(Pt 3):947–56.Beynnon BD, Good L, RisbergMA.
The effect of bracing on proprioception of kneeswith anterior cruciate ligament injury.
J Orthop Sports Phys Ther 2002;32(1):11–5.Branstrom H, Malmgren-Olsson EB, Barnekow-Bergkvist M.
Balance performance in patients with whiplash associated disorders and patients with prolonged musculoskeletal disorders.
Adv Physiother 2001;3:120–7.Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, et al.
Rapid modulation of human cortical motor outputs following ischaemic nerve block.
Brain 1993;116(Pt 3):511–25.Brumagne S, Cordo P, Verschueren S.
Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing.
Neurosci Lett 2004;366:63–6.Brumagne S, Cordo PP, Lysens RMDP, Verschueren SP, Swinnen SP.
The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain.
Spine 2000;25(8):989–94.Cantello R, Gianelli M, Civardi C, Mutani R.
Magnetic brain stimulation: the silent period after the motor evoked potential.
Neurology 1992;42(10):1951–9.Cebolla AM, Palmero-Soler E, Dan B, Cheron G.
Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential.
NeuroImage 2011;54(2):1297–306. doi:10.1016/j.neuroimage.2010.08.060.Chen JL, Penhune VB, Zatorre RJ.
The role of auditory and premotor cortex in sensorimotor transformations.
Ann N Y Acad Sci 2009;1169:15–34 [The Neurosciences and Music III Disorders and Plasticity].Chen R, Lozano AM, Ashby P.
Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings.
Exp Brain Res 1999;128(4):539–42.Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al.
Intracortical inhibition and facilitation in different representations of the human motor cortex.
J Neurophysiol 1998;80(6):2870–81.Cheron G, Borenstein S.
Gating of the early components of the frontal and parietal somatosensory evoked potentials in different sensory-motor interference modalities.
Electroencephalogr Clin Neurophysiol 1991;80(6):522–30.Cheron G, Borenstein S.
Mental movement simulation affects the N30 frontal component of the somatosensory evoked potential.
Electroencephalogr Clin Neurophysiol 1992;84(3):288–92.Desmedt JE, Cheron G.
Central somatosensory conduction in man: neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes.
Electroencephalogr Clin Neurophysiol 1980;50(5–6):382–403.Desmedt JE, Cheron G.
Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal man: differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components.
Electroencephalogr Clin Neurophysiol 1981;52(6):553–70.Desmedt JE, Huy NT, Bourguet M.
The cognitive P40, N60 and P100 components of somatosensory evoked potentials and the earliest electrical signs of sensory processing in man.
Electroencephalogr Clin Neurophysiol 1983;56(4): 272–82.Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al.
Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits.
Exp Brain Res 1998;119:265–8.Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, et al.
Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex.
Exp Brain Res 1999;129(4):494–9.Falla D.
Unravelling the complexity of muscle impairment in chronic neck pain.
Man Ther 2004;9(3):125–33.Fisher RJ, Nakaura Y, Bestmann S, Rothwell JC, Bostock H.
Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking.
Exp Brain Res 2002;143:240–8.Friemert B, Bach C, Schwarz W, Gerngross H, Schmidt R.
Benefits of active motion for joint position sense.
Knee Surg Sports Traumatol Arthrosc 2006;14(6): 564–70.Fryer G, Morris T, Gibbons P.
Paraspinal muscles and intervertebral dysfunction: part one.
J Manipulative Physiol Ther 2004;27(4):267–74.Gandevia SC, Burke D.
Projection to the cerebral cortex from proximal and distal muscles in the human limb.
Brain 1988;111:389–403.Gandevia SC, Burke D, McKeon B.
he projection of muscle afferents from the hand to cerebral cortex in man.
Brain 1984;107:1–13.Gilman S.
Joint position sense and vibration sense: anatomical organisation and assessment.
J Neurol Neurosurg Psychiatry 2002;73(5):473–7.Haavik-Taylor H, Murphy B.
Altered cortical integration of dual somatosensory input following the cessation of a 20 min period of repetitive muscle activity.
Exp Brain Res 2007a;178(4):488–98.Haavik-Taylor H, Murphy B.
Transient modulation of intracortical inhibition following spinal manipulation.
Chiropractic J Aust 2007b;37:106–16.Haavik-Taylor H, Murphy B.
Altered Central Integration of Dual Somatosensory Input After Cervical Spine Manipulation
J Manipulative Physiol Ther. 2010 (Mar); 33 (3): 178–188Haavik-Taylor H, Murphy B.
The Effects of Spinal Manipulation on Central Integration of Dual Somatosensory Input
Observed After Motor Training: A Crossover Study
J Manipulative Physiol Ther. 2010 (May); 33 (4): 261–272Haavik, H and Murphy, B.
Subclinical Neck Pain and the Effects of Cervical Manipulation on Elbow Joint Position Sense
J Manipulative Physiol Ther. 2011 (Feb); 34 (2): 88–97Haavik-Taylor H, Holt K, Murphy B.
Exploring the Neuromodulatory Effects of the Vertebral Subluxation and Chiropractic Care
Chiropractic Journal of Australia 2010 (Mar); 40 (1): 37–44Haavik-Taylor H, Murphy B.
Cervical Spine Manipulation Alters Sensorimotor Integration:
A Somatosensory Evoked Potential Study
Clin Neurophysiol. 2007 (Feb); 118 (2): 391–402Haavik-Taylor H, Murphy B.
Altered sensorimotor integration with cervical spine manipulation.
J Manipulative Physiol Ther 2008;31(2):115–26.Haavik Taylor, H.; Murphy, B.
The Effects of Spinal Manipulation on Central Integration of Dual Somatosensory
Input Observed After Motor Training: A Crossover Study
J Manipulative Physiol Ther. 2010 (May); 33 (4): 261–272Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, et al.
Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans.
J Physiol 2002;538(Pt 1):253–61.Hazneci B, Yildiz Y, Sekir U, Aydin T, Kalyon TA.
Efficacy of isokinetic exercise on joint position sense and muscle strength in patellofemoral pain syndrome.
Am J Phys Med Rehabil 2005;84(7):521–7.Hestboek L, Leboeuf-Yde C.
Are chiropractic tests for the lumbo-pelvic spine reliable and valid? A systematic critical literature review.
J Manipulative Physiol Ther 2000;23(4):258–75.Hodges PW, Richardson CA.
Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds.
Arch Phys Med Rehabil 1999;80(9):1005–12.Hodges PW, Richardson CA, Jull G.
Evaluation of the relationship between laboratory and clinical tests of transversus abdominis function.
Physiother Res Int 1996;1(1):30–40.Hopper DM, Creagh MJ, Formby PA, Goh SC, Boyle JJ, Strauss GR.
Functional measurement of knee joint position sense after anterior cruciate ligament reconstruction.
Arch Phys Med Rehabil 2003;84(6):868–72.Inghilleri M, Berardelli A, Cruccu G, Manfredi M.
Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction.
J Physiol 1993;466:521–34.Ishii Y, Terajima K, Terashima S, Bechtold JE, Laskin RS.
Comparison of joint position sense after total knee arthroplasty.
J Arthroplasty 1997;12(5):541–5.Ivanenko YP, Solopova IA, Levik YS.
The direction of postural instability affects postural reactions to ankle muscle vibration in humans.
Neurosci Lett 2000;292:103–6.Jull G, Falla D, Treleaven J, Hodges P, Vicenzino B.
Retraining cervical joint position sense: the effect of two exercise regimes.
J Orthop Res 2007;25(3): 404–12.Jull G, Kristjansson E, Dall’Alba P.
Impairment in the cervical flexors: a comparison of whiplash and insidious onset neck pain patients.
Man Ther 2004;9(2):89–94.Kanovsky´ P, Bare M, Rektor I.
The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings.
Clin Neurophysiol 2003;114(6):981–91.Karanjia PN, Ferguson JH.
Passive joint position sense after total hip replacement surgery.
Ann Neurol 1983;13(6):654–7.Karlberg M, Persson L, Magnusson M.
Reduced postural control in patients with chronic cervicobrachial pain syndrome.
Gait Posture 1995;3:241–9.Knox J, Cordo P, Skoss R, Durrant S, Hodges P.
Illusory changes in head position induced by neck muscle vibration can alter the perception of elbow position.
Behav Neurosci 2006a;120(6):1211–7.Knox JJ, Coppieters MW, Hodges PW.
Do you know where your arm is if you think your head has moved?
Exp Brain Res 2006b;173(1):94–101. Epub 2006 Mar 25.Knox JJ, Hodges PW.
Changes in head and neck position affect elbow joint position sense.
Exp Brain Res 2005;165(1):107–13.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al.
Corticocortical inhibition in human motor cortex.
J Physiol 1993;471: 501–19.Kukowski B, Haug B.
Quantitative evaluation of the silent period, evoked by transcranial magnetic stimulation during sustained muscle contraction, in normal man and in patients with stroke.
Electromyogr Clin Neurophysiol 1992;32(7–8):373–8.Lackner J, DiZio P.
Vestibular, proprioceptive, and haptic contribution to spatial orientation.
Annu Rev Psychol 2005;56:115–47.Larsen R, Lund H, Christensen R, Rogind H, Danneskiold-Samsoe B, Bliddal H.
Effect of static stretching of quadriceps and hamstring muscles on knee joint position sense.
Br J Sports Med 2005;39(1):43–6.Leach RA.
Manipulation terminology in the chiropractic, osteopathic, and medical literature. 2nd ed.
The Chiropractic Theories: a synopsis of Scientific Research.
Baltimore: Williams and Wilkins; 1986. p. 15.Learman KE, Myers JB, Lephart SM, Sell TC, Kerns GJ, Cook CE.
Effects of spinal manipulation on trunk proprioception in subjects with chronic low back pain during symptom remission.
J Manipulative Physiol Ther 2009;32:118–26.Marks R.
Further evidence of impaired position sense in knee osteoarthritis.
Physiother Res Int 1996;1(2):127–36.Marshall P, Murphy B.
The effect of sacroiliac joint manipulation on feed-forward activation times of the deep abdominal musculature.
J Manipulative Physiol Ther 2006;29(3):196–202.Marshall P, Murphy B.
Delayed abdominal muscle onsets and self-report measures of pain and disability in chronic low back pain.
J Electromyogr Kinesiol 2010;20(5):833–9.Marshall PW, Murphy BA.
Muscle activation changes after exercise rehabilitation for chronic low back pain.
Arch Phys Med Rehabil 2008;89(7):1305–13.Mauguiere F.
Somatosensory evoked potentials: normal responses, abnormal waveforms and clinical applications in neurological diseases.
In: Niedermeyer E, editor. Electroencephalography: basic principles, clinical applications,
and related fields.
Baltimore: Williams and Wilkins; 1999.Mauguiere F, Desmedt JE, Courjon J.
Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning.
Brain 1983;106(Pt 2):271–311.McCabe CS, Cohen H, Blake DR.
Somaesthetic disturbances in fibromyalgia are exaggerated by sensory–motor conflict: implications for chronicity of the disease?
Rheumatology 2007;46:1587–92.McCabe CS, Haigh RC, Halligan PW, Blake DR.
Simulating sensory–motor incongruence in healthy volunteers: implications for a cortical model of pain.
Rheumatology 2005;44:509–16.Michaelson P, Michaelson M, Jaric S, Latash ML, Sjolander P, Djupsjobacka M.
Vertical posture and head stability in patients with chronic neck pain.
J Rehabil Med 2003;35(5):229–35.Murphy B, Dawson N, Slack J.
Sacroiliac joint manipulation decreases the H-reflex.
Electroencephalogr Clin Neurophysiol 1995;35:87–94.Nuwer MR, Aminoff M, Desmedt J, Eisen AA, Goodin D, Matsuoka S, et al.
IFCN recommended standards for short latency somatosensory evoked potentials.
Report of an IFCN committee.
International Federation of Clinical Neurophysiology.
Electroencephalogr Clin Neurophysiol 1994;91(1):6–11.O’Connor BL, Visco DM, Brandt KD, Albrecht M, O’Connor AB.
Sensory nerves only temporarily protect the unstable canine knee joint from osteoarthritis. Evidence that sensory nerves reprogram the central nervous system after cruciate ligament transection.
Arthritis Rheum 1993;36(8):1154–63.Okuda T, Ochi M, Tanaka N, Nakanishi K, Adachi N, Kobayashi R.
Knee joint position sense in compressive myelopathy.
Spine 2006;31(4):459–62.Palmgren PJ, Sandstrom PJ, Lundqvist FJ, Heikkila H.
Improvement After Chiropractic Care in Cervicocephalic Kinesthetic
Sensibility and Subjective Pain Intensity in Patients
with Nontraumatic Chronic Neck Pain
J Manipulative Physiol Ther 2006 (Feb); 29 (2): 100–106Paulus I, Brumagne S.
Altered interpretation of neck proprioceptive signals in persons with subclinical recurrent neck pain.
J Rehabil Med 2008;40(6): 426–32.Persson L, Karlberg M, Magnusson M.
Effects of different treatments on postural performance in patients with cervical root compression: a randomized prospective study assessing the importance of the neck in postural control.
J Vestib Res 1996;6(6):439–53.Radebold A, Cholewicki J, Polzhofer GK, Greene HS.
Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain.
Spine 2001;26(7):724–30.Reider B, Arcand MA, Diehl LH, Mroczek K, Abulencia A, Stroud CC, et al.
Proprioception of the knee before and after anterior cruciate ligament reconstruction.
Arthroscopy 2003;19(1):2–12.Ribeiro F, Mota J, Oliveira J.
Effect of exercise-induced fatigue on position sense of the knee in the elderly.
Eur J Appl Physiol 2007;99(4):379–85.Rossi S, della Volpe R, Ginanneschi F, Ulivelli M, Bartalini S, Spidalieri R, et al.
Early somatosensory processing during tonic muscle pain in humans: relation to loss of proprioception and motor ‘defensive’ strategies.
Clin Neurophysiol 2003;114(7):1351–8.Rossini PM, Babiloni F, Bernardi G, Cecchi L, Johnson PB, Malentacca A, et al.
Abnormalities of short-latency somatosensory evoked potentials in parkinsonian patients.
Electroencephalogr Clin Neurophysiol 1989;74(4): 277–89.Rossini PM, Gigli GL, Marciani MG, Zarola F, Caramia M.
Non-invasive evaluation of input–output characteristics of sensorimotor cerebral areas in healthy humans.
Electroencephalogr Clin Neurophysiol 1987;68(2):88–100.Rubin AM, Woolley SM, Dailey VM, Goebel JA.
Postural stability following mild head or whiplash injuries.
Am J Otol 1995;16(2):216–21.Sainsburg RG, Kalakanis CD.
Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms.
J Neurophysiol 1999;81:1045–56.Shakespeare DT, Stokes M, Sherman KP, Young A.
Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain.
Clin Physiol 1985;5(2): 137–44.Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC.
Signals of motor command bias joint position sense in the presence of feedback from proprioceptors.
J Appl Physiol 2009;106:950–8.Stapley PJ, Beretta MV, Toffola ED, Schieppati M.
Neck muscle fatigue and postural control in patients with whiplash injury.
Clin Neurophysiol 2006; 117(3):610–22.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R.
Development of motor system dysfunction following whiplash injury.
Pain 2003;103(1–2):65–73 [see comment].Strimpakos N, Sakellari V, Gioftsos G, Kapreli E, Oldham J.
Cervical joint position sense: an intra- and inter-examiner reliability study.
Gait Posture 2006;23(1): 22–31.Suter E, McMorland G.
Decrease in elbow flexor inhibition after cervical spine manipulation in patients with chronic neck pain.
Clin Biomech 2002;17(7): 541–4.Suter E, McMorland G, Herzog W, Bray R.
Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain.
J Manipulative Physiol Ther 1999;22(3):149–53.Suter E, McMorland G, Herzog W, Bray R.
Conservative lower back treatment reduces inhibition in knee-extensor muscles: a randomized controlled trial.
J Manipulative Physiol Ther 2000;23(2):76–80.Swinkels A, Dolan P.
Regional assessment of joint position sense in the spine.
Spine 1998;23(5):590–7.Takayama H, Muratsu H, Doita M, Harada T, Kurosaka M, Yoshiya S.
Proprioceptive recovery of patients with cervical myelopathy after surgical decompression.
Spine 2005a;30(9):1039–44.Takayama H, Muratsu H, Doita M, Harada T, Yoshiya S, Kurosaka M.
Impaired joint proprioception in patients with cervical myelopathy.
Spine 2005b;30(1): 83–6.Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguiere F, Fiaschi A.
Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow.
Brain 2000;123(Pt 1):42–50.Tinazzi M, Zanette G, Polo A, Volpato D, Manganotti P, Bonato C, et al.
Transient deafferentation in humans induces rapid modulation of primary sensory cortex not associated with subcortical changes: a somatosensory evoked potential study.
Neurosci Lett 1997;223(1):21–4.Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC.
Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex.
Electroencephalogr Clin Neurophysiol 1996;101(4):263–72.Tsauo J-Y, Cheng P-F.
The effect of sensorimotor training on knee proprioceptive and function for patients with knee osteoarthritis: a preliminary report.
Clin Rehab 2008;22:448–57.van Vliet PM, Heneghan NR.
Motor control and the management of musculoskeletal dysfunction.
Manual Therapy Conference Proceedings from the 2nd International Conference
on Movement Dysfunction Pain and Performance.
Evidence and Effect 2006 2006/8;11(3):208.Waberski TD, Buchner H, Perkuhn M, Gobbele R, Wagner M, Kucker W, et al.
N30 and the effect of explorative finger movements: a model of the contribution of the motor cortex to early somatosensory potentials.
Clin Neurophysiol 1999;110(9):1589–600.Wall JT, Xu J, Wang X.
Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body.
Brain Res Rev 2002;39(2–3):181–215.Zhu Y, Haldeman S, Hsieh CY, Wu P, Starr A.
Do cerebral potentials to magnetic stimulation of paraspinal muscles reflect changes in palpable muscle spasm, low back pain, and activity scores?
J Manipulative Physiol Ther 2000;23(7): 458–64.Zhu Y, Haldeman S, Starr A, Seffinger MA, Su SH.
Paraspinal muscle evoked cerebral potentials in patients with unilateral low back pain.
Spine 1993;18(8): 1096–102.Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W.
Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation.
J Physiol 1998;511(Pt 1):181–90.

Heidi Haavik Heidi Haavik is a chiropractor and a neurophysiologist who has worked in the area of human neurophysiology for the past ten years. She has utilized techniques such as somatosensory evoked electroencephalography and transcranial magnetic brain stimulation to investigate the effects of chiropractic manipulation of dysfunctional spinal segments on somatosensory processing, sensorimotor integration and motor cortical output. Dr. Haavik graduated from the New Zealand College of Chiropractic in 1999, and was awarded her PhD degree by the University of Auckland in 2008. She is currently the Director of Research at the New Zealand College of Chiropractic where she has established two human neurophysiology research laboratories. Dr. Haavik is also an Adjunct Professor at the University of Ontario, Institute of Technology in Oshawa, Canada and is a member of the World Federation of Chiropractic’s Research Council. Dr. Haavik has received numerous research awards and has published a number of papers in chiropractic and neurophysiology journals. She has presented her work to both chiropractic and neuroscience communities around Australasia, North America and Europe. She is on the Editorial Board of the Journal of Manipulative and Physiological Therapeutics and Journal of Chiropractic Education. She was named Chiropractor of the year in 2007 by both the New Zealand Chiropractic Association and the New Zealand College of Chiropractic Alumni Association.

Bernadette A. Murphy
Bernadette A. Murphy graduated from the Canadian Memorial Chiropractic College in 1989 before moving to New Zealand where she completed her MSc (1992) and PhD (1998) in Human Neurophysiology at the University of Auckland. She was a fulltime faculty member in the Department of Sport and Exercise Science from 1999 to 2007, where she established an MSc in Exercise Rehabilitation. In January 2008, she returned to Canada and took a position in the Faculty of Health Sciences at the University of Ontario Institute of Technology (UOIT). She is a Professor and Director of Health Sciences and Kinesiology, and heads the Human Neurophysiology and Rehabilitation Laboratory. The overall theme of her research is neural adaptation in humans and the role of physical interventions such as manipulation and exercise in aiding the re-establishment of appropriate neuromuscular connections. She was the Recipient of a $100,000 Vice-Chancellor’s Early Career Research Award at Auckland in 2002 which funded much of her initial work into neuromuscular alterations in chronic low back pain, as well as several grants from the Australian Spinal Research Foundation which enabled her to investigate disordered sensorimotor integration in neck and back pain patients. Her current basic science research is funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada. She is on the Editorial Board of ISRN Rehabilitation and the Journal of the Canadian Chiropractic Association. She has supervised numerous Masters and PhD students and reviews for several journals including Neuroimage, Journal of Neurophysiology, Spine, Clinical Neurophysiology, Experimental Brain Research, JEK and JMPT.

Return to SUBLUXATION
Since 5-03-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |