

The Role of Nutrients in the Pathogenesis and
Treatment of Migraine Headaches: ReviewThis section was compiled by Frank M. Painter, D.C.
Make comments or suggestions to Frankp@chiro.org




FROM: Biomed Pharmacother. 2018 (Jun); 102: 317–325 ~ FULL TEXT
Elyas Nattagh-Eshtivania, Mahmood Alizadeh Sanib, Monireh Dahria, Faezeh Ghalichia, Abed Ghavamia, Pishva Arjanga, Ali Tarighat-Esfanjanic
Faculty of Nutrition and Food Sciences,
Tabriz University of Medical Sciences,
Tabriz, Iran.
nattaghe@tbzmed.ac.ir
OBJECTIVE: Migraine as a disabling neurovascular disease affects 6% of men and 18% of women worldwide. The deficiency of many nutrients including magnesium, niacin, riboflavin, cobalamin, coenzymes Q10, carnitine, α-lipoic acid and vitamin D is associated with migraine. Some researchers postulate that mitochondrial dysfunction and impaired antioxidant status can cause migraine. Also increase in homocysteine level can lead to migraine attacks; therefore, some Nutraceuticals play a vital role in migraine prevention. Thus, the aim of the current study was to review randomized controlled trials (RCT) assessing the effect of nutritional supplements on migraine patients.
METHODS: English articles in the following databases were searched: MEDLINE, AMED, EMBASE and Cochrane Library. In this manuscript, RCTs published during 1990-2017 were reviewed.
RESULTS: Evidences indicate that supplementation with magnesium, carnitine, riboflavin, niacin, CoQ10, vitamin D, Vitamin B12 and alpha lipoic acid have prophylactic and therapeutic effects on migraine patients.
CONCLUSION: Due to the possible side effects of pharmacological drugs and drug addictions, the use of nutrient compounds alone or in combination with routine cures have been proposed. However, further constructive studies are required.
KEYWORDS: Headache; Keyword; Migraine; Mitochondrial dysfunction; Nutrients
From the FULL TEXT Article:
Introduction
Migraine is a primary headache disorder diagnosed by recurrent and moderate to severe headaches. These unilateral and pulsating headaches last from 4 to 72 hours [1]. Associated symptoms include nausea, vomiting, sensitivity to light, sound and odor. Physical activity may increase the intensity of pain [2]. Migraine attacks may be with or without aura (a short period of visual disturbance signaling headache occurrence). Occasionally, an aura sometimes occurs with headaches [3]. Migraine is the second main cause of headaches after tension type headaches. Migraine is a debilitating brain disorder with serious social and financial consequences for the individual and the society [4]. The incidence of migraine is higher among women due to hormonal influences [5]. Although the main cause of migraine is unknown, various factors such as genetics and environmental factors, are involved in the onset of migraine attacks [6]. Mutation in the MTHFR gene, abnormal level of vitamin D, production of inflammatory agents around the nerves and cerebrospinal fluid, low serotonin level, increased calcitonin gene related peptide (CGRP), matrix metalloproteinase 9 (MMP-9), homocysteine and nitric oxide (NO) levels, mitochondrial dysfunction and decreased level of metabolic enzymes are among the most important causes of migraine [7–11]. In migraine-susceptible people, vasoactive peptides such as CGRP and substance P, are released from trigeminovascular neurons. These peptides exacerbate vasodilation and cause neurogenic inflammation which may lead to vasodilation and leakage of blood vessels [12]. Vasodilation and neurogenic inflammation increase activation of trigeminovascular neurons and modulate transmission of pain impulses in the brain. Studies have indicated that inflammatory factors, such as tumor necrosis factor-α (TNF-α), increase CGRP transcription [13].
Migraine drug treatments aim to prevent headache attack or reduce the intensity and frequency of attacks, particularly when they are characterized by intense pain. Triptans can be considered as important drugs for acute treatment; they effect serotonin (5-HT) 1B/D/F receptors located on presynaptic trigeminal nerve endings of vascular smooth muscle and the central nervous system (CNS) [14–16]. In addition to tryptan, various other drugs including beta blockers, tricyclic antidepressants, calcium channel blockers, NSAIDs, and anticonvulsants are used in treating migraine [16, 17].
In addition to preventive treatments, some minerals such as (Mg), coenzymes Q10 (CoQ10), a-lipoic), vitamins (B2, B3, B12, D) and carnitine, are often considered as nutrients rather than drugs and are effective in migraine prevention [18–22].
Researchers have measured the baseline levels of riboflavin, vitamin D, folate, CoQ10 and magnesium in migraine patients. A high percentage of patients have CoQ10, vitamin D, riboflavin, magnesium deficiencies. Interestingly, young women and girls are more likely to experience CoQ10 deficiency and boys are susceptible to vitamin D deficiency. Additionally, an association between migraine and cardiovascular diseases and mortality is mentioned among women. Patients suffering from chronic migraines at regular intervals are in risk of CoQ10, magnesium, vitamin D and riboflavin deficiency, compared to those with episodic migraines with infrequent intervals. Since there is no comprehensive study reviewing the effects of dietary supplements on migraine patients, the purpose of this review was to determine the effect of mineral, coenzyme and vitamin deficiencies in the pathogenesis of migraine headaches and their potential therapeutic effect on migraine.
Magnesium
Magnesium is the second frequent intracellular cation present in all tissues. Magnesium plays many roles in the human body. It contributes to intracellular energy storage and expenditure, acts as a cofactor in many enzymes, is required for nucleic acid synthesis and is involved in cell division and growth, as well as regulation of ion channels, receptors and the transport system. Migraine is likely considered as a brain excitability disorder [23]. Magnesium deficiency may increase the sensitivity of migraine neuro-inflammation, calcium channel and N-methyl- D-aspartate (NMDA) receptor blockade, glutamate and nitric oxide activity, serotonin receptor affinity, and endogenous hormone regulation [24]. Magnesium has an important role in the regulation of NMDA glutamate receptors which are involved in pain transmission inside the nervous system and controlling brain blood flow [25, 26]. Magnesium blocks NMDA receptors and prevents the entry of calcium into cells [27, 28]. As such, low magnesium level accelerates activation of NMDA receptors which provoke the entry of calcium into cells and effects neurons and cerebral vascular muscles. Therefore, magnesium acts as an NMDA receptor antagonist. Studies have shown that NMDA receptors play an important role in the onset and progression of Cortical Spreading Depression (CSD) [29, 30]. The CSD theory is related to the extension of migraine aura [31–33]. One of the important mechanisms that has been considered to increase the sensitivity of the brain to this phenomenon is alteration of mitochondrial metabolism. Magnesium deficit may lead to CSD through alteration of oxidative phosphorylation and neuronal polarization in the mitochondria [34].

Table 1 Therefore, by counteracting vasospasm, inhibiting platelet accumulation, stabilizing cell membranes and decreasing the formation of inflammatory mediators, magnesium may beneficially target different aspects of the neurogenic inflammation which occur during migraine and eventually improve mitochondrial oxidative phosphorylation, 5-HT neurotransmission and the NO system [35]. One of the primary scientific studies by Nuclear magnetic resonance spectroscopy reported the role of magnesium in migraine and magnesium level decrease in patients when compared to healthy controls [36]. Also, several studies have shown that serum level of magnesium in migraine patients is lower than healthy subjects [37–43]. Intravenous (IV) magnesium administration is routinely offered for acute migraine, as well as prophylaxis, while oral magnesium supplementation is prescribed for prophylaxis. The American Academy of Neurology (AAN) has revealed the effectiveness of oral magnesium usage in migraine prevention (level B evidence). A meta-analysis [44] assessing the effectiveness of IV magnesium in acute migraine treatment suggested level of U for IV magnesium. The suggested dose of magnesium supplement is 400 mg per day, and can be raised up to 1200 mg, if tolerated. Possible gastrointestinal adverse effects of magnesium supplementation are abdominal pain, nausea and diarrhea [35]. Among the various forms of magnesium supplements, magnesium glycinate and other amino acid-chelated forms are likely to be tolerated [35]. Table 1 demonstrates the effects of magnesium on migraine symptoms in various clinical trials.
Riboflavin

Table 2 Riboflavin plays an important role in the metabolism of carbohydrates, fats, and proteins. Riboflavin, or vitamin B2, is considered as an essential component and precursor of riboflavin 5'-phosphate, known as Flavin mononucleotide (FMN) and Flavin adenine dinucleotide (FAD) [51]. This vitamin participates in the electron transport chain (ETC) and is required for the activity of flavoenzymes. Several factors may contribute to the pathogenesis of migraine, such as mitochondrial dysfunction resulting in oxygen metabolism insufficiency and changes in mitochondrial energy metabolism [52]. As a result, decrease in mitochondrial phosphorylation potential in between attacks has been observed among patients with migraine. Many studies have reported that vitamin B2 in high doses could be effective in migraine prophylaxis. Patients may not have enough Vitamin B2, so this vitamin could be a potential treatment for migraine. Even though evidences obtained from clinical trials aren’t strong, both the AAN (level B evidence) [53] and the Canadian Headache Society recommend its consumption in adults with migraine, because it is well tolerated and side effects are limited and mild [54]. The recommended dose of riboflavin in adult migraineurs is about 400 mg per day. Based on studies, riboflavin has not been shown useful in migraine prevention in children and therefore is not recommended. Table 2 summarizes clinical trials regarding Riboflavin for treating migraine.
Coenzyme Q10
Coenzyme Q10 is a naturally hydrophobic substance and is an essential element of the mitochondrial electron transport chain [64]. CoQ10 is a substance that is both synthesized in the body and absorbed from food sources; however, its total absorption is inadequate for pathological conditions [65]. CoQ10 has many roles in the body, including: transferring electrons throughout the inner mitochondrial membrane from the NADH dehydrogenase complex (complex I) and the Succinate-Q-reductase complex (complex II) to cytochrome C [66], acting as an antioxidant and helping protect the myocardium from postischemic renewed damages. Elevated level of MMP-9 is associated with blood-brain barrier (BBB) dysfunction and inflammation of nerves exacerbate migraine attacks [67]. In addition, animal studies have shown that BBB dysfunction and other MMP-9-related mechanisms develop the onset of CSD which is the main mechanism of migraine attacks [68]. Imamura et al showed that MMP-9 level is higher in migraine patients than healthy subjects [69].

Table 3 Active oxygen species, especially H2O2, are one of the most important factors in the expression and regulation of Matrix metalloproteinase. In addition, Tumor Necrotizing Factor-α and Interlukine-6 regulate the expression of MMPs [70]. CoQ10 is one of the most important antioxidants that acts against H2O2 and reduces the expression of cytokines and MMPs [65]. CoQ10 also improves exercise tolerance, muscle weakness, reduces serum pyruvate and lactate levels, and accelerates post-exercise recovery of phosphocreatine in patients with mitochondrial encephalomyopathies [71, 72]. In the United States, CoQ10 is known as an over-the-counter (OTC) dietary supplement. In patients with mitochondrial dysfunction, under certain conditions, CoQ10 is associated with increased oxidative stress and acts as a useful therapeutic agent. Several studies have shown that serum lactate and pyruvate level are higher in migraine patients than healthy subjects [73]. On the other hand, CoQ10 supplementation improves muscle and brain energy metabolism in patients with mitochondrial cytopathies [74]. The AAN considers CoQ10 useful in migraine prevention (grade C quality evidence) [53], and the Canadian Headache Society guidelines strongly recommend CoQ10 as a migraine preventative agent [54]. Although the effective dose of CoQ10 is unclear, 1–3 mg/kg per day is recommended [75]. Table 3 summarizes RCTs using CoQ10 as a therapeutic nutrient for treating migraine.
Vitamin D
Vitamin D is a fat-soluble vitamin present in slight amounts in food. It is usually added to food and is available as a supplement. When the skin is exposed to sunlight, vitamin D is produced in the body. Vitamin D plays important roles in the body such as increasing calcium absorption, developing healthy bones, protecting older people from osteoporosis, immune system reinforcement and decreasing inflammation. A number of case studies have reported the positive effects of vitamin D supplementation in headache and migraine [81, 82]. A case study performed on two groups of women with migraine associated with menstruation and premenstrual syndrome indicated that patients had inadequate vitamin D levels. After two months of treatment, it was observed that vitamin D and calcium supplementation (1600-1200 IU per day) significantly decreased migraine attacks and premenstrual symptoms [83, 84].

Table 4 In another study on postmenopausal patients with migraine and low level of vitamin D, vitamin D and calcium supplementation reduced the frequency and duration of migraine attacks. In a study on eight patients with chronic tension-type headache, vitamin D deficiency, and osteomalacia, demonstrated that daily intake of vitamin D and calcium supplementation (1500 IU vitamin D3 and 1000 mg calcium) improved symptoms of headaches during 4–6 weeks, while after one week of treatment, serum level of calcium became normal. The exact relationship between vitamin D deficiency and headache is unclear. The most important mechanisms involved in headache include possible sensitization of second and third neurons due to continuous stimulation of sensory receptors of periosteal covering and central sensitization. Low serum level of magnesium is another possible mechanism associated with vitamin D deficiency [85]. A possible mechanism for the pathogenesis of tension-type headache is the abnormal metabolism of magnesium. Since the intestinal absorption of magnesium is dependent on vitamin D [85], thus reduction of magnesium absorption due to vitamin D deficit may lead to tension-type headache. Another mechanism associated with tension-type headache is the presence of vitamin D receptors, 1-hydroxylase (the enzyme responsible for the formation of the vitamin D active form), vitamin D binding protein in the brain and particularly hypothalamus [11]. Table 4 summarizes studies investigating the effects of vitamin D in people with headache.
Vitamin B3
Niacin, is an organic compound known as nicotinic acid, and is an essential nutrient. Niacin and nicotinamide are two of the various forms of the vitamin B3 complex. Limitation of scientific information has caused complexity in the relationship between niacin and migraine. Niacin is a vitamin that plays an important role in dilatation and widening of blood vessels. Because migraine headaches are related to the contraction of blood vessels in the brain, Niacin is not generally considered to be effective for migraine prevention. However, low plasma level of serotonin has been involved in the pathogenesis of migraine. Niacin may act as a negative feedback regulator in the kynurenine pathway to shunt tryptophan into the serotonin pathway, which eventually leads to higher plasma serotonin level [90]. Niacin has been studied as a potential treatment for migraine. In contrast, few studies have reported side effects for niacin in cases of headaches, particularly migraine. Additionally, headaches, but not specifically migraine headaches, have been reported as infrequent side effects of niacin consumption. The effectiveness of niacin in treating headaches requires further randomized controlled trials. In acute migraine headaches, activation of the trigeminovascular complex causes several symptoms. On the other hand, this complex leads to intracranial vasoconstriction followed by, migraine aura and headache due to vasodilation of the extra cranial vessels and activation of the perivascular nociceptive nerves.
Intravenous and oral administration of niacin, may prevent the symptoms of migraine by dilating the intracranial vessels and subsequent contractions of the extracranial vessels. According to results, niacin could be considered as a peripheral vasodilator, however, its impact on the main central mechanisms involved in migraine headaches (cerebral blood) has not been completely investigated. By inducing the production of prostaglandin D2 (PGD2) in the skin, Niacin causes lateral dilation and cutaneous blushing which leads to an increase in the levels of PGD2, 9α, 11β-PGF2 and other metabolites in the plasma [91]. Administration of niacin at doses of 500 mg orally or topically in the form of methyl nicotinate significantly increases the release of prostaglandin D2 in the skin and its metabolites in the plasma [91, 92]. It is not entirely clear whether prostaglandin D2 affects the intracranial arteries, however, since niacin aborts acute migraine attacks, it seems applicable. Bicknell and Prescott [92], demonstrated that niacin has an important role in the vasodilation of the cerebral and spinal vessels, so intravenous injection increases the rate of intracranial blood flow without any alteration in blood pressure. Unfortunately, there is not enough evidence for the effect of niacin on increasing the rate of blood flow. From the perspective of tension-type headaches, intravenous niacin is beneficial due to its central vasodilator properties. Central mechanisms such as the trigeminal system are responsible for the underlying pathophysiology of chronic tension-type and migraine headaches [93]. There is also cerebrospinal or intracranial venous pressure in chronic tension-type headaches [93].

Table 5 Although chronic tension-type migraine headaches are not the same, they are very similar, which can be due to the escalating pathophysiological procedures [94]. Therefore, based on the same hypothesized mechanism of action described previously, niacin may be helpful in relieving the acute phase of tension headaches. A number of studies have reported prophylactic beneficial effects for niacin when administered orally. Recently, it has been proved that mitochondrial energy metabolism disorders play an important role in triggering migraine headaches [95]. Niacin may be a useful migraine preventive agent due to increasing substrate availability to complex I, maintaining sufficient mitochondrial energy metabolism [96]. In addition, riboflavin and COQ10 are also responsible for the complex I reinforcement of the mitochondrial respiratory chain [55, 56, 64]. Niacin improves mitochondrial energy metabolism and increasing blood flow and oxygenation to the skeletal muscles and prevents tension-type headaches. The overall effect of reducing episodes of muscular tension and soreness could be related to the reduction of lactic acid concentration. Niacin improves the mitochondrial energy metabolism by reducing the concentration of lactic acid. On the other hand, studies have shown that niacin supplementation decreases blood lactate and pyruvate concentration in over 50% of patients with mitochondrial encephalopathy, myopathy, lactic acidosis, and stroke-like episodes [97]. This mechanism may be accurate in patients with migraine, since plasma level of lactate and pyruvate are higher in patients with migraine than patients with tension-type headaches or normal subjects [98]. Table 5 summarizes studies
Vitamine B12
Vitamin B12, also called cobalamin, is one of the members of the vitamin B family. Methyl cobalamin and adenosyl cobalamin are the active forms of vitamin B12 in mammals. Besides the two forms mentioned, circulating vitamin B12 is also present as hydroxycobalamin. Vitamin B12 is involved in several pathways. Studies have demonstrated that Hydroxycobalamin has scavenging action against NO [107]. Nitric oxide is involved in pain transmission, hyperalgesia, chronic pain, inflammation, central sensitization and mostly the cyclic guanosine mono phosphate (cGMP) dependent pathway [9, 108]. Based on this hypothesis, vitamin B12 acts as a scavenger against NO, thus it plays an important role in migraine prophylaxis [109]. In an open trial assessing the effect of hydroxycobalamin in prevention migraine, intranasal administration of hydroxocobalamin decreased the frequency of attacks by about 50% in 53% of migraine patients [110]. As mentioned earlier, other vitamins, such as riboflavin, niacin and coenzymes, such as coenzyme Q10, have been suggested for their role in migraine prevention, based on the hypothesis that they improve mitochondrial production of adenosine triphosphate (ATP). Thus, we can understand a common mechanism between these compounds and vitamin B12. In fact, elevated NO level is able to inhibit the respiratory chain by binding to complex I and III and cytochrome C oxidase [111, 112]. Similar effects have been mentioned for NO donors, which determine the production of proxy nitrite, a toxic subcellular components present in the mitochondria [113]. It has also been noted that the mitochondria is able to produce NO radicals [114]. Riboflavin is one of the components involved in the synthesis of B12 [115], both of these vitamins have revealed anti-nociceptive and anti-inflammatory effects in animal models [116].
Evidences indicate an association between gastric damage and migraine. Gastric mucus damage, observed in migraine patients, could be due to excessive consumption of NSAIDs, which are used in migraine attacks. Excessive use of NSAIDs causes gastrointestinal distress, reduces gastric mucus production along with bleeding, and causes gastric ulceration [117]. Gastric damage could also cause intrinsic factor deficiency and thus decrease vitamin B12 absorption and finally affect the important metabolic functions regulated by vitamin B12. When 225 migraine patients were evaluated for Helicobacter pylori, 40% of results were positive. After eradicating H. pylori, the intensity, duration, and frequency of migraine attacks were significantly reduced [118, 119]. Several studies have mentioned an association between vitamin B12 pathway dysfunction and headache pathogenesis. Low serum levels of vitamin B12, folate and B6 are correlated with high levels of homocysteinemia, and plasma homocysteine level may be reduced by folic acid supplementation [120]. Vitamin B12 and folate are involved in the remethylation and synthesis of S-adenosylmethionine (SAMe). [121] Vitamin B12 and folate serum level reduction are observed in a majority of patients suffering from migraine [122]. Moreover, basal level of homocysteinemia, is considered to be a reliable marker of vitamin B12 deficiency which is higher among these patients, especially in migraineurs with aura. After compensating deficiencies, migraine index values are significantly reduced in respect to basal level. Until now the possible correlation between migraine severity and blood homocysteine level and the possible role of hyperhomocysteinemia as a causative factor in the predisposition of migraine has not been fully investigated [123].
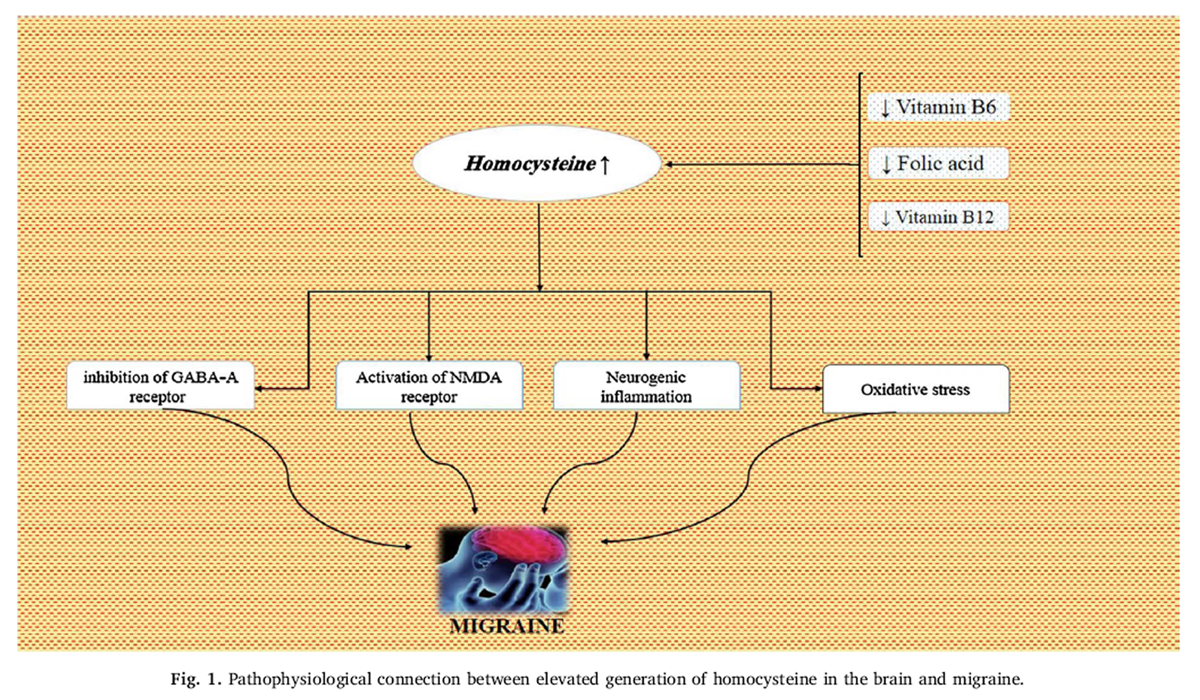
Figure 1 Increased level of homocysteine in the brain may act as a trigger or amplifier via various putative mechanisms. Homocysteine acts as an antagonist to gamma-amino butyric acid (GABA)-A receptor [124], whereas few anti-migraine preventive drugs such as amitriptyline are strong GABAergic agonists [124], thus homocysteine may negatively modulate pain threshold in migraine patients. Since treatment with (NMDA) receptor antagonists is effective in inhibiting CSD [125], whereas homocysteine is a potent excitatory neurotransmitter that acts via activating NMDA receptor [126]. It is mentioned that elevated homocysteine level in the brain may augment negative electrophysiological hyperactivity. In addition, homocysteine has inflammatory properties. The final potential connection between brain homocysteine and migraine is oxidative stress. Homocysteine could increase oxidative stress by inhibiting the function of key antioxidant enzymes, such as extracellular superoxide dismutase [127], which in turn is associated with migraine [128]. Further studies are essential to substantiate putative mechanisms (Figure 1).
Alpha lipoic acid
Alpha-lipoic acid (ALA) is a nutritional coenzyme involved in the energy metabolism of proteins, carbohydrates and fats, which has physiological functions in blood glucose disposal, and is able to scavenge a number of free radicals [129]. ALA is normally synthesized in animal origins and is essential for aerobic metabolism. It is also manufactured and available as a dietary supplement with antioxidant and pharmaceutical properties. Similar to riboflavin, niacin and COQ10, alpha lipoic acid enhances mitochondrial oxygen metabolism and ATP production [130]. In a small double-blind trial, supplementation with 600 mg of ALA once a day for three months significantly reduced the frequency of migraine attacks. However, this improvement was not statistically significant when compared to the changes observed in the placebo group. Additional research is needed to determine the effectiveness of ALA in preventing migraines.
L-carnitine
Carnitine (4-N-trimethylammonium-3-hydroxybutyric acid) plays a critical role in energy production, transportation of long-chain fatty acids across the inner mitochondrial membrane for β-oxidation and ATP production [131]. L-carnitine deficiency changes the oxidation of fatty acids and increases toxins that are originated from nociceptive triggers [132]. In fact, carnitine deficiency decreases beta-oxidation. Migraines and migraine triggers are associated with oxidative stress [133]. As mentioned earlier, antioxidants help give an end to oxidative stress and migraines [134]. L-carnitine, as a cofactor, has an important role in the transportation of free fatty acids (FFA) from the cytosol to the mitochondria. Free fatty acids degrade to Acyl-CoA by h-oxidation and enter the tricarboxylic acid (TCA) cycle. A large amount of oxygen is consumed in this reaction and ATP is synthesized in ETC and oxidative phosphorylation. Oxygen is reduced to H2O at the end of the TCA cycle and decreases oxygen concentration leading to reduced ROS formation [135].
L-carnitine prevents oxidative damage and regulates nitric oxide, cellular respiration and the activity of enzymes involved in defending against oxidative damage [131]. Additionally, L-carnitine has a protective effect on the activity of mitochondrial enzyme succinate dehydrogenase as well as antioxidant enzymes, catalase and superoxide dismutase against 3-NPA-induced neurotoxicity [131]. Although he number of migraine attacks, it has been associated with low level of carnitine in patients with renal failure on dialysis. Carnitine supplementation improves headache pains [136]. Another explanation for the association between migraine and carnitine deficiency is improvement of carnitine level via riboflavin replacement [137]. Few studies have examined the effect of carnitine on migraine patients. Tarighat et al evaluated the effects of magnesium, L-carnitine, and concurrent magnesium– L-carnitine supplementation in migraine prophylaxis. In this single-blind clinical trial, subjects were assigned into four groups: 500 mg/day magnesium oxide (n=33), 500mg/day L-carnitine (n=35), 500 mg/day magnesium oxide and 500 mg/day L-carnitine concurrently (n=30), or the control group (n=35). According to their results, L-carnitine supplementation significantly decreased migraine frequency, severity, index and migraine days [22]. In another study, Kabbouche et al reported that L-carnitine supplementation reduced migraine frequency and severity [132]. Despite, the number of studies indicating positive results for L-carnitine supplementation in migraines, in a triple-blind crossover study, Hagen et al showed no significant differences in headache outcomes between acetyl-carnitine and placebo [138].
Conclusion
Nowadays complementary and alternative medicines are widely used. Considering the complex pathogenesis of migraine, various drugs have been used for its treatment. However, these drugs have possible side effects. In patients who suffer from these side effects and are not treated efficiently by prophylactic drugs, considering a nutraceutical agent for migraine prevention might be a wise choice. New approaches for improving headache symptoms in migraine patients include using nutrient compounds such as Magnesium, CoQ10, ALA, L-carnitine and vitamins (B2, B3, B12 and D), all of which have minimal adverse effects. These nutrients reduce the frequency and severity of migraine attacks via positive effects on mitochondrial function, reducing inflammatory factors and improving antioxidant status. Using effective nutrients along with prescribed drugs leads to decreased dosage of drugs required for the treatment of headaches and may reduce the side effects drugs.
References:
J. Michael, D.A.G.P. Aminoff, A. David, D. Greenburg, P. Roger, R. Simon, Clinical Neurology, McGraw-Hill, New York, 2009.
H.C.C.o.t.I.H. Society, The International Classification of Headache Disorders, (beta Version), Cephalalgia, (2013).
W. Pryse-Phillips, Companion to Clinical Neurology, Oxford University Press, 2009.
T.J. Steiner, L.J. Stovner, T. Vos, R. Jensen, Z. Katsarava, Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain 19 (1) (2018) 17–20.
S.-J. Wang, Epidemiology of migraine and other types of headache in Asia, Curr. Neurol. Neurosci. Rep. 3 (2) (2003) 104–108.
E.J. Mulder, C. Van Baal, D. Gaist, M. Kallela, J. Kaprio, D.A. Svensson, D.R. Nyholt, N.G. Martin, A.J. MacGregor, L.F. Cherkas, Genetic and environmental influences on migraine: a twin study across six countries, Twin Res. Hum. Genetics 6 (5) (2003) 422–431.
M. Ishii, S. Shimizu, Y. Sakairi, A. Nagamine, Y. Naito, Y. Hosaka, Y. Naito, T. Kurihara, T. Onaya, H. Oyamada, MAOA, MTHFR, and TNF-β genes polymorphisms and personality traits in the pathogenesis of migraine, Mol. Cell. Biochem. 363 (1–2) (2012) 357–366.
P.L. Durham, Calcitonin gene?related peptide (CGRP) and migraine, Headache 46 (s1) (2006).
L. Neeb, U. Reuter, Nitric oxide in migraine, CNS Neurol. Disord. Drug Targets 6 (4) (2007) 258–264.
M. Sparaco, M. Feleppa, R. Lipton, A. Rapoport, M. Bigal, Mitochondrial dysfunction and migraine: evidence and hypotheses, Cephalalgia 26 (4) (2006) 361–372.
S. Prakash, N.C. Mehta, A.S. Dabhi, O. Lakhani, M. Khilari, N.D. Shah, The prevalence of headache may be related with the latitude: a possible role of Vitamin D insufficiency? The J. Headache Pain 11 (4) (2010) 301–307.
C. Sun-Edelstein, A. Mauskop, Foods and supplements in the management of migraine headaches, Clin. J. Pain 25 (5) (2009) 446–452.
E.J. Bowen, T.W. Schmidt, C.S. Firm, A.F. Russo, P.L. Durham, Tumor necrosis factor?α stimulation of calcitonin gene?related peptide expression and secretion from rat trigeminal ganglion neurons, J. Neurochem. 96 (1) (2006) 65–77.
R. Cady, D.W. Dodick, Diagnosis and treatment of migraine, Mayo Clinic Proceedings, Elsevier, 2002 pp. 255-261.
M.D. Ferrari, K.I. Roon, R.B. Lipton, P.J. Goadsby, Oral triptans (serotonin 5-HT 1B/1D agonists) in acute migraine treatment: a meta-analysis of 53 trials, Lancet 358 (9294) (2001) 1668–1675.
M. Schürks, H.-C. Diener, P. Goadsby, Update on the prophylaxis of migraine, Curr. Treat. Options Neurol. 10 (1) (2008) 20–29.
S. Modi, D.M. Lowder, Medications for migraine prophylaxis, Am. Fam. Phys. 73 (1) (2006) 72–78.
C. Sun-Edelstein, A. Mauskop, Role of magnesium in the pathogenesis and treatment of migraine, Expert Rev. Neurother. 9 (3) (2009) 369–379.
J. Prousky, D. Seely, The treatment of migraines and tension-type headaches with intravenous and oral niacin (nicotinic acid): systematic review of the literature, Nutrition J. 4 (1) (2005) 3.
A. Bianchi, S. Salomone, F. Caraci, V. Pizza, R. Bernardini, C.C. D’Amato, Role of Magnesium, Coenzyme Q10, Riboflavin, and Vitamin B12 in Migraine Prophylaxis, (2004).
R. Mahdavi, E.A. Tarighat, M.M. Ebrahimi, M. Talebi, M.S. Ghaem, Effects of Oral Magnesium for Migraine Prophylaxis, (2009).
A.T. Esfanjani, R. Mahdavi, M.E. Mameghani, M. Talebi, Z. Nikniaz, A. Safaiyan, The effects of magnesium, l-carnitine, and concurrent magnesium–l-carnitine supplementation in migraine prophylaxis, Biol. Trace Elem. Res. 150 (1–3) (2012) 42–48.
M.J. Laires, C.P. Monteiro, M. Bicho, Role of cellular magnesium in health and human disease, Front Biosci 9 (262) (2004) 76.
F.R. Taylor, Nutraceuticals and headache: the biological basis, Headache 51 (3) (2011) 484–501.
A.C. Foster, G.E. Fagg, Taking apart NMDA receptors, Nature 329 (6138) (1987) 395–396.
Q. Huang, A. Gebrewold, A. Zhang, B.T. Altura, B.M. Altura, Role of excitatory amino acids in regulation of rat pial microvasculature, Am. J. Physiol.-Regul., Integr. Comp. Physiol. 266 (1) (1994) R158–R163.
S.J. Tepper, Complementary and alternative treatments for childhood headaches, Curr. Pain Headache Rep. 12 (5) (2008) 379–383.
E. Coan, G. Collingridge, Magnesium ions block an N-methyl-D-aspartate receptormediated component of synaptic transmission in rat hippocampus, Neurosci. Lett. 53 (1) (1985) 21–26.
N. Gorelova, V. Koroleva, T. Amemori, V. Pavlik, J. Bureš, Ketamine blockade of cortical spreading depression in rats, Electroencephalogr. Clin. Neurophysiol. 66 (4) (1987) 440–447.
M.D. Ferrari, Biochemistry of migraine, Pathol. Biol. 40 (4) (1992) 287–292.
A.C. Charles, S.M. Baca, Cortical spreading depression and migraine, Nat. Rev. Neurol. 9 (11) (2013) 637–644.
C. Ayata, Cortical spreading depression triggers migraine attack: pro, Headache 50 (4) (2010) 725–730.
S. Bhaskar, K. Saeidi, P. Borhani, H. Amiri, Recent progress in migraine pathophysiology: role of cortical spreading depression and magnetic resonance imaging, Eur. J. Neurosci. 38 (11) (2013) 3540–3551.
K. Welch, N.M. Ramadan, Mitochondria, magnesium and migraine, J. Neurol. Sci. 134 (1) (1995) 9–14.
O. Daniel, A. Mauskop, Nutraceuticals in acute and prophylactic treatment of migraine, Curr. Treat. Options Neurol. 18 (4) (2016) 14.
N. Ramadan, H. Halvorson, A. Vande-Linde, S.R. Levine, J. Helpern, K. Welch, Low brain magnesium in migraine, Headache 29 (9) (1989) 590–593.
V. Gallai, P. Sarchielli, G. Coata, C. Firenze, P. Morucci, G. Abbritti, Serum and salivary magnesium levels in migraine. Results in a group of juvenile patients, Headache 32 (3) (1992) 132–135.
F. Facchinetti, G. Sances, P. Borella, A.R. Genazzani, G. Nappi, Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium, Headache 31 (5) (1991) 298–301.
J. Schoenen, J. Sianard-Gainko, M. Lenaerts, Blood magnesium levels in migraine, Cephalalgia 11 (2) (1991) 97–99.
A. Mauskop, B.T. Altura, R.Q. Cracco, B.M. Altura, Intravenous magnesium sulphate relieves migraine attacks in patients with low serum ionized magnesium levels: a pilot study, Clin. Sci. 89 (6) (1995) 633–636.
V. Pfaffenrath, P. Wessely, C. Meyer, H. Isler, S. Evers, K. Grotemeyer, Z. Taneri, D. Soyka, H. Göbel, M. Fischer, Magnesium in the prophylaxis of migraine?a double?blind, placebo?controlled study, Cephalalgia 16 (6) (1996) 436–440.
A. Trauninger, Z. Pfund, T. Koszegi, J. Czopf, Oral magnesium load test in patients with migraine, Headache 42 (2) (2002) 114–119.
P. Aloisi, A. Marrelli, C. Porto, E. Tozzi, G. Cerone, Visual evoked potentials and serum magnesium levels in juvenile migraine patients, Headache 37 (6) (1997) 383–385.
H. Choi, N. Parmar, The use of intravenous magnesium sulphate for acute migraine: meta-analysis of randomized controlled trials, Eur. J. Emerg. Med. 21 (1) (2014) 2–9.
A. Peikert, C. Wilimzig, R. Köhne-Volland, Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study, Cephalalgia 16 (4) (1996) 257–263.
?. Demirkaya, O. Vural, B. Dora, M.A. Topçuo?lu, Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks, Headache 41 (2) (2001) 171–177.
F. Wang, S.K. Van Den Eeden, L.M. Ackerson, S.E. Salk, R.H. Reince, R.J. Elin, Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double?blind, placebo?controlled trial, Headache 43 (6) (2003) 601–610.
M. Bigal, C. Bordini, S. Tepper, J. Speciali, Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study, Cephalalgia 22 (5) (2002) 345–353.
O. Sadeghi, M. Nasiri, F.A. Bayatiyani, H. Rasad, N. Pahlavani, Z. Maghsoudi, G. Askari, Migraine and magnesium, Rev. Evid. (2018).
E. Köseoglu, A. Talasl?oglu, A.S. Gönül, M. Kula, The effects of magnesium prophylaxis in migraine without aura, Magnes. Res. 21 (2) (2008) 101–108.
J.T. Pinto, R.S. Rivlin, Riboflavin (Vitamin B2), Handbook of Vitamins, 5th ed., Taylor and Francis, Boca Raton (FL), 2013, pp. 191–265.
W.R. Yorns, H.H. Hardison, Mitochondrial dysfunction in migraine, Seminars in Pediatric Neurology, Elsevier, 2013 pp. 188-193.
S. Holland, S. Silberstein, F. Freitag, D.W. Dodick, C. Argoff, E. Ashman, Evidencebased guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the quality standards subcommittee of the American academy of Neurology and the American Headache Society, Neurology 78 (17) (2012) 1346.
T. Pringsheim, W. Davenport, G. Mackie, I. Worthington, M. Aubé, S.N. Christie, J. Gladstone, W.J. Becker, Canadian Headache Society guideline for migraine prophylaxis, Can J Neurol Sci 39 (2 Suppl. 2) (2012) S1–59.
J. Schoenen, M. Lenaerts, E. Bastings, High-dose riboflavin as a prophylactic treatment of migraine: results of an open pilot study, Cephalalgia 14 (5) (1994) 328–329.
J. Schoenen, J. Jacquy, M. Lenaerts, Effectiveness of high-dose riboflavin in migraine prophylaxis a randomized controlled trial, Neurology 50 (2) (1998) 466–470.
P.S. Sándor, J. Áfra, A. Ambrosini, J. Schoenen, Prophylactic Treatment of Migraine With β-Blockers and Riboflavin: Differential Effects on the Intensity Dependence of Auditory Evoked Cortical Potentials, Headache 40 (1) (2000) 30–35.
C. Boehnke, U. Reuter, U. Flach, S. Schuh-Hofer, K. Einhäupl, G. Arnold, High?dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre, Eur. J. Neurol. 11 (7) (2004) 475–477.
M. Maizels, A. Blumenfeld, R. Burchette, A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial, Headache 44 (9) (2004) 885–890.
S.C. MacLennan, F.M. Wade, K.M. Forrest, P.D. Ratanayake, E. Fagan, J. Antony, High-dose ribof lavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial, J. Child Neurol. 23 (11) (2008) 1300–1304.
M. Condo, A. Posar, A. Arbizzani, A. Parmeggiani, Riboflavin prophylaxis in pediatric and adolescent migraine, J. Headache Pain 10 (5) (2009) 361–365.
J. Bruijn, H. Duivenvoorden, J. Passchier, H. Locher, N. Dijkstra, W.-F. Arts, Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial, Cephalalgia 30 (12) (2010) 1426–1434.
A. Rahimdel, A. Zeinali, P. Yazdian-anari, R. Hajizadeh, E. Arefnia, Effectiveness of vitamin B2 versus sodium valproate in migraine prophylaxis: a randomized clinical trial, Electron. Phys. 7 (6) (2015) 1344.
T. Rozen, M. Oshinsky, C. Gebeline, K. Bradley, W. Young, A. Shechter, S. Silberstein, Open label trial of coenzyme Q10 as a migraine preventive, Cephalalgia 22 (2) (2002) 137–141.
M. Sanoobar, S. Eghtesadi, A. Azimi, M. Khalili, B. Khodadadi, S. Jazayeri, M.R. Gohari, N. Aryaeian, Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial, Nutr. Neurosci. 18 (4) (2015) 169–176.
F.L. Crane, P. Navas, The diversity of coenzyme Q function, Mol. Aspects Med. 18 (1997) 1–6.
M. Ashina, J. Tvedskov, K. Lipka, J. Bilello, M. Penkowa, J. Olesen, Matrix metalloproteinases during and outside of migraine attacks without aura, Cephalalgia 30 (3) (2010) 303–310.
S. Jander, M. Schroeter, O. Peters, O.W. Witte, G. Stoll, Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain, J. Cereb. Blood Flow Metab. 21 (3) (2001) 218–225.
K. Imamura, T. Takeshima, E. Fusayasu, K. Nakashima, Increased plasma matrix metalloproteinase?9 levels in migraineurs, Headache 48 (1) (2008) 135–139.
M. Bahar, S. Khaghani, P. Pasalar, M. Paknejad, M.R. Khorramizadeh, H. Mirmiranpour, S.G. Nejad, Exogenous coenzyme Q10 modulates MMP-2 activity in MCF-7 cell line as a breast cancer cellular model, Nutr. J. 9 (1) (2010) 62.
Y. Nishikawa, M. Takahashi, S. Yorifuji, Y. Nakamura, S. Ueno, S. Tarui, T. Kozuka, T. Nishimura, Long?term coenzyme Q10 therapy for a mitochondrial encephalomyopathy with cytochrome c oxidase deficiency A 31P NMR study, Neurology 39 (3) (1989) 399-399.
Y. Ihara, R. Namba, S. Kuroda, T. Sato, T. Shirabe, Mitochondrial encephalomyopathy (MELAS): pathological study and successful therapy with coenzyme Q 10 and idebenone, J. Neurol. Sci. 90 (3) (1989) 263–271.
Y. Altunkaynak, M. Ozturk, D.H. Ertem, B. Guveli, F.U. Okay, Z. Yildirim, B. Mutluay, A.C. Dirican, E. Altunkaynak, A. Koksal, Serum lactic acid and pyruvic acid levels in patients with migraine and tension type headache, Dusunen Adam. 26 (3) (2013) 276.
W.J. Koroshetz, B.G. Jenkins, B.R. Rosen, M.F. Beal, Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10, Ann. Neurol. 41 (2) (1997) 160–165.
S.J. Tepper, Nutraceutical and other modalities for the treatment of headache, Continuum (Minneap Minn) 21 (4, Headache) (2015) 1018–1031.
P. Sandor, L. Di Clemente, G. Coppola, U. Saenger, A. Fumal, D. Magis, L. Seidel, R. Agosti, J. Schoenen, Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial, Neurology 64 (4) (2005) 713–715.
A.D. Hershey, S.W. Powers, A.L.B. Vockell, S.L. LeCates, P.L. Ellinor, A. Segers, D. Burdine, P. Manning, M.A. Kabbouche, Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine, Headache 47 (1) (2007) 73–80.
S.K. Slater, T.D. Nelson, M.A. Kabbouche, S.L. LeCates, P. Horn, A. Segers, P. Manning, S.W. Powers, A.D. Hershey, A randomized, double-blinded, placebocontrolled, crossover, add-on study of CoEnzyme Q10 in the prevention of pediatric and adolescent migraine, Cephalalgia 31 (8) (2011) 897–905.
A. Shoeibi, N. Olfati, M.S. Sabi, M. Salehi, S. Mali, M.A. Oryani, Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: an open-label, add-on, controlled trial, Acta Neurol. Belg. 117 (1) (2017) 103–109.
M. Dahri, M. Hashemilar, M.A. Jafarabadi, A. Tarighat-Esfanjani, Efficacy of coenzyme Q10 for the prevention of migraine in women: a randomized, doubleblind, placebo-controlled study, Eur. J. Integr. Med. 16 (2017) 8–14.
T. Mottaghi, F. Khorvash, G. Askari, M.R. Maracy, R. Ghiasvand, Z. Maghsoudi, B. Iraj, The relationship between serum levels of vitamin D and migraine, J. Res. Med. Sci. 18 (Suppl. 1) (2013) S66.
R. Iannacchero, A. Costa, A. Squillace, L. Gallelli, U. Cannistrà, G. De Sarro, P060. Vitamin D deficiency in episodic migraine, chronic migraine and medicationoveruse headache patients, J. Headache Pain 16 (S1) (2015) A184.
S. Thys-Jacobs, Vitamin D and calcium in menstrual migraine, Headache 34 (9) (1994) 544–546.
S. Thys-Jacobs, Alleviation of migraines with therapeutic vitamin D and calcium, Headache 34 (10) (1994) 590–592.
S. Prakash, N.D. Shah, Chronic tension?type headache with vitamin D deficiency: casual or causal association? Headache 49 (8) (2009) 1214–1222.
D. Mitsikostas, D. Tsaklakidou, N. Athanasiadis, A. Thomas, The prevalence of headache in Greece: correlations to latitude and climatological factors, Headache 36 (3) (1996) 168–173.
M.K. Turner, W.M. Hooten, J.E. Schmidt, J.L. Kerkvliet, C.O. Townsend, B.K. Bruce, Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain, Pain Med. 9 (8) (2008) 979–984.
K.V. Knutsen, M. Brekke, S. Gjelstad, P. Lagerløv, Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway, Scand. J. Prim. Health Care 28 (3) (2010) 166–171.
M. Kjærgaard, A.E. Eggen, E.B. Mathiesen, R. Jorde, Association between headache and serum 25?Hydroxyvitamin D; the tromsø study: tromsø 6, Headache 52 (10) (2012) 1499–1505.
D.A. Velling, D.W. Dodick, J.J. Muir, Sustained-release niacin for prevention of migraine headache, Mayo Clinic Proceedings, Elsevier, 2003 pp. 770-771..
J.D. Morrow, W.G. Parsons, L. Roberts 2nd, Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid, Prostaglandins 38 (2) (1989) 263–274.
J.D. Morrow, J.A. Awad, J.A. Oates, L.J. Roberts, Identification of skin as a major site on prostaglandin D2 release following oral administration of niacin in humans, J. Invest. Dermatol. 98 (5) (1992) 812–815.
J. Hannerz, T. Jogestrand, Relationship between chronic tension?type headache, cranial hemodynamics, and cerebrospinal pressure: Study involving provocation with sumatriptan, Headache 44 (2) (2004) 154–159.
R. Cady, C. Schreiber, K. Farmer, F. Sheftell, Primary headaches: a convergence hypothesis, Headache 42 (3) (2002) 204–216.
S.J. Tepper, A. Rapoport, F. Sheftell, The pathophysiology of migraine, Neurologist 7 (5) (2001) 279–286.
B. Marriage, M.T. Clandinin, D.M. Glerum, Nutritional cofactor treatment in mitochondrial disorders, J. Am. Diet. Assoc. 103 (8) (2003) 1029–1038.
K. Majamaa, H. Rusanen, A.M. Remes, J. Pyhtinen, I.E. Hassinen, Increase of blood NAD+ and attenuation of lactacidemia during nicotinamide treatment of a patient with the MELAS syndrome, Life Sci. 58 (8) (1996) 691–699.
H. Okada, S. Araga, T. Takeshima, K. Nakashima, Plasma lactic acid and pyruvic acid levels in migraine and tension?type headache, Headache 38 (1) (1998) 39–42.
M. Atkinson, Migraine headache: some clinical observations on the vascular mechanism and its control, Ann. Intern. Med. 21 (6) (1944) 990–997.
J.W. Goldzieher, G.L. Popkin, Treatment of headache with intravenous sodium nicotinate, J. Am. Med. Assoc. 131 (2) (1946) 103–105.
R. Grenfell, Treatment of migraine with nicotinic acid, Am. Pract. Dig. Treat. 3 (9) (1949) 542–544.
R. Grenfell, Treatment of tension headache, Am. Pract. Dig. Treat. 2 (11) (1951) 933–936.
Z. Morgan, Nicotinic acid therapy in vasoconstriction type of headache, Md. State Med. J. 2 (7) (1953) 377.
Z.R. Morgan, A newer method of nicotinic acid therapy in headache of the vasoconstrictive type, J. Am. Geriatr. Soc. 3 (8) (1955) 545–551.
J. Hall, Enhancing niacin’s effect for migraine, Cortlandt Forum (1991) 47.
J.E. Prousky, E. Sykes, Two case reports on the treatment of acute migraine with niacin: its hypothetical mechanism of action upon calcitonin-gene related peptide and platelets, J. Orthomol. Med. 18 (2) (2003) 108–110.
M. Rajanayagam, C. Li, M. Rand, Differential effects of hydroxocobalamin on NO?mediated relaxations in rat aorta and anococcygeus muscle, Br. J. Pharmacol. 108 (1) (1993) 3–5.
L.L. Thomsen, H.K. Iversen, T.A. Brinck, J. Olesen, Arterial supersensitivity to nitric oxide (nitroglycerin) in migraine sufferers, Cephalalgia 13 (6) (1993) 395–399.
P.-H. Van der Kuy, F. Merkus, J. Lohman, Jt. Berg, P. Hooymans, Hydroxocobalamin, a nitric oxide scavenger, in the prophylaxis of migraine: an open, pilot study, Cephalalgia 22 (7) (2002) 513–519.
P.H. Van Der Kuy, J. Lohman, A quantification of the placebo response in migraine prophylaxis, Cephalalgia 22 (4) (2002) 265–270.
L.L. Pearce, M.W. Epperly, J.S. Greenberger, B.R. Pitt, J. Peterson, Identification of respiratory complexes I and III as mitochondrial sites of damage following exposure to ionizing radiation and nitric oxide, Nitric Oxide 5 (2) (2001) 128–136.
S. Shiva, P.S. Brookes, R.P. Patel, P.G. Anderson, V.M. Darley-Usmar, Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase, Proc. Natl. Acad. Sci. 98 (13) (2001) 7212–7217.
G.C. Brown, V. Borutaite, Nitric Oxide, Cytochrome C and Mitochondria, Biochemical Society Symposia, Portland Press Limited, 1999 pp. 17–25.
T.M. Sarkela, J. Berthiaume, S. Elfering, A.A. Gybina, C. Giulivi, The modulation of oxygen radical production by nitric oxide in mitochondria, J. Biol. Chem. 276 (10) (2001) 6945–6949.
P. Renz, Riboflavin as precursor in the biosynthesis of the 5, 6-dimethylbenzimidazole- moiety of vitamin B12, FEBS Lett. 6 (3) (1970) 187–189.
D.S. França, A.L. Souza, K.R. Almeida, Sl.S. Dolabella, C. Martinelli, M.M. Coelho, B vitamins induce an antinociceptive effect in the acetic acid and formaldehyde models of nociception in mice, Eur. J. Pharmacol. 421 (3) (2001) 157–164.
S. Gabriel, L. Jaakkimainen, C. Bombardier, J. Carson, Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Commentary, Ann. Intern. Med. 116 (1992).
A. Gasbarrini, A. De Luca, G. Fiore, M. Gambrielli, F. Franceschi, V. Ojetti, E. Torre, G. Gasbarrini, P. Pola, M. Giacovazzo, Beneficial effects of helicobacter pylori eradication on migraine, Hepato-Gastroenterol. 45 (21) (1998) 765–770.
M. Seyyedmajidi, S.-A. Banikarim, A. Ardalan, S.-H. Hozhabrossadati, A. Norouzi, J. Vafaeimanesh, Helicobacter pylori and Migraine: Is Eradication of Helicobacter pylori Effective in Relief of Migraine Headache? Casp. J. Neurol. Sci. 2 (4) (2016) 29–35.
D.S. Wald, L. Bishop, N.J. Wald, M. Law, E. Hennessy, D. Weir, J. McPartlin, J. Scott, Randomized trial of folic acid supplementation and serum homocysteine levels, Arch. Intern. Med. 161 (5) (2001) 695–700.
G. Lippi, C. Mattiuzzi, T. Meschi, G. Cervellin, L. Borghi, Homocysteine and migraine. A narrative review, Clin. Chim. Acta 433 (2014) 5–11.
V. Pizza, R. De Magistris, A. Nasta, C. D’amato, A. Bianchi, Migraine and B12 vitamin, Cephalalgia 22 (2002) 27.
V. Pizza, A. Agresta, D. Cassano, C.C. d’Amato, A. Capasso, The role of homocysteine in the pathogenesis of migrane, Curr. Neurobiol. 4 (2013).
D. Lominadze, N. Tyagi, U. Sen, A. Ovechkin, S.C. Tyagi, Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor, Mol. Cell. Biochem. 371 (1–2) (2012) 89–96.
T.R. Anderson, R.D. Andrew, Spreading depression: imaging and blockade in the rat neocortical brain slice, J. Neurophysiol. 88 (5) (2002) 2713–2725.
K.S. McCully, Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation, Ann. Clin. Lab. Sci. 39 (3) (2009) 219–232.
M. Yamamoto, H. Hara, T. Adachi, Effects of homocysteine on the binding of extracellular?superoxide dismutase to the endothelial cell surface, FEBS Lett. 486 (2) (2000) 159–162.
C. Bernecker, C. Ragginer, G. Fauler, R. Horejsi, R. Möller, S. Zelzer, A. Lechner, M. Wallner-Blazek, S. Weiss, F. Fazekas, Oxidative stress is associated with migraine and migraine?related metabolic risk in females, Eur. J. Neurol. 18 (10) (2011) 1233–1239.
P. Data, Alpha-lipoic acid, Arzneimittelforschung 45 (1995) 872–874.
R. Matalon, D.A. Stumpf, K. Michals, R.D. Hart, J.K. Parks, S.I. Goodman, Lipoamide dehydrogenase deficiency with primary lactic acidosis: favorable response to treatment with oral lipoic acid, J. Pediatr. 104 (1) (1984) 65–69.
Gülçin, Antioxidant and antiradical activities of L-carnitine, Life Sci. 78 (8) (2006) 803–811.
M.A. Kabbouche, S.W. Powers, A.L.B. Vockell, S.L. LeCates, A.D. Hershey, Carnitine palmityltransferase II (CPT2) deficiency and migraine headache: two case reports, Headache 43 (5) (2003) 490–495.
D. Tuncel, F.I. Tolun, M. Gokce, S. ?mrek, H. Ekerbiçer, Oxidative stress in migraine with and without aura, Biol. Trace Elem. Res. 126 (1–3) (2008) 92–97.
Y. Eren, E. Dirik, S. Ne?elio?lu, Ö. Erel, Oxidative stress and decreased thiol level in patients with migraine: cross-sectional study, Acta Neurol. Belg. 115 (4) (2015) 643–649.
V. Rodwell, D. Bender, K.M. Botham, P.J. Kennelly, P.A. Weil, Harpers Illustrated Biochemistry, 30th ed, McGraw Hill Professional, 2015.
M. Wanic-Kossowska, Protective role of carnitine in acetate metabolism of patients with uremia treated by hemodialysis, Pol. Arch. Med. Wewn. 97 (6) (1997) 534–540.
W.J. Triggs, C.R. Roe, W.J. Rhead, S.K. Hanson, S.-N. Lin, L.J. Willmore, Neuropsychiatric manifestations of defect in mitochondrial beta oxidation response to riboflavin, J. Neurol. Neurosurg. Psychiatry 55 (3) (1992) 209–211.
K. Hagen, E. Brenner, M. Linde, G.B. Gravdahl, E.A. Tronvik, M. Engstrøm, U. Sonnewald, G. Helde, L.J. Stovner, T. Sand, Acetyl-l-carnitine versus placebo for migraine prophylaxis: a randomized, triple-blind, crossover study, Cephalalgia 35 (11) (2015) 987–995.

Return to NUTRITION
Return to ALTERNATIVE HEADACHE TREATMENTS
Since 4-22-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |