

Non-specific Neck Pain in Schoolchildren:
Prognosis and Risk Factors for Occurrence
and Persistence. A 4-year Follow-up StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Pain. 2008 (Jul 15); 137 (2): 316–322 ~ FULL TEXT
Minna Stahl • Hannu Kautiainen • Ashraf El-Metwally • Arja Hakkinen
Jari Ylinen • Jouko J. Salminen • Marja Mikkelsson
Department of Physical and Rehabilitation Medicine,
University Hospital of Turku,
P.O.Box 52, 20520
Turku, Finland.
Minna.Stahl@tyks.fiThis study investigated the natural course of neck pain (NP) in 9-12-year-olds during a 4-year follow-up. Risk factors for the occurrence and persistence of weekly NP were explored separately for boys and girls. At baseline, 1756 schoolchildren completed a questionnaire eliciting musculoskeletal pain symptoms, other physical, and psychological symptoms and frequency of physical activity, and were tested for joint hypermobility. Symptoms during the preceding three months were asked using a five-level frequency classification. Re-evaluation was performed after one and four years using identical questionnaires. During follow-up, 24% reported none, 71% fluctuating, and 5% persistent weekly NP. The frequency of NP at baseline was linearly related to weekly NP during follow-up in both genders (P<0.001). Furthermore, a significant increasing linear trend towards a more persistent course of NP was seen in children with weekly other musculoskeletal and/or other physical and psychological symptoms at baseline. Among originally neck pain-free pre-/early adolescents, weekly other musculoskeletal pain symptoms (only in girls) and other physical and psychological symptoms (in both genders) predicted the occurrence of weekly NP during follow-up. In conclusion, neck pain in schoolchildren tends to fluctuate, but there also seems to exist a subgroup (5%) with persistent NP already in pre-/early adolescents, or even earlier. Co-occurrence of frequent other musculoskeletal symptoms and/or markers of psychological stress with frequent NP are risk indicators for a more persistent course, at least within next few years. Since adult chronic NP problems might originate in childhood, further studies are needed, including preventive interventions.
KEYWORDS: Neck pain; Children; Adolescents; Risk factors; Prognosis; Epidemiology
From the Full-Text Article:
Introduction
Chronic neck pain (NP) is a common health problem among adults. [3, 7, 29, 39] According to a Finnish study, 5% of men and 7% of women suffer from chronic NP. [3] From the beginning of the 1990s, the prevalence of NP has steadily increased among Finnish adolescents [18], and similar trends have also been reported in some other Western countries. [21] This has raised speculations that NP might become a significant health problem in the near future. According to Brattberg [5], chronic musculoskeletal pain problems may appear already at the age of 8–14 years, perhaps even earlier. Although there is some evidence that adolescents with neck symptoms are at a higher risk of having NP in adulthood [19, 31], similar prognostic studies in younger children are lacking. Thus, there are still gaps in the literature regarding factors that can predict the development and persistence of neck symptoms in school-aged children. Such information would be highly important for creating scientifically- based primary and secondary prevention programs for youth, which might have implications for adults as well.
This study is a continuation of the previous, prospective, population-based follow-up surveys, which showed that NP was the most persistent and recurrent musculoskeletal pain in school-aged children. [10, 24] In addition, we have reported that newly-developed neck symptoms in originally musculoskeletal pain-free children were mainly self-limiting and that NP became more common among girls than boys in adolescence. [33] The aim of this study was to conduct an in-depth investigation about the course of NP in different frequency groups from pre-/early adolescence stage to mid-adolescence and to explore possible risk factors for its occurrence and persistence separately for boys and girls.
Methods
Study population
This study took place in Lahti, a city in Southern Finland with a population of 94,827 (1995). Nineteen of the 21 primary schools in the town accepted the invitation to participate. Due to unsuitability of some of the study methods, the city’s special schools were excluded: the Steiner school, the hospital school, and the schools for hearing impaired, physically disabled, and mentally handicapped children. The study was conducted in March 1995, and all the pupils from the third and fifth grades present on the survey day participated. The initial sample consisted of 1,756 children, 867 from the third (mean age 9.8 [SD = 0.4]) and 889 from the fifth grade (mean age 11.8 [SD = 0.4] years), representing 83% of all schoolchildren in these grades in the city of Lahti.
The pupils were re-evaluated after one and four years, in March 1996 and 1999. There were 1268 (72%) schoolchildren (597 boys and 671 girls) who completed a study questionnaire on all three occasions, at baseline and at the two follow-ups. These schoolchildren constituted our study population. The number of dropouts during the follow-up period was 488 (264 boys and 224 girls) owing to absence from school on the survey day, changing schools, moving away, and refusal to participate. The study population and those who were lost to follow-up were similar in age and NP frequency distribution.
Measurements
On all three study occasions, the subjects completed identical questionnaires including pain questionnaire designed to assess musculoskeletal pain symptoms in schoolchildren and additional questions about other symptoms and frequency of physical activity. Joint hypermobility was also tested at baseline.
The pain questionnaire included site-specific questions of musculoskeletal pain in seven different areas of the body (A: neck, B: upper limb, C: chest, D: lower limb, E: upper back, F: lower back, and G: buttock). Beside the pain questions, there were body maps with front and back views, divided into seven different body parts to indicate the anatomic areas A–G of the question. For analysis, the body parts E, F, and G were combined to represent “back pain”. Musculoskeletal pain symptoms were asked by using a five-level frequency classification (pain seldom or never, once a month, once a week, more than once a week, almost daily) during the preceding three months (i.e. from Christmas until the day of the study). For analysis, pain frequencies once a week, more than once a week and almost daily were combined and renamed as “at least once a week/weekly” class. Based on the frequency of NP reported during the follow-up, three different types of courses of NP were identified: pain-free (no NP at any evaluation point), fluctuating (NP frequency varied from no pain to once a month to at least once a week), and persistent (NP at least once a week at all three evaluation points).
The development of the pain questionnaire, its reliability and concurrent validity had been measured earlier in a sub-sample of the population. [23, 24] The main outcome variable of this study, neck pain, was considered in three different frequency categories (none/once a month/ at least once a week) and the test–retest reliability in stability for this threefold neck pain classification over a week time-interval was good (j = 0.87).
Additional questions evaluated six physical and psychological symptoms: headache, abdominal pain, depressive mood, daytime tiredness, difficulty falling asleep, and waking up during the night. These symptoms were asked using the same frequency categorization as for musculoskeletal pain symptoms. For analysis, all symptoms appearing at least once a week were considered positive.
The frequency of physical activity was elicited with the question:”How often do you exercise vigorously for at least half an hour?” The response alternatives were “not at all”, “1–2 times”, “3–4 times”, and “5–7 times per week”. Based on the frequency of the reported physical activity, children were categorized into 2 groups (less than 3 times a week, and 3 or more times a week).
Joint hypermobility was assessed at baseline using the Beighton’s method
(score 0 to 9, one point for each side of the body for tests A–D and one point for test E):(A) passive dorsiflexion of the little fingers beyond 90°,
(B) passive apposition of the thumbs to the flexor aspect of the forearm,
(C) hyperextension of the elbows beyond 10°,
(D) hyperextension of the knees beyond 10°, and
(E) forward flexion of the trunk, with knees straight, so that the palms of the hands rest easily on the floor. [4]The intra-and inter-observer reliability of this method had also been measured earlier in a sub-sample of the population with j coefficients of 0.75 and 0.78, respectively. [23] Beighton score of 6 was chosen as the cut-off point for hypermobility on the basis of the distribution of the results.
Statistical methods
Variables with normal (Gaussian) distribution of the descriptive values were expressed by mean and standard deviations (SD); statistical comparisons between the groups were made using the t-test or analysis of variance (ANOVA). Measures with discrete distributions were expressed as counts (%) and analyzed by v2, Fisher’s exact test or Cochran–Armitage– Trend test with Monte Carlo P-values. Risk factors for the occurrence of NP during the follow-up were analyzed using generalizing estimating equations (GEE) models with an exchangeable correlation structure. Hommel’s method was applied to adjust levels of significance for multiple testing if appropriate.
Results
Courses of NP from pre-/early adolescence to midadolescence
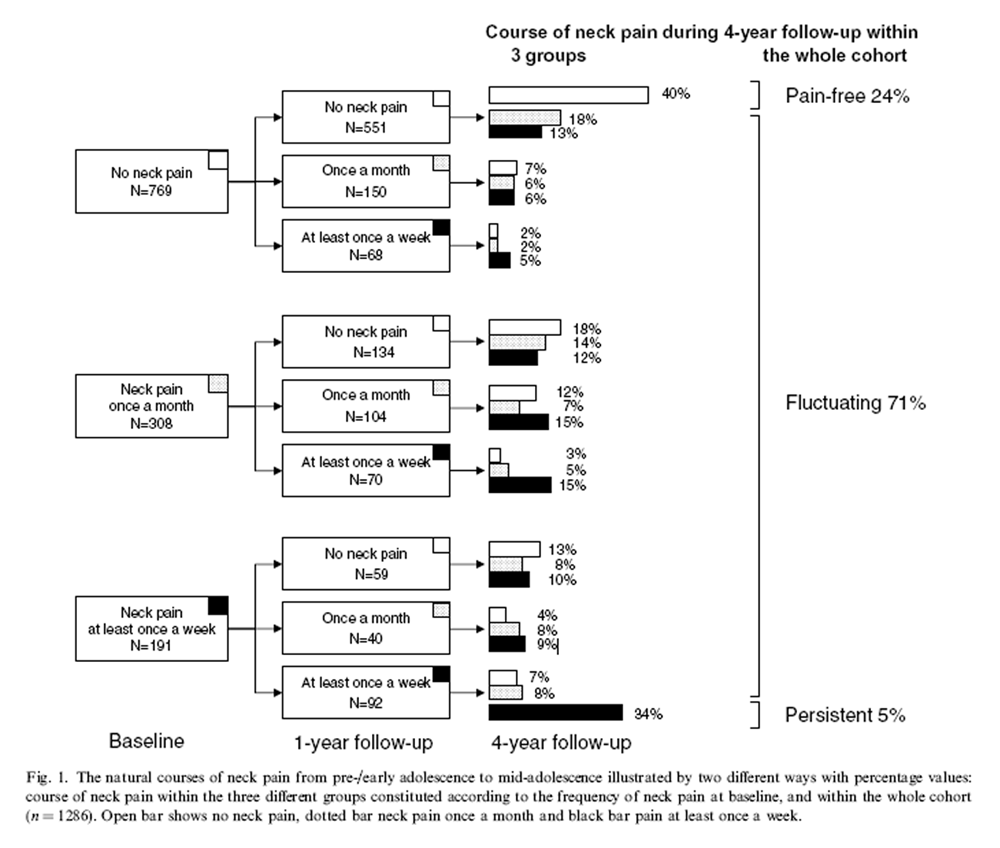
Figure 1 The population was divided into three subgroups depending on the self-reported NP frequency at baseline: no NP (61%), NP once a month (24%), and NP at least once a week (15%). Figure 1 shows the natural courses of NP during the 4-year follow-up within these three groups, and in the whole cohort: 304 [24% (95% CI 22 to 27)] stayed pain-free, 896 [71% (68 to 73)] had a fluctuating course of NP, and 64 [5% (4 to 6)] persistent NP at least once a week.
Relationship between the frequency of NP at baseline and during the follow-up

Figure 2 Of those schoolchildren reporting no NP at baseline, 219 [28% (95% CI 25 to 32)] reported weekly NP during the follow-up (at either one or both re-evaluation points). Of those reporting NP once a month at baseline, 152 [49% (95% CI 44 to 55)] had weekly NP during the follow-up. Of those reporting weekly NP at baseline, 128 [67% (95% CI 60 to 72)] also had weekly NP during the follow-up. There was a significant linearity (P < 0.001) between the different baseline NP frequency groups and weekly NP during the follow-up in both genders (Figure 2). Girls who reported weekly NP at baseline were 5.9 (95% CI 4.0 to 8.7) times more likely to also have weekly NP during the follow-up compared with girls without NP at baseline. The corresponding odds ratio for boys was 4.6 (95% CI 3.2 to 7.4).
Baseline factors related to the persistent course type

Table 1 Comparisons were made between the twelve different baseline variables and the three different NP course groups separately for boys and girls (Table 1). In both sexes, there was a significant increasing linear trend towards a more persistent course of NP when having weekly other musculoskeletal pain and/or other selfreported physical and psychological symptoms at baseline. Joint hypermobility and frequency of physical activity in pre-/early adolescence were not related to the later course of NP.
Factors predicting occurrence of weekly NP

Table 2 Schoolchildren reporting no neck symptoms at baseline (N = 769) were chosen for the GEE models to determine factors predicting occurrence of weekly NP during the follow-up. Analyses were performed separately for boys and girls (Table 2). The occurrence of weekly NP increased during the follow-up time in both genders. Having other physical and psychological symptoms with a frequency of at least once a week at baseline predicted the occurrence of weekly NP in both genders. Furthermore, having weekly other musculoskeletal pain symptoms predicted the occurrence of weekly NP among girls. The number of reported other musculoskeletal pain (1 to 4) or physical and psychological symptoms (1 to 6) increased the odds ratio, respectively.
Discussion
In this study, for the first time ever, the long-term (4 years) prognosis and risk factors of NP have been evaluated in a pre-/early adolescent to mid-adolescent population. As has been noted before, NP, already quite prevalent in preadolescents, becomes more prevalent in adolescence with female over-representation. [9, 13, 16, 18, 22, 27, 32, 33, 37, 38]. This study confirms our earlier findings (in an originally musculoskeletal pain-free cohort) that NP is mostly a fluctuating phenomenon among school-aged children. [33] However, there is a subpopulation (5%) showing a persistent course of neck symptoms beginning already at ages 9 to 12, perhaps even earlier. Siivola et al. [31] reported that neck symptoms in adolescence predicted NP in early adulthood. Interestingly, according to Cote et al. [8], about 5% of adults suffer from highly disabling chronic NP. These findings raise a suspicion that life-long chronic non-specific NP problems have their origins in childhood. Thus, further longitudinal studies extending from childhood to adulthood are needed.
We have previously reported that the frequency of NP fairly well reflects the intensity of pain. [33] Moreover, the findings of the current study indicated that the frequency of NP is an important predictive factor for the persistence of the pain.
Since a gender difference becomes evident in the prevalence rates of NP in adolescence, we were interested to explore the role of possible earlier risk factors separately among boys and girls. We found that self-reported frequent other musculoskeletal pain and/or other physical and psychological symptoms such as headache, abdominal pain, day tiredness, depressive mood and sleep difficulties were risk factors for both the occurrence and persistence of weekly NP during the next four years. The physical and psychological symptoms asked in our study are known only rarely associated with any organic disease in schoolchildren and chiefly considered expressions of psychological stress and often called psychosomatic symptoms. [1, 2, 6, 11, 35] Our risk factor findings are in accordance with previous reports: co-occurrence of other musculoskeletal pains with NP has been recognized in adolescent populations. [16, 30, 33, 37] Grimmer et al. [16] reported that any bodily pain was experienced by more than 50% of 13–17-year-old adolescents during the previous week and, further, that the prevalence of headache was decreasing with a concomitant increase in neck and upper back pain over the 5-year study period. Psychosomatic symptoms have displayed the strongest association with NP compared to all other studied potential risk factors in earlier cross-sectional studies. [36, 37]
In the study by Diepenmaat et al. [9], an association existed between NP, stress and depressive symptoms among 12–16-year-old adolescents. Feldman et al. [13] demonstrated that a lower mental health score was a risk factor for the development of weekly NP in a one-year follow-up among adolescents. Parallel findings have been reported among school-aged children with low back pain. [20] Mikkelsson et al. [26], for one, suggested in their 1-year follow-up of pre-/early adolescents that depressive symptoms and sleep problems may contribute to regional NP becoming widespread. In the study by Siivola et al. [31] psychosomatic stress symptoms in adolescence predicted neck and shoulder pain in early adulthood.
In our study, neither general joint hypermobility nor the level of physical activity affected the course of NP in schoolchildren. We must note that in using the Beighton’s method, as we did to detect joint hypermobility, the specific mobility of the neck cannot be determined. Several studies have been conducted to investigate the relationship between hypermobility and musculoskeletal symptoms in schoolchildren, but none of them has concentrated specifically on NP and the results have been contradictory. [4, 10, 14, 15, 23–25, 28] Similarly, inconsistent findings have been published regarding the association between physical activity and NP. This might be attributed to the large variation of methods used to measure physical activity, which prevents proper comparisons. [13, 22, 27, 37]
The strength of this study is its prospective, population based setting with a response rate of 72% during follow-up. [12] Furthermore, the outcome measure (NP) was based on self-reports, precisely defined, and the frequency was evaluated. [12, 17] The fluctuation of NP due to seasonal variation was precluded, since the baseline study and both follow-ups were conducted in wintertime. [34] In these data, girls were over-represented among the participants included in the analysis vs. those lost to follow-up. However, it is likely that this had no impact on the results, since the analyses were performed separately for both genders. Another weakness is that the follow-up did not cover the entire follow- up time, but only the period of three months at one and four years. Yet, we considered it appropriate to call NP persistent if its reported frequency was at least once a week at all three evaluation points.
By solely relying on the self-report of symptoms from our study population, we have most likely detected also neck symptoms that are milder than those seen in clinical settings as only small proportion of the children seek help for their neck pain. This might have led to underestimation of the strength of the risk association – i.e. the true measurements of risk association, for more severe non-specific neck pain, would be greater than what we report in this paper. It always must be remembered that children and adolescents with frequent neck pain seeking medical help must be carefully examined and followed, because there might also be few cases with underlying severe disease. Once severe underlying causes for neck pain have been excluded, it might be helpful to ask about the risk factors found in this study in order to distinguish children most likely to have self-limiting neck symptoms from those who are at a higher risk of having more persistent NP, and who might hence benefit from early secondary preventive interventions.
In conclusion, our results suggest that neck symptoms in school-aged children tend to fluctuate in the majority of cases, yet there seems to be a subgroup (5%) of children with persistent NP already at ages 9 to 12, perhaps even earlier. Co-occurrence of frequent other musculoskeletal symptoms and/or markers of psychological stress with weekly NP are risk indicators for a more persistent course of NP from pre-/early adolescence to mid-adolescence. Our findings raise a suspicion that adult chronic NP problems might have their origins in childhood and therefore further studies in young age groups, including preventive interventions, are needed.
Acknowledgements
The Medical Research Fund of The Rheumatic Foundation Hospital, the University Hospital of Turku and the Central Hospital of Jyva¨skyla¨ supported this study. We thank Mrs. Tuija Sulonen, RN, for assistance in collecting the data at follow-up, Mr. Olli Heinonen, Paavo Nurmi Keskus, for offering a desk and a computer for the writing process of this article and Mrs. Marja Vajaranta for revising the language.
References:
Alfven G.
Psychological tension headaches among children. A survey.
Lakartidningen 1986;45:3827–8. in Swedish.Aro H, Paronen O, Aro S.
Psychosomatic symptoms among 14–16 year old Finnish adolescents.
Soc Psychiatry 1987;22:171–6.Aromaa A, Koskinen S.
Health and functional capacity in finland: baseline results of the Health 2000 Health Examination Survey.
Helsinki, Finland: National Public Health Institute; 2002.
Publication B3/2002.Beighton P, Solomon L, Soskolne CL.
Articular mobility in an African population.
Ann Rheum Dis 1973;32:413–8.Brattberg G.
Do pain problems in young school children persist into early adulthood? A 13-year follow-up.
Eur J Pain 2004;8:187–99.Bury RG.
A study of 111 children with recurrent abdominal pains.
Aust Paediatr J 1987;23:117–9.Cassou B, Derriennic F, Monfort C, Norton J, Touranchet A.
Chronic neck and shoulder pain, age, and working conditions: longitudinal results from a large random sample in France.
Occup Environ Med 2002;59:537–44.Cote P, Cassidy JD, Carroll L.
The Saskatchewan Health and Back Pain Survey.
The Prevalence of Neck Pain and Related Disability in Saskatchewan Adults
Spine (Phila Pa 1976). 1998 (Aug 1); 23 (15): 1689–1698Diepenmaat ACM, van der Wal MF, de Vet HCW, Hirasing RA.
Neck/shoulder, low back, and arm pain in relation to computer use, physical activity, stress, and depression among dutch adolescents.
Pediatrics 2006;117:412–16.El-Metwally A, Salminen JJ, Auvinen A, Kautiainen H, Mikkelsson M.
Prognosis of non-specific musculoskeletal pain in preadolescents: a prospective 4-year follow-up study till adolescence.
Pain 2004;110:550–9.El-Sheikh M, Buckhalt JA, Mark Cummings E, Keller P.
Sleep disruptions and emotional insecurity are pathways of risk for children.
J Child Psychol Psychiatry 2007;48:88–96.Fejer R, Kyvik KO, Hartvigsen J.
The prevalence of neck pain in the world population: a systemic critical review of the literature.
Eur Spine J 2006;15:834–48.Feldman D, Shrier I, Rossignol M, Abenhaim L.
Risk factors for the development of neck and upper limb pain in adolescents.
Spine 2002;27:523–8.Gedalia A, Press J.
Articular symptoms in hypermobile schoolchildren: a prospective study.
J Paediatr 1991;119:944–6.Gedalia A, Press J, Buskila D.
Joint hypermobility and fibromyalgia in schoolchildren.
Ann Rheum Dis 1993;52:494–6.Grimmer K, Nyland L, Milanese S.
Repeated measures of recent headache, neck and upper back pain in Australian adolescents.
Cephalalgia 2006;26:843–51.Goodman J, McGrath P.
The epidemiology of pain in children and adolescents: a review.
Pain 1991;46:247–64.Hakala P, Rimpela¨ A, Salminen JJ, Virtanen SM, Rimpela¨ M.
Back, neck and shoulder pain in Finnish adolescents: national cross-sectional surveys.
BMJ 2002;325:743–5.Hertzberg A.
Prediction of cervical and low back pain based on routine school health examinations.
Scan J Prim Health Care 1985;3:247–53.Jones G, Watson K, Silman A, Symmons D, Macfarlane G.
Predictors of low back pain in British school children: a population-based prospective Cohort study.
Pediatrics 2003;111:822–8.King A, Wold B, Tudor-Smith C, Harel Y,
The health of youth. A Cross-National Survey,
WHO Regional Publications 1996, Eur Ser 1996;69:68–9.Kujala U, Taimela S, Viljanen T.
Leisure physical activity and various pain symptoms among adolescents.
Br J Sports Med 1999;33:325–8.Mikkelsson M, Salminen JJ, Kautiainen H.
Joint hypermobility is not a contributing factor to musculoskeletal pain in pre-adolescents.
J Rheumatol 1996;23:1963–7.Mikkelsson M, Salminen JJ, Kautiainen H.
Non-specific musculoskeletal pain in preadolescents. Prevalence and 1-year persistence.
Pain 1997;73:29–35.Mikkelsson M, Salminen JJ, Sourander A, Kautiainen H.
Contributing factors to the persistence of musculoskeletal pain in preadolescents: a prospective 1-year follow-up study.
Pain 1998;77:67–72.Mikkelsson M, Sourander A, Salminen JJ, Kautiainen H, Piha J.
Widespread pain and neck pain in schoolchildren. A prospective one-year follow-up study.
Acta Paediatr 1999;88: 1119–24.Niemi S, Levoska S, Kemila¨ J, Rekola K,
Keinanen-Kiukaanniemi S. Neck and shoulder symptoms and leisure time activities in high school students.
JOSPT 1996;24:25–9.Qvindesland A, Jonsson H.
Articular hypermobility in Icelandic 12-year-olds.
Rheumatology 1999;38:1014–6.Picavet HSJ, Schouten JSAG.
Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC3-study.
Pain 2003;102:167–78.Salminen JJ.
The adolescent back. A field survey of 370 Finnish school children.
Acta Paediatr Scand 1984;73:1–122.Siivola S, Levoska S, Latvala K, Hoskio E, Vanharanta H, Keinanen-Kiukaanniemi S.
Predictive factors for neck and shoulder pain: a longitudinal study in young adults.
Spine 2004;29:1662–9.Smedbraten B, Natvig B, Rutle O, Bruusgaard D.
Self-reported bodily pain in school children.
Scand J Rheumatol 1998;27:273–6.Stahl M, Mikkelsson M, Kautiainen H, Hakkinen A, Ylinen J, Salminen JJ.
Neck pain in adolescence. A 4-year follow-up of pain-free preadolescents.
Pain 2004;110:427–31.Takala E-P, Viikari-Juntura E, Moneta G, Saarenmaa K, Kaivanto K.
Seasonal variation in neck and shoulder symptoms.
Scand J Work Environ Health 1992;18:257–61.Tamminen TM, Bredenberg P, Escartin T, Kaukonen P, Puura K, Rutanen M, et al.
Psychosomatic symptoms in preadolescent children.
Psychother Psychosom 1991;56:70–7.van Gent C, Dols J, de Rover C, Hira Sing R, de Vet C.
The weight of schoolbags and the occurrence of neck, shoulder and back pain in young adolescents.
Spine 2003;28:916–21.Vikat A, Rimpelä M, Salminen JJ, et al.
Neck or Shoulder Pain and Low Back Pain in Finnish Adolescents
Scand J Public Health. 2000 (Sep); 28 (3): 164–173Wedderkopp N, Leboeuf-Yde C, Andersen LB, Froberg K, Hansen HS.
Back Pain Reporting Pattern in a Danish Population-based Sample of Children and Adolescents
Spine (Phila Pa 1976). 2001 (Sep 1); 26 (17): 1879–1883Webb R, Brammah T, Lunt M, Urwin M, Allison T, Symmons D.
Prevalence and predictors of intense, chronic, and disabling neck and back pain in the UK general population.
Spine 2003;28:1195–202.
Return to PEDIATRICS
Return to NECK AND BACK PAIN
Since 1-25-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |