

Pubertal Development and Growth are Prospectively Asociated
with Spinal Pain in Young People (CHAMPS study-DK)This section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: European Spine Journal 2019 (Jul); 28 (7): 1565–1571 ~ FULL TEXT
Jeffrey J. Hebert, Charlotte Leboeuf-Yde, Claudia Franz, Arnaud Lardon, Lise Hestbæk, Neil Manson, Niels Wedderkopp
Faculty of Kinesiology,
University of New Brunswick,
Fredericton,
NB, E3B 5A3, Canada.
J.Hebert@unb.caPURPOSE: To examine the prospective associations of pubertal development and linear growth with spinal pain frequency and duration in children.
METHODS: We recruited students from 10 public primary schools. Over 42 months, pubertal development was assessed four times and categorized according to Tanner stages 1-5, and height was measured on seven occasions. Occurrences of spinal pain were reported weekly via text messaging. We constructed variables for spinal pain duration (total weeks with pain) and frequency (number of episodes). Potential associations between pubertal development and growth were examined with generalized estimating equations and reported with incident rate ratios (IRRs). All models were adjusted for potential confounders.
RESULTS: Data from 1021 children (53% female; mean [SD] age = 9.4 [1.4] years), with median participation duration of 39 months, were included. Advancing pubertal development was associated with increased spinal pain duration (IRR [95% CI] = 1.90 [1.45, 2.49] to 5.78 [4.03, 8.29]) and frequency of pain episodes (IRR [95% CI] = 1.32 [1.07, 1.65] to 2.99 [2.24, 3.98]). Similar associations were observed for each 1-cm change in height in 6 months with spinal pain duration (IRR [95% CI] = 1.19 [1.15, 1.23]) and frequency (IRR [95% CI] = 1.14 [1.11, 1.17]). The relations between pubertal development and spinal pain, as well as growth and spinal pain, were largely independent.
CONCLUSIONS: In young people, pubertal development and linear growth are likely to be independent risk factors for the development of spinal pain. Pubertal development demonstrates evidence of dose-response in its relationship with spinal pain. This knowledge may assist healthcare providers with clinical decision-making when caring for pediatric patients. These slides can be retrieved under Electronic Supplementary Material.
KEYWORDS: Back pain; Body height; Growth and development; Puberty; Risk factors
From the Full-Text Article:
Introduction
Spinal pain is the largest cause of disability worldwide [1], yet its etiology is unknown in most cases. [2-4] Spinal pain is common in young people [5, 6], often presenting in childhood and increasing in prevalence with age; 1 in 3 9-year-old children and half of 15-year-old adolescents experience spinal pain in the previous month. [7] The occurrence of spinal pain in youth is notable as it often tracks into adulthood. [8]
Little is known about risk factors for the development of spinal pain in youth. [9, 10] Previous studies have reported a relationship between spinal pain and pubertal development. [11-13] Puberty is characterized by the rapid developmental physical and psychological changes occurring during the transition from childhood and adulthood. [14] One of the most striking changes during puberty is linear growth (change in height), with mean growth during peak height velocity of 9 cm and 10 cm per year occurring in girls and boys, respectively. [15] Linear growth is a potential cause of spinal pain owing to rapid mechanical loading changes on the spine. [16-19]
Two recent systematic reviews reported conflicting evidence for a causal association between pubertal development and spinal pain. [20, 21] However, several important limitations of the primary research were identified, including the lack of longitudinal studies of children at various stages of pubertal development, and tracking of children over sufficient time periods to account for variations in growth and development. Additionally, the measures of spinal pain were prone to recall bias, and statistical analyses were often suboptimal for the characteristics of the data. Consequently, the authors called for additional longitudinal research to investigate the role of pubertal development and its components.
Therefore, this study examined the prospective associations of pubertal development and linear growth with spinal pain frequency and duration in children. We hypothesized that advancing pubertal development and greater changes in height would be associated with increased spinal pain frequency and duration in children.
Methods
Study design
This prospective cohort study was nested in the Childhood Health, Activity, and Motor Performance School Study Denmark (CHAMPS study-DK). The CHAMPS study-DK is a quasi-experimental trial designed to identify the effects of physical education on physical activity, cardiovascular health, and motor performance of primary school students. [22] All 19 public primary schools in the Svendborg region of Denmark were invited to participate in the study. Ten schools elected to participate. Students from six schools received an intensive physical education program comprising 270 min per week, while students from the remaining four schools received the usual physical education program (90 min per week).
In the current study, all participating students were merged in a common cohort. Linear growth was measured at baseline, 6, 12, 18, 30, and 42 months. Estimates of pubertal development were obtained at baseline, 12, 30, and 42 months. Spinal pain outcomes were measured on a weekly basis for the duration of the study. Limitations in human resources and equipment required that participating children entered the study on a rolling basis, with participating schools were progressively enrolled over the course of a school year. Therefore, the median (IQR) participation time of individual students was 39.0 (34.6–42.2) months. Additional details about the study have been reported previously [23].
Study participants
The study sample included all primary school students enrolled in the first through sixth grades in the participating schools. Parents provided written consent for all participating children and children gave verbal consent prior to enrollment. Ethical approval was provided by the Regional Scientific Committee of Southern Denmark (ID S20080047), and the study was registered with the Danish Data Protection Agency (J.nr. 2008-41-2240).Anthropometric measures and pubertal development Measures of height and weight were obtained with children barefoot and wearing light clothes. Height was measured to the nearest .5 cm using a portable stadiometer (SECA 214, Seca Corporation, Hanover, MD, USA), and weight was measured to the nearest .1 kg with a calibrated Tanita BWB- 800S digital scale (Tanita Corporation, Tokyo, Japan). Linear growth was reported as the change in height occurring between each of the six time points. Body mass index was classified as normal, overweight, or obese using age- and sex-specific norms developed by the International Obesity Task Force. [24]
We measured pubertal development with Tanner stages. [25] As part of a structured interview, Tanner stage was self-assessed by children with the assistance of explanatory text and visual representations of pubic hair development in boys and breast development in girls. [26] Pubertal development was reported on a scale from 1 to 5, with higher scores indicating later pubertal stages. Stage 1 represents prepubertal status, while stages 2–4 denote increasing levels of adolescent development, and stage 5 indicates adult development. Tanner scores 4 and 5 were collapsed into a common category owing to the low prevalence of these scores early in the study.Spinal pain outcomes
Spinal (neck, mid-back, and/or lower back) pain was measured using a Web-based SMS text messaging system (SMSTrack ApS, Esbjerg, Denmark) each week for the duration of the study, except during school holidays. Every Sunday, parents were sent SMS messages inquiring about the presence or absence of spinal pain experienced by their child that week. Surrogate parental reporting was used to address concerns over the validity of self-reporting by children. [27, 28] All responses were uploaded to an online database; nonsensical responses resulted in a telephone call to parents for clarification. This approach is reliable and valid when compared to information about back pain obtained from structured clinical interviews. [29]
From these data, we constructed two spinal pain outcomes: spinal pain duration and frequency. Pain duration was characterized by the total weeks of spinal pain reported during each period (i.e., between each measure of growth and pubertal development). We estimated spinal pain frequency by measuring the number of pain episodes occurring during each study period. Episodes were defined by the occurrence of spinal pain that was preceded by one or more pain-free weeks immediately prior to a pain report.
Statistical analysis
Data were analyzed using Stata v15 software (StataCorp, College Station, TX, USA). We investigated the longitudinal associations between (1) pubertal development and (2) linear growth and each spinal pain outcome using generalized estimating equation models. Each model included a negative binomial family, a log link with an exchangeable correlation matrix, and robust standard errors to account for the clustered nature of the data.
Exposures for pubertal development comprised the Tanner stage score at each of the four time points (baseline, 12, 30, and 42 months). Linear growth exposures were represented by the change in height occurring between each of the seven time points (baseline, 6, 12, 18, 30, and 42 months). Spinal pain outcomes were the measures of duration (weeks with pain) and frequency (episode count) occurring between exposure measurements. Separate analyses were conducted for each spinal pain outcome, and results were reported with incidence rate ratios (IRR) and 95% confidence intervals. Alpha was .05 for all analyses.
We explored for but identified no significant interactions between sex and Tanner score or sex and height change and therefore included no interaction terms in the final models. Sex and school type (usual or intensive physical education) were included as covariates in all models. We did not adjust for age owing to its collinearity with linear growth and pubertal development.
We further explored the associations between linear growth and spinal pain by modeling parameter estimates and confidence intervals for 2, 3, 4, and 5 cm changes in height during a 6-month period. To investigate the independent associations between pubertal development and spinal pain, independent of growth, we constructed additional models controlling for change in height. Conversely, we controlled for pubertal development to investigate the independent associations between linear growth and spinal pain.
Results

Table 1
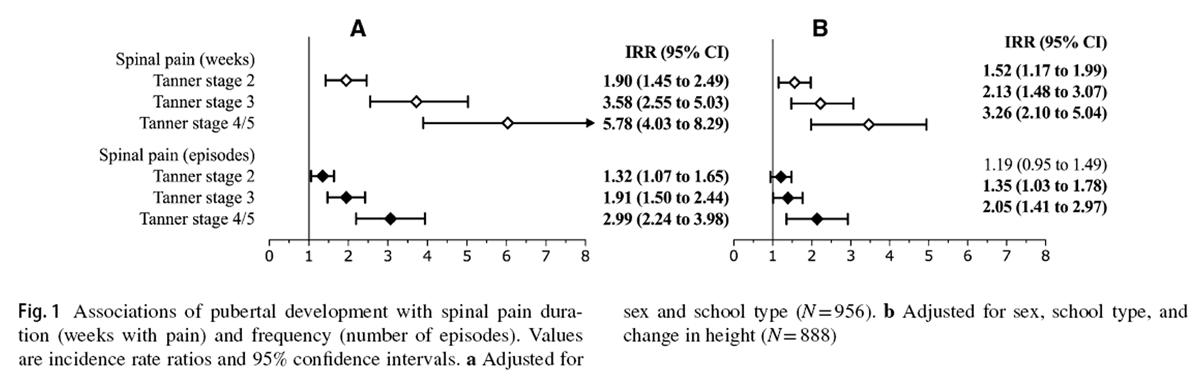
Figure 1

Figure 2 Descriptive statistics at baseline and follow-up for demographic and anthropometric variables, as well as pubertal development, are presented in Table 1. During the study, 885 (52.7%) children reported at least one episode of spinal pain. Among children who experienced spinal pain, the median (IQR) total symptom duration was 2 (1–5) weeks. Figures 1 and 2 report the associations between pubertal development and linear growth, respectively, for each spinal pain outcome variable.
Pubertal development and spinal pain
Advancing pubertal development was associated with spinal pain duration (Fig. 1a). Relative to Tanner stage 1 development, children at Tanner stage 2 (IRR [95% CI] = 1.90 [1.45 to 2.49]), stage 3 (IRR [95% CI] = 3.58 [2.55 to 5.03]), and stage 4/5 (IRR [95% CI] = 5.78 [4.03 to 8.29]) experienced greater durations of spinal pain.
Similarly, Tanner stages 2 (IRR [95% CI] = 1.32 [1.07 to 1.65]), 3 (IRR [95% CI] = 1.91 [1.50 to 2.44]), and 4/5 (IRR [95% CI] = 2.99 [2.24 to 3.98]) were associated with a greater frequency of spinal pain episodes (Fig. 1a). Controlling for linear growth resulted in modest reductions to the magnitude of associations between pubertal development and spinal pain duration and frequency, with nearly all parameter estimates remaining significant (Fig. 1b).
Linear growth and spinal pain
Greater changes in height were associated with increased spinal pain duration (IRR [95% CI] = 1.19 [1.15 to 1.23]) and frequency of episodes (IRR [95% CI] = 1.14 [1.11 to 1.17]) (Fig. 2a). This translates into a 19% increased risk for an additional week in which spinal pain was reported and 14% greater risk for an additional pain episode per 1 cm of linear growth in a 6-month period. Estimated risks associated with 2-, 3-, 4-, and 5-cm height increases in 6 months are displayed in Fig. 2a. After controlling for pubertal status, all associations between growth and spinal pain duration and frequency remained significant (Fig. 2b).
Discussion
The current study advances the understanding of spinal pain in young people. Both pubertal development and linear growth were associated with spinal pain. Boys and girls with more advanced pubertal development and those undergoing greater growth experienced increased spinal pain frequency and duration. Moreover, these relationships were largely independent; controlling for growth had little impact on the relations between pubertal development and spinal pain, and controlling for pubertal development had little impact on the relations between linear growth and spinal pain. We found no evidence for a modifying role of sex in these relationships but did identify evidence of dose–response between pubertal development and spinal pain duration and frequency. This means that pubertal development and linear growth are potential risk factors for spinal pain in both girls and boys that they may explain unique aspects of risk.
It should be noted that pubertal development and linear growth are non-modifiable factors. Nevertheless, their associations with spinal pain have clinical relevance. Recognizing the role of puberty and growth may help clinicians to manage young people with spinal pain. Traditionally, the occurrence of spinal pain in youth was thought to be a rare and concerning presentation, owing to its potential for pathological etiology. [30] However, current evidence suggests spinal pain in young people to be a relatively common and usually benign condition [31], even in the case of chronic pain. [32] Therefore, recognition of the relations between pubertal development, growth, and spinal pain can assist clinicians in setting appropriate expectations and provide reassurance and advice to pediatric patients and parents— recommendations common to all clinical guidelines for the management of non-specific back pain. [33]
It is also important for clinicians to remain vigilant and identify concerning features of spinal pain in young people that should raise clinical suspicion for serious pathology. [34] Pediatric patients presenting with spinal pain and ‘red flags’ such as recent trauma, fever, weight loss, previous malignancy, or other findings such as pain during lumbar extension that may indicate spondylolysis/spondylolisthesis, warrant additional evaluation and/or referral. [31] Information about sedentary and physical activity behavior, as well as the recognition of psychosocial factors associated with future disability and potential learned behaviors, may inform the management of spinal pain in this population. [31, 35]
Our study results are consistent with the two previous population-based longitudinal studies that identified relationships between pubertal development and back pain among Dutch and American adolescents (odds ratios [95% CI] 1.34 [1.13 to 1.57] to 1.61 [1.30 to 1.99]). [12] However, those studies found no significant relationships between growth (‘growth spurt’) and back pain (odds ratios [95% CI] 1.04 [.89 to 1.21] to 1.13 [.98 to 1.31]). Compared to the current study, those participants were approximately 1.5 years older at baseline (mean age = 11.1–11.6 years).
The current investigation addressed several important limitations of the prior longitudinal studies. While those studies included only a single measure of pubertal development and growth at baseline, we obtained serial measures of pubertal development at four time points and linear growth at seven time points over the 42-month study period. The back pain outcomes in previous studies relied on participants’ ability to accurately self-report their frequency of pain over the preceding 3 months. We limited potential for recall bias by intensively measuring spinal pain each week and constructed variables comprising two pain characteristics: frequency and duration.
In addition, most adolescents in the previous studies were already in a mid-pubertal developmental stage at baseline, and therefore it is possible that some effects of early pubertal development were missed. In contrast, a large proportion of children in the current study were prepubertal (51% Tanner stage 1), or in the early stage of pubertal development (38% Tanner stage 2) at baseline. Despite the differences in the samples and methodology, the results with respect to the relationship between pubertal development and spinal pain were consistent, thus increasing the confidence in this finding.
The existence of an association between exposure and outcome does not infer a cause and effect relationship. In the context of the Bradford Hill criteria [36], however, the current study results support several elements of causality between pubertal development and spinal pain. As discussed, our results are consistent with previous longitudinal evidence and thus provide evidence of replication. Additionally, we identified evidence of dose–response, with more advanced pubertal development associated with increased spinal pain duration and frequency.
Temporality is potentially the most challenging causal criteria to establish in spinal pain research. Spinal pain is classified as a chronic disease [37, 38], characterized by recurrent episodes [39] that occur rarely or frequently. [40] It is, therefore, difficult to distinguish between the onset of disease and a new episode of recurrent pain, and challenging to assemble a true inception cohort of disease-free individuals. Consequently, most studies of spinal pain investigate the episodes or patterns of pain and not the cause of the disease itself. [41] Most children in the current study were younger than the expected age of onset for spinal pain. [42] Therefore, the temporal sequencing of exposure and outcome was likely intact for many participants (i.e., the onset of puberty preceding the occurrence of spinal pain).
The primary strengths of the current study were the prospective design, intensive monitoring of spinal pain, long-term follow-up, and multilevel analyses that accounted for the longitudinal nature of the data and potential confounding. Study limitations include measurement issues related to the exposure and outcome variables. Compared to self-assessment, the grading of pubertal stages is more accurate when performed by physicians as part of a clinical examination. However, compared to prior studies using self-assessment [43], our measures of pubertal development were conducted as part of a structured interview, and participants were provided with standardized text and illustrations to assist with decision-making, which may have improved the accuracy of these assessments. Nevertheless, misclassification resulting from the assessment of pubertal development represents a potential source of error in our study.
Although our intensive measures of spinal pain frequency and duration likely helped to limit recall bias, we did not capture all aspects of pain. Knowledge of additional pain characteristics and consequences such as pain intensity, functional limitations, and the need for healthcare utilization will help to further the understanding of pubertal development and linear growth. Finally, there may be other factors that were not considered in the current study that explain the relationships between pubertal development, growth, and spinal pain. These limitations are potential sources of residual confounding in our analyses.
Future research is needed to investigate the role of other features of pubertal development and their relations with spinal pain. Specifically, the examination of psychological and hormonal characteristics of development may advance the understanding of the role of puberty in the development of spinal pain in youth.
Acknowledgements
The authors gratefully acknowledge the valuable work of numerous students who assisted with data collection in the CHAMPS study-DK. We also thank the participating children, their parents, and teachers in the schools involved in the project. We are grateful for the cooperation with The Svendborg Project, Sport Study Sydfyn, and the Municipality of Svendborg. Finally, we wish to acknowledge members of the CHAMPS study-DK not listed as coauthors in this paper: E. Jespersen, M. Heidemann, and C.T. Rexen.
Funding
The TRYG Foundation, University College Lillebaelt, University of Southern Denmark, The Nordea Foundation, The IMK foundation, The Region of Southern Denmark, The Egmont Foundation, The A.J. Andersen Foundation, The Danish Rheumatism Association, Østifternes Foundation, Brd. Hartmann’s Foundation, TEAM Denmark, The Danish Chiropractor Foundation, and The Nordic Institute of Chiropractic and Clinical Biomechanics. The funding sources played no role in the design, conduct, or reporting of this study.
Conlict of interest
Prof. Hebert receives salary support from the Canadian Chiropractic Research Foundation and the New Brunswick Health Research Foundation. The authors declare no additional conflicts of interest.
References:
Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators.
Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases
and Injuries, 1990-2015: a Systematic Analysis for the Global Burden of Disease Study 2015
Lancet. 2016 (Oct 8); 388 (10053): 1545–1602Deyo RA, Rainville J, Kent DL (1992)
What can the history and physical examination tell us about low back pain?
JAMA 268:760–765Manek NJ, MacGregor AJ (2005)
Epidemiology of back disorders: prevalence, risk factors, and prognosis.
Curr Opin Rheumatol 17:134–140.
https ://doi.org/10.1097/01.bor.00001 54215.08986 .06van Tulder M, Koes B, Bombardier C (2002)
Low back pain.
Best Pract Res Clin Rheumatol 16:761–775.
https ://doi.org/10.1053/ berh.2002.0267Disease GBD, Injury I, Prevalence C (2017)
Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases
and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016.
Lancet 390:1211–1259.
https ://doi.org/10.1016/S0140 -6736(17)32154 -2Stallknecht SE, Strandberg-Larsen K, Hestbæk L, et al. .
Spinal Pain and Co-occurrence with Stress and General Well-being Among Young Adolescents:
A Study Within the Danish National Birth Cohort
European Journal of Pediatrics 2017 (Jun); 176 (6): 807–814Kjaer P, Wedderkopp N, Korsholm L, Leboeuf-Yde C.
Prevalence and Tracking of Back Pain From Childhood to Adolescence
BMC Musculoskelet Disord. 2011 (May 16); 12: 98Hestbaek L, Leboeuf-Yde C, Kyvik KO:
Is Comorbidity in Adolescence a Predictor for Adult Low Back Pain? A Prospective Study of a Young Population
BMC Musculoskelet Disord 2006 (Mar 16); 7: 29Kamper SJ, Yamato TP, Williams CM (2016)
The prevalence, risk factors, prognosis and treatment for back pain in children and adolescents:
an overview of systematic reviews.
Best Pract Res Clin Rheumatol 30:1021–1036.
https ://doi.org/10.1016/j.berh.2017.04.003Dolphens M, Vansteelandt S, Cagnie B, Vleeming A, Nijs J, Vanderstraeten G, Danneels L (2016)
Multivariable Modeling of Factors Associated with Spinal Pain in Young Adolescence
European Spine Journal 2016 (Sep); 25 (9): 2809–2821Hulsegge G, van Oostrom SH, Picavet HS, Twisk JW, Postma DS, Kerkhof M (2011)
Musculoskeletal complaints among 11-year-old children and associated factors: the PIAMA birth cohort study.
Am J Epidemiol 174:877–884.
https://doi.org/10.1093/aje/kwr20 5Janssens KA, Rosmalen JG, Ormel J, Verhulst FC, Hunfeld JA, Mancl LA. (2011)
Pubertal status predicts back pain, overtiredness, and dizziness in American and Dutch adolescents.
Pediatrics 128:553–559.
https ://doi.org/10.1542/peds.2010-2364Wedderkopp N, Andersen LB, Froberg K, Leboeuf-Yde C (2005)
Back pain reporting in young girls appears to be puberty-related.
BMC Musculoskel Disord 6:52.
https ://doi.org/10.1186/1471-2474-6-52Wheeler MD (1991)
Physical changes of puberty.
Endocrinol Metab Clin North Am 20:1–14Tanaka T, Suwa S, Yokoya S, Hibi I (1988)
Analysis of linear growth during puberty.
Acta Paediatr Scand Suppl 347:25–29Salminen JJ, Erkintalo M, Laine M, Pentti J (1995)
Low back pain in the young. A prospective three-year follow-up study of subjects with and without low back pain.
Spine 20:2101–2107 discussion 2108)Fairbank JC, Pynsent PB, Van Poortvliet JA, Phillips H (1984)
Influence of anthropometric factors and joint laxity in the incidence of adolescent back pain.
Spine 9:461–464Feldman DE, Shrier I, Rossignol M, Abenhaim L (2001)
Risk factors for the development of low back pain in adolescence.
Am J Epidemiol 154:30–36Hasler CC (2013)
Back pain during growth.
Swiss Med Wkly 143:w13714.
https ://doi.org/10.4414/smw.2013.13714Lardon A, Leboeuf-Yde C, Le Scanff C, Wedderkopp N.
Is Puberty a Risk Factor For Back Pain in the Young? A Systematic Critical Literature Review
Chiropractic & Manual Therapies 2014 (Oct 15); 22 (1): 27Swain M, Kamper SJ, Maher CG, Broderick C, McKay D, Henschke N (2018)
Relationship between growth, maturation and musculoskeletal conditions in adolescents: a systematic review.
Br J Sports Med.
https ://doi.org/10.1136/bjspo rts-2017-09841 8Craig P, Cooper C, Gunnell D, Haw S, Lawson K, Macintyre S, Ogilvie D. (2012)
Using natural experiments to evaluate population health interventions: new Medical Research Council guidance.
J Epidemiol Community Health 66:1182–1186.
https ://doi.org/10.1136/jech-2011-20037 5Wedderkopp N, Jespersen E, Franz C, Klakk H, Heidemann M, Christiansen C/ (2012)
Study protocol. The childhood health, activity, and motor performance school study Denmark (the CHAMPS-study DK).
BMC Pediatr 12:128.
https ://doi.org/10.1186/1471-2431-12-128Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000)
Establishing a standard definition for child overweight and obesity worldwide: international survey.
BMJ 320:1240–1243Tanner JM (1962)
Growth at adolescence, with a general consideration of the effects of hereditary and environmental
factors upon growth and maturation from birth to maturity.
Blackwell Scientific Publications, OxfordDuke PM, Litt IF, Gross RT (1980)
Adolescents’ self-assessment of sexual maturation.
Pediatrics 66:918–920Baranowski T, Smith M, Baranowski J, Wang DT, Doyle C, Lin LS, Hearn MD, Resnicow K (1997)
Low validity of a sevenitem fruit and vegetable food frequency questionnaire among third-grade students.
J Am Diet Assoc 97:66–68.
https ://doi.org/10.1016/S0002 -8223(97)00022 -9Peterson L, Harbeck C, Moreno A (1993)
Measures of children’s injuries: self-reported versus maternal-reported events with temporally proximal versus delayed reporting.
J Pediatr Psychol 18:133–147Johansen B, Wedderkopp N (2010)
Comparison between data obtained through real-time data capture by SMS and a retrospective telephone interview.
Chiropr Osteopat 18:10.
https ://doi.org/10.1186/1746-1340-18-10Davis PJ, Williams HJ (2008)
The investigation and management of back pain in children.
Arch Dis Child Educ Pract Ed 93:73–83.
https ://doi.org/10.1136/adc.2006.11553 5Jakes AD, Phillips R, Scales M (2015)
Teenagers with back pain.
BMJ 350:h1275.
https ://doi.org/10.1136/bmj.h1275Bhatia NN, Chow G, Timon SJ, Watts HG (2008)
Diagnostic modalities for the evaluation of pediatric back pain: a prospective study.
J Pediatr Orthop 28:230–233.
https ://doi.org/10.1097/BPO.0b013 e3181 651bc 8Koes BW, van Tulder M, Lin CW, Macedo LG, McAuley J, Maher C.
An Updated Overview of Clinical Guidelines for the Management of Non-specific Low Back Pain
in Primary Care
European Spine Journal 2010 (Dec); 19 (12): 2075–2094Shah SA, Saller J (2016)
Evaluation and diagnosis of back pain in children and adolescents.
J Am Acad Orthop Surg 24:37–45. https ://doi.org/10.5435/JAAOS -D-14-00130Franz C, Moller NC, Korsholm L, Jespersen E, Hebert JJ, Wedderkopp N (2017)
Physical activity is prospectively associated with spinal pain in children (CHAMPS study-DK).
Sci Rep 7:11598.
https ://doi.org/10.1038/s4159 8-017-11762 -4Hill AB (1965)
The environment and disease: association or causation?
Proc R Soc Med 58:295–300Itz CJ, Geurts JW, van Kleef M, Nelemans P.
Clinical Course of Non-specific Low Back Pain:
A Systematic Review of Prospective Cohort Studies Set in Primary Care
European Journal of Pain 2013 (Jan); 17 (1): 5–15Pengel LH, Herbert RD, Maher CG, Refshauge KM (2003)
Acute low back pain: systematic review of its prognosis.
BMJ 327:323.
https ://doi.org/10.1136/bmj.327.7410.323Lemeunier N, Leboeuf-Yde C, Gagey O (2012)
The natural course of low back pain: a systematic critical literature review.
Chiropractic & Manual Therapies 2018 (Jan 9); 20: 33
Hancock MJ, Maher CM, Petocz P, Lin CW, Steffens D, Luque-Suarez A, Magnussen JS (2015)
Risk factors for a recurrence of low back pain.
Spine J 15:2360–2368.
https ://doi.org/10.1016/j.spine e.2015.07.007Ardakani EM, Leboeuf-Yde C, Walker BF.
Failure to Define Low Back Pain as a Disease or an Episode Renders Research on Causality
Unsuitable: Results of a Systematic Review
Chiropractic & Manual Therapies 2018 (Jan 9); 26: 1Jones GT, Macfarlane GJ (2005)
Epidemiology of low back pain in children and adolescents.
Arch Dis Child 90:312–316.
https ://doi.org/10.1136/adc.2004.05681 2Rasmussen AR, Wohlfahrt-Veje C, Tefre de Renzy-Martin K, Hagen CP, Tinggaard J. (2015)
Validity of self-assessment of pubertal maturation.
Pediatrics 135:86–93.
https ://doi.org/10.1542/peds.2014-0793
Return to PEDIATRICS
Return to NECK AND BACK PAIN
Since 1-03-2020


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |