Chiropractic Management of Postoperative Spine Pain:
A Report of 3 CasesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2013 (Sep); 12 (3): 168–175 ~ FULL TEXT
OPEN ACCESS Christopher M. Coulisa, and Anthony J. Lisi
VA Connecticut Healthcare System,
West Haven, CT;
University of Bridgeport College of Chiropractic,
Bridgeport, CT.

OBJECTIVE: The purpose of this case series is to describe chiropractic care including spinal manipulation for 3 patients with postsurgical spine pain.
CLINICAL FEATURES: Three patients with postsurgical spine pain (1 cervical fusion, 1 lumbar discectomy, and 1 lumbar laminectomy) presented for chiropractic treatment at a major US medical center. Treatment included spinal manipulation and/or flexion-distraction mobilization based on patient response to joint loading strategies.
INTERVENTION AND OUTCOMES: Two patients were treated with high-velocity, low-amplitude spinal manipulation; and 1 patient was treated with flexion-distraction mobilization. Treatment frequency and duration were 4 treatments over 4 weeks for case 1, 17 treatments over 7 years for case 2, and 5 treatments over 5 weeks for case 3. Subjective improvement was noted using numeric pain scores and functional changes; and upon completion, the patients reported being "satisfied" with their overall outcome. One episode of transient benign soreness was noted by 1 patient. No additional adverse events or effects were noted.
CONCLUSION: In these 3 cases, patients with postsurgical spine pain responded positively to chiropractic care. Spinal manipulation/mobilization was tolerated without significant adverse effects.
KEYWORDS: Adverse effect; Chiropractic; Manipulation; Postoperative period
From the FULL TEXT Article:
Introduction
Chronic pain after surgery is common. [1, 2] It has been reported that about 1 in 5 patients who have undergone various surgical procedures experiences severe postoperative pain or only poor to fair pain relief despite pain management therapies. [2] With regard to spinal surgery, it is estimated that 15% to 61% of patients report persistent or recurrent pain postsurgically depending on the specific intervention. [3–5] Moreover, up to two-thirds of all chronic pain patients enrolled in pain centers in the United States are believed to experience failed back surgery syndrome. [6] Data suggest that chronic back pain post–spinal surgery should be treated nonoperatively unless progressive neurologic deficits exist. [7]
The prevalence of postsurgical patients presenting to chiropractic practices ranges from 2.3% to 12%. [8–10] However, the available evidence regarding safety and effectiveness of chiropractic treatment of postsurgical spinal pain is limited to only case reports. O’Shaughnessy et al [11] presented 8 cases of persistent low back pain (LBP) or pelvic pain post–lumbar total disk replacement with positive outcome after high-velocity, low-amplitude (HVLA) spinal manipulation; adverse events were benign and similar to those experienced by patients without prior spinal surgery. Kruse and Cambron [12] treated 32 postsurgical patients with flexion-distraction mobilization, reporting no adverse events and noting benefit in all patients regardless of surgical procedure used. Morningstar and Strauchman [13] treated 3 post–lumbar spine fusion patients with manipulation under anesthesia followed by 8 weeks of physical therapy, reporting durable subjective and functional improvement without adverse events; and Estadt [14] demonstrated positive response to a trial of lumbar manipulation and rehabilitative exercises in a patient who previously underwent lumbar microdiscectomy. Polkinghorn and Colloca [15] presented a single case of cervical spine pain post–discectomy and fusion that tolerated instrumental adjustment without adverse reactions. McGregor and Cassidy [16] presented 3 cases of sacroiliac syndrome post–lumbar fusion that responded to HVLA manipulation. Shaw [17] reported a case of back and leg pain post–lumbar discectomy that improved after HVLA manipulation and physical therapy modalities. Gluck [18] described a case of back and leg pain after 2 lumbar discectomies that responded to flexion-distraction and extensive active rehabilitation. Finally, Lisi and Bhardwaj [19] reported resolution of back pain in one case of postsurgical chronic cauda equina syndrome treated by HVLA manipulation.
With the rate of spinal surgery continuing to increase, [20–22] the incidence of postsurgical spine pain is also expected to rise. The purpose of this article is to report on clinical decision making and presence of adverse events in the use of HVLA spinal manipulation and/or flexion-distraction mobilization in 3 cases of postsurgical spine pain that were referred to the chiropractic clinic at a major US medical center.
Case reports
Case 1
A 31–year-old man presented with acute LBP and bilateral lower extremity pain. Prior surgical intervention included L5 laminectomy, left L4–5 nerve root decompression, and right L5–S1 nerve root decompression performed 2.5 years previously for L4–5 and L5–S1 disk herniation. This provided good relief for several years until the patient attempted to unload and stack firewood. At that time, the patient noted immediate severe lumbar spine pain with radiation into his bilateral lower extremities to the lateral aspect of his feet (left > right), prompting consultation to our clinic. Upon presentation, he stated that his condition was provoked with sitting, bending over, and coughing/sneezing and was worse in the morning, whereas mild relief was achieved with lying down, diclofenac, and Flexeril (cyclonezaprine; McNeil Consumer and Specialty Pharmaceuticals, Fort Washington, PA). He reported associated left ankle weakness and paresthesia of the left shin; however, he denied bowel or bladder retention or incontinence, saddle anesthesia, or constitutional symptoms. He rated his condition using a verbal numeric pain scale (NPS) as 6/10 on average and 8/10 at worst and described it as a dull, throbbing, nagging pain with intermittent burning radiating pain into both legs. He prioritized his lower extremity pain over the LBP. Functionally, his condition limited his ability to sit, walk, bend, and pilot helicopters. Prior treatment of his back pain included physical therapy, acupuncture, nonsteroidal anti-inflammatory drugs, and narcotics, without relief. His medical history included posttraumatic stress disorder, gastroesophogeal reflux disease, bilateral dry eye syndrome, emphysema, chondromalacia of both knees, and attention-deficit/hyperactivity disorder.
Examination revealed an antalgic posture and gait. The patient displayed difficulty arising from a seated position. Lumbar spine range of motion was limited in both flexion and extension; a preference for extension was noted as centralization was achieved with repeated motion. Adverse nerve root tension was noted with supine and seated straight leg raise (SLR) on the right, crossed leg raise was unremarkable, deep tendon reflexes (DTRs) were 2 + at the Achilles and patella bilateral and symmetric, and strength was 5/5 throughout the lower extremities bilaterally; there was no evidence of muscular atrophy or fasciculation. Hypoesthesia was noted along the left lateral leg and foot. Long tract signs were not present. Articular stiffness and pain were noted in the lower lumbar spine with associated hypertonicity and palpable tenderness in the adjacent thoracolumbar paraspinal muscles. Sacroiliac joint and hip provocation was unremarkable for reproduction of the patient’s chief complaint. Distal pulses were present bilaterally and symmetrically. Magnetic resonance imaging of the lumbar spine demonstrated a disk protrusion at L4–5 and a disk extrusion at L5–S1 extending to the right lateral recess causing effacement of the right nerve root.
The patient was given a working diagnosis of discogenic LBP and right S1 radiculopathy. He was assessed for appropriateness of HVLA spinal manipulation by provocation testing involving the application of graded preloading consistent with the manipulative procedure, which caused pain. At each visit, this provocation testing maneuver resulted in increased lower extremity pain; thus, manipulation was not performed. Provocation testing with flexion-distraction mobilization was tolerated; thus, this was used, targeting the lower lumbar spine. He was also given a home exercise program that included end-range loading consistent with his directional preference and spinal stabilization exercises.
The patient received 4 treatments over 4 weeks, noting benefit of both his low back and lower extremity pain without any adverse reaction. His pain levels decreased to 4/10 on average and 7/10 at worst, noting a decrease in the frequency and duration of acute episodes. He also reported functional improvement; he was able to tolerate scuba diving, piloting a helicopter, and driving 200 miles without exacerbation of his condition. He stated that his symptoms were more localized to the lower lumbar spine with decreased intensity of pain in the leg; however, there was no change in the left lateral leg paresthesia. At reevaluation, he demonstrated increased lumbar spine range of motion with increased tolerance to flexion; however, adverse nerve root tension continued to be present on the right during SLR, and hypoesthesia was still present at the left lateral leg and foot. The patient was satisfied with his response to treatment and wished to continue with his home exercise program and return for treatment on an as-needed basis.
Case 2
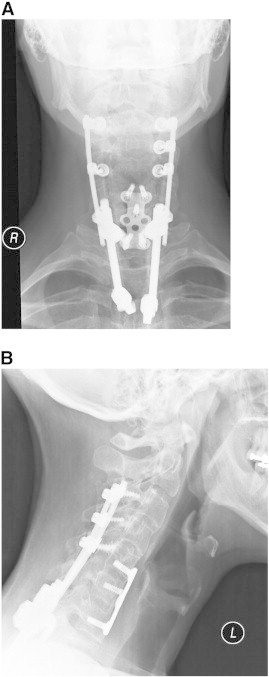
A 47–year-old man presented with a history of chronic neck, upper back, and right upper extremity pain after a cervical spine fracture secondary to a 3–story fall. Prior surgical intervention included C3–T3 fusion with instrumentation and allograft performed 7 years previously (Fig 1A and B). Despite treatment, the patient reported constant neck, thoracic, and right upper extremity pain with associated right-hand weakness and dysesthesia. Upon presentation, he was unable to identify specific provocative factors because all movements, positions, and activities exacerbated his condition. He noted transient relief with Naprosyn (Roche Pharmaceuticals, Nutley, NJ). He reported significant difficulty with right-hand grip, manipulation of simple objects, and fine motor control with both hands secondary to severe finger weakness (extension and opposition). He denied associated headache, ataxia, dizziness, chest pain/shortness of breath, or constitutional symptoms. However, he noted daily urinary urgency; this was monitored by his neurologist. He rated his condition using NPS as 6/10 on average and 8/10 at worst and described it as a deep throbbing ache. Functionally, he had a decreased ability to work and perform activities of daily living. Prior treatment included intensive rehabilitation, trigger point injections, and pharmacotherapy (nonsteroidal anti-inflammatory drugs, and opioid and nonopioid analgesics). His medical history was remarkable for neurogenic bowel/bladder, neurogenic erectile dysfunction, bilateral ulnar neuropathy, ocular migraines, obstructive sleep apnea, and chronic pain syndrome.
Examination revealed a well-nourished, well-groomed man that did not appear to be in distress. Cervical range of motion was limited in all planes without increased cervical, thoracic, or right upper extremity pain. Repeated passive cervical spine range of motion failed to centralize or peripheralize his condition. Adverse nerve root tension was noted with right upper limb tension test; DTRs were 1 + and bilateral at the biceps, triceps, and brachioradialis and 2 + bilateral at the patella and Achilles. Chronic, nonprogressive weakness, graded 3/5, was noted with right wrist extension, 1/5 with finger extension and abduction, and 2/5 with finger flexion. Atrophy was noted of the forearm muscles to the hands bilateral. Pathological reflexes were not present in the upper or lower extremities. Articular stiffness and pain were noted along the upper cervical and midthoracic spine (regions adjacent to the fusion construct). Hypertonicity and tenderness were noted at the bilateral suboccipital muscles, cervical paraspinal muscles, and interscapular muscles. Bilateral shoulder provocation testing was unremarkable for reproduction of the patient’s complaint. Magnetic resonance imaging of the cervical spine demonstrated multiple postsurgical changes with anterior fixation from C5 to C7 and posterior fixation from C3 to T3.
He was given a working diagnosis of facetogenic cervical and thoracic pain with residual neuromotor deficits. He was able to tolerate articular provocation and premanipulative positioning; therefore, he was treated with HVLA manipulation to the cervical and thoracic spine with the therapeutic goal of analgesia and improving segmental range of motion; more specifically, the patient received HVLA “thrust” manipulation to the C1–2 segments bilaterally while supine and the T3–6 segments while prone. Myofascial release to the hypertonic and painful musculature including the suboccipital, cervical paraspinal, and rhomboid muscles was also used.
The patient has been treated 17 times over the past 7 years. He continues to experience exacerbations periodically that respond to a short trial of care, usually consisting of 2 to 3 treatments. Posttreatment, he typically reports a return to baseline pain and functional levels evidenced by a greater than 2–point decrease in numeric rating scale, increased cervical spine range of motion, and improved ability to perform his activities of daily living. However, no benefit is noted regarding the upper extremity paresthesia and weakness; the upper limb tension test continues to demonstrate signs of adverse nerve root tension, and forearm atrophy continues to be observed. No adverse effects have been reported after any treatment.
Case 3
A 60–year-old man presented with a history of chronic LBP and right lower extremity pain. L3–5 laminectomy was performed 27 years prior after experiencing an L3/4 and L4/5 disk herniation secondary to falling off of a truck. Since that time, he noted periods of episodic and variable pain resulting in numerous presentations to the emergency department for assessment and medications. Upon presentation to our clinic, he reported that he had noted a 2–week exacerbation of lumbar spine pain with radiation to the anterior right thigh, medial leg, and foot after stumbling and falling off of a curb while walking. His condition was provoked with sitting for more than 15 minutes, riding a bike, walking more than 100 yards, sneezing/coughing, and quick movements and appeared to be worse towards the end of the day. He was unable to identify palliative factors, positions, or activities. He reported associated right lower extremity numbness and subjective weakness resulting in 4 collapses during the past 2 weeks; however, he denied foot drop, bowel/bladder dysfunction, saddle anesthesia, or constitutional symptoms. He rated his condition using an NPS as 7/10 on average and 10/10 at worst and described it as throbbing in the lumbar spine with intermittent stabbing pain in to the right lower extremity. Functionally, he was limited to driving limited distances and to short periods of sitting and had difficulty with self-care. Prior treatment included physical therapy and medications (tricyclic antidepressants, acetaminophen, meloxicam, cyclobenzaprine, and opioids). Medical history was remarkable for depressive disorder, hypertension, obesity, nicotine/alcohol/cocaine dependence, cardiomyopathy, erectile dysfunction, and abdominal pain.
Examination revealed a well-nourished, well-groomed man in distress. He had difficulty maintaining a seated posture and required frequent change of position. Lumbar spine range of motion was limited in both flexion and extension. A preference for extension was noted; however, neither centralization nor peripheralization was present. Neurological examination demonstrated a positive right femoral nerve stress test, SLR was unremarkable, lower extremity strength was 5/5, DTRs were trace bilateral at the patella and Achilles, and sensation to light touch was intact in the lower extremities bilateral. Long tract signs were not present. There was tightness of the thoracolumbar paraspinal muscles with tender points. The lower lumbar articulations were hypomobile and somewhat painful on provocation. Sacroiliac and hip provocation was unremarkable. Distal pulses were intact, strong bilateral. Magnetic resonance imaging of the lumbar spine demonstrated disk protrusion at L2–3, L3–4, L4–5, and L5–S1 with moderate to severe spinal stenosis and postoperative changes with minimal scarring.
He was given a working diagnosis of postlaminectomy syndrome and chronic right L4 radiculopathy. He was assessed for appropriateness of HVLA spinal manipulation and flexion-distraction mobilization and was able to tolerate premanipulative positioning and articular provocation. Subsequently, he was treated with flexion-distraction mobilization targeting the lumbar spine and side-posture HVLA manipulation of the lower lumbar spine at each visit. This combination of mobilization (aimed at decreasing gross regional stiffness) immediately preceding manipulation (aimed at analgesia and increased segmental range of motion) during the same visit is often used pragmatically.
He received 5 treatments over a 5–week period, reporting only temporary relief of his low back and right anterior thigh pain; at each follow-up, his pain level was continually rated 6/10 to 7/10. However, he noted durable functional improvement: he tolerated driving 600 miles without exacerbation of his symptoms and reported increased walking distance prior to the onset of pain. He reported mild lumbar spine soreness after the initial treatment that lasted the remainder of the day; this resolved thereafter. He did not experience any other adverse reactions from additional treatment. Reevaluation demonstrated increased lumbar spine range of motion. However, there continued to remain a positive right femoral nerve stress test result indicating persistent right L4 radiculopathy, continued lumbar paraspinal tenderness, and lower lumbar articular stiffness and pain. The patient was satisfied with his response to treatment and wished to follow up as needed for exacerbation of lumbar spine or lower extremity pain or if he noted deterioration of his functional gains. All 3 patients provided consent for their health information to be published in this case series.
Discussion
It is believed that approximately 7.5% of patients experiencing chronic LBP elect to undergo spinal surgery. [23] Despite surgical intervention, up to 61% of patients continue to experience spinal pain after surgery. [3–5] These patients may continue to seek treatment and are faced with numerous options for pain management and functional restoration, including chiropractic care. Aspergren and Burt [8] demonstrated that 3.8% of patients consulting chiropractic physicians had undergone at least one previous spinal surgery, whereas Hurwitz et al [9] noted that 2.3% of patients consulting a doctor of chiropractic had a history of at least one previous back surgery, and Stern et al10 found that 12% of cases of LBP and leg pain treated at a chiropractic teaching clinic had a history of previous low back surgery.
However, very little evidence exists to guide chiropractic clinicians in the treatment of such patients; and in particular, the safety and effectiveness of spinal manipulation/mobilization in the postsurgical spine have yet to be demonstrated. In this article, 3 cases of postsurgical spinal pain were treated with HVLA spinal manipulation and/or flexion-distraction mobilization. Duration of time postsurgery ranged from 2 to 27 years. Treatment duration was 1 ×/wk for 4 and 5 weeks for case 1 and 3, respectively, and several isolated trials of 2 to 3 treatments over the past 7 years for case 2. In this study, we observed improvement in numeric rating scale scores and function. Additionally, in each trial, the patients reported being “satisfied” with their overall outcome. The only adverse effect reported was 1 episode of transient benign soreness after the initial treatment in 1 case; this resolved within 24 hours. No other complications were seen in any other patient.
Depending on specific clinical characteristics, postsurgical joints may be considered a relative contraindication to HVLA manipulation. [24] Subsequently, administering spinal manipulation to a post–spinal surgery patient requires knowledge of surgical procedures and a greater degree of diagnostic acumen and manipulative skill than is required for the management of uncomplicated LBP. [25–27] In this study we used a pragmatic approach to the treatment of the postoperative spine. Management decisions were based on knowledge of postsurgical spine biomechanics and examination findings that supported the use of HVLA manipulation and/or mobilization as a treatment option.
In case 1, the patient was diagnosed with lumbar discogenic pain status post–L5 laminectomy, left L4–5 nerve root decompression, and right L5–S1 nerve root decompression. The incidence of recurrent disk herniation after discectomy ranges from 2% to 18%. [28] High-velocity, low-amplitude manipulation has been shown to be a nonoperative treatment option for lumbar radiculopathy secondary to a herniated disk [29] and for chronic lumbar discogenic pain with radiculopathy postmicrodiscectomy. [14] Unfortunately, this patient was not able to tolerate the procedure, as each attempt at HVLA spinal manipulation resulted in peripheralization of his complaint. It has been reported that movements/positions causing peripheralization of a patient’s symptoms are contraindicated, whereas movements/positions that centralize symptoms are appropriate. [30, 31] Therefore, he was treated with flexion-distraction mobilization, which has been found to decrease intradiscal pressure, [32] and end-range loading consistent with his directional preference. [33]
In case 2, the patient presented with chronic neck pain post–cervical and thoracic fusion. Chronic neck pain post–cervical spine fusion is common and may be attributed to numerous factors including surgery at the wrong level, insufficient removal of herniated or degenerative tissues, unrecognized second disk herniation, recurrence of herniation, unrecognized displaced sequestration, new disk herniation at a different level, epidural fibrosis/local arachnoiditis, or symptomatic arthritis of the posterior joints. [34] Additionally, in the untreated adjacent levels, increased motion and elevated intradiscal pressures have been associated with an increased risk of adjacent level syndrome, [35] believed to be the cause of 25% of post–cervical fusion pain. [36] Moreover, current evidence suggests that the zygoapophyseal joint is the most common cause of posttraumatic chronic neck pain. [37] The patient’s symptoms were believed to be facetogenic in nature. Subsequently, he was treated with HVLA manipulation to the cervical and thoracic spine, as he tolerated facet loading and premanipulative positioning without increased pain or radiation distally. He reported subjective benefit and functional gain posttreatment without the presence of adverse effect. To our knowledge, this is the first reported case of cervical spine HVLA manipulation post–cervical spine fusion.
In case 3, the patient presented with signs and symptoms consistent with postlaminectomy syndrome and chronic L4 radiculopathy. The patient did not demonstrate centralization or peripheralization of his symptoms with repeated end-range loading, and joint provocation maneuvers reproduced his complaint. Current evidence suggests that joint manipulation may be used if segmental provocation maneuvers reproduced all or part of a patient's pain and centralization of pain is not found on end-range loading examination. [38, 39] Current evidence regarding chiropractic manipulation and postlaminectomy syndrome appears to be limited to one case series involving only flexion-distraction mobilization. [12] However, this may be the result of prior work failing to note the specific surgical procedure that was performed. Future studies of manipulation and/or mobilization in this cohort should be more precise in describing the particular surgical procedure that was performed prior to manual intervention. In this case, the patient was treated with both flexion-distraction and spinal manipulation to the lower lumbar spine. The patient reported posttreatment soreness lasting 24 hours after the initial encounter; this resolved thereafter. No additional adverse events were noted.
Although numerous randomized controlled trials have shown spinal manipulation and/or mobilization to be safe and effective for the treatment of chronic neck pain and LBP, these trials invariably have excluded patients who have undergone prior spine surgery. This case series, along with the others described above, [11–19] provides grade 4 evidence supporting the hypothesis that such treatment may also be safe and effective for patients experiencing spinal pain after spine surgery. When revision surgery is not indicated and nonpharmacologic treatment options are sought, spinal manipulation and/or mobilization is often considered. Further work is needed to determine if this is an appropriate consideration for this patient population. In particular, future studies should report outcomes for patients with various subtypes of surgical decompression, fusion constructs, and/or arthroplasty.
Until more compelling data are presented, we propose that a clinician’s knowledge of the postsurgical spine and a patient’s response to provocation testing appear to have face validity for selecting manual therapy procedures.
Limitations
Results of this study are limited to the patients described. Secondly, it is possible that the improvement noted in each case was related to the concurrent use of analgesics and/or anti-inflammatory medications. However, use of medication in each case was initiated prior to manual treatment, was reported to be ineffective, and was discontinued during the trial of care. Thirdly, the positive outcomes experienced may have been attributed to the natural course of chronic back and neck pain. Contemporary viewpoints infer that acute exacerbations of chronic pain are common and generally return to baseline after a period of time. Although we cannot discount this, the likelihood that each of the 3 cases “self-resolved” within the period of care seems small. It is also possible that the gains noted from treatment were temporary and the patient’s symptoms returned to baseline after the trial was completed, as no long term follow-up was performed. Furthermore, the lack of adverse events in our study may be attributed to lack of disclosure by the patient and lack of long-term follow-up. As this is a case study/series, outcomes cannot be attributed to the interventions used; and one cannot assess safety or risk.
Conclusion
This study presents 3 cases of spinal pain post–spine surgery in which chiropractic spinal manipulation and/or mobilization was used without significant adverse effects and with reported positive clinical outcomes. This adds to the existing literature of previously reported cases; yet, the overall evidence base on this topic remains quite limited. Because spinal manipulation and/or mobilization may be considered as a nonoperative, nonpharmacologic option for pain management in such patients, more advanced studies are needed.
Funding sources and potential conflicts of interest
No funding sources or conflict of interest were reported for this study.
Disclaimer:
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
References:
Dolin S.J., Cashman J.N., Bland J.M.
Effectiveness of acute postoperative pain management: I. Evidence from published data.
Br J Anaesth. 2002;89:409–423Perkins F.M., Kehlet H.
Chronic pain as an outcome of surgery: a review of predictive factors.
Anesthesiology. 2000;93(4):1123–1133Carragee E.J., Han M.Y., Suen P.W., Kim D.
Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence.
J Bone Joint Surg Am. 2003;85(1):102–108Maigne J.Y., Planchon C.A.
Sacroiliac joint pain after lumbar fusion. A study with anesthetic blocks.
Eur Spine J. 2005;14(7):654–658Fritsch E.W., Heisel J., Rupp S.
The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments.
Spine. 1996;21(5):626–633Hua S.E., Levy R.M.
Spinal cord stimulation for failed back surgery syndrome.
In: Benzon H.T., Srinivasa N.R., Borsook D., Molloy R.E., Strichartz G., editors.
Essentials of pain medicine and regional anesthesia.
Churchill Livingston; Philadelphia, PA: 1999. pp. 237–241.Phillips F.M., Cunningham B.
Managing chronic pain of spinal origin after lumbar surgery: the role of decompressive surgery.
Spine. 2002;27(22):2547–2553Aspegren D.D., Burt A.L.
A study of postspinal surgery cases in chiropractic offices.
J Manipulative Physiol Ther. 1994;17(2):88–92Hurwitz E.L., Coulter I.D., Adams A.H., Genovese B.J., Shekelle P.G.
Use of chiropractic services from 1985 through 1991 in the United States and Canada.
Am J Pub Health. 1998;88(5):771–776Stern P.J., Cote P., Cassidy J.D.
A series of consecutive cases of low back pain with radiating leg pain treated by chiropractors.
J Manipulative Physiol Ther. 1995;18(6):335–342O’Shaughnessy J, Drolet M, Roy JF, Descarreaux M.
Chiropractic management of patients post–disc arthroplasty: eight case reports.
Chiropr Osteopat. 2010. Apr 21;18:7Kruse R.A., Cambron J.
Chiropractic Management of Postsurgical Lumbar Spine Pain:
A Retrospective Study of 32 Cases
J Manipulative Physiol Ther 2011 (Jul); 34 (6): 408–412Morningstar M.W., Strauchman M.N.
Manipulation under anesthesia for patients with failed back surgery: retrospective report of three cases with 1-year follow-up.
J Chiropr Med. 2012;11(1):30–35Estadt G.M.
Chiropractic/rehabilitation management of post-surgical disc herniation: a retrospective case report.
J Chiropr Med. 2004;3(3):108–115Polkinghorn B.S., Colloca C.J.
Chiropractic treatment of postsurgical neck syndrome with mechanical force, manually assisted short-lever spinal adjustments.
J Manipulative Physiol Ther. 2001;24(9):589–595McGregor M., Cassidy J.D.
Post-surgical sacroiliac joint syndrome.
J Manipulative Physiol Ther. 1983;6(1):1–11Shaw TW.
Chiropractic rehabilitation of the retraumatized post surgical lumbar spine with radiculopathy.
J Am Chiropr Assoc: Mar 1996(33:3): 71–74.Gluck N.I.
Passive care and active rehabilitation in a patient with failed back surgery syndrome.
J Manipulative Physiol Ther. 1996;19(1):41–47Lisi A.J., Bhardwaj M.K.
Chiropractic high velocity low amplitude spinal manipulation in the treatment of a patient with chronic cauda equina syndrome: an evidence-based case report.
J Manipulative Physiol Ther. 2004;27(9):574–578Wang M.C., Kreuter W., Wolfla C., Maiman D., Deyo R.
Trends and variations in cervical spine surgery in the United States.
Spine. 2009;34(9):955–961Weinstein J., Lurie J., Olson P., Bronner K., Fisher E.
United States’ trends and regional variations in lumbar spine surgery; 1992-2003.
Spine. 2006;31(23):2707–2714Gray D., Deyo R., Kreuter W.
Population-based trends in volumes and rates of ambulatory lumbar spine surgery.
Spine. 2006;31(17):1957–1963Carey T.S., Evans A., Hadler N., Kalsbeek W., McLaughlin C., Fryer J.
Care seeking among individuals with chronic low back pain.
Spine. 1995;20(3):312–317Triano JJ.
Biomechanics of Spinal Manipulative Therapy
Spine J. 2001 (Mar); 1 (2): 121–130Haldeman S., Chapman-Smith D., Peterson D., editors.
Guidelines for Chiropractic Quality Assurance and Practice Parameters
Aspen Publishers; Gaithersburg, MD: 1993.Triano J.J., McGregor M., Skogsberg D.R.
Use of chiropractic manipulation in lumbar rehabilitation.
J Rehabil Res Dev. 1997;34:394–404Triano J.J.
The mechanics of spinal manipulation.
In: Herzog W., editor. Clinical biomechanics of spinal manipulation.
Churchill Livingstone; Philadelphia: 2000.McGirt M.J., Ambrossi G.L., Datoo G.
Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal.
Neurosurgery. 2009;64(2):338–344. discussion 344–5McMorland G, Suter E, Casha S, du Plessis SJ, Hurlbert RJ.
Manipulation or Microdiskectomy for Sciatica?
A Prospective Randomized Clinical Study
J Manipulative Physiol Ther. 2010 (Oct); 33 (8): 576–584McKenzie R.
The lumbar spine: mechanical diagnosis and therpay.
Spinal Publications; Waikanae, New Zealand: 1981.Lisi A.J.
The centralization phenomenon in chiropractic spinal manipulation of discogenic low back pain and sciatica.
J Manipulative Physiol. 2001;24(9):596–602Gudavalli M., Cox J., Cramer G., Baker J., Patwardhan A.
Intervertebral disc pressure changes during low back treatment procedures.
BED-Advances in Bioengineering. 1998;39:187–188.Long A., Donelson R., Fung T.
Does it matter which exercise? A randomized controlled trial or exercise for low back pain.
Spine. 2004;29(23):2593–2602Chan C., Peng P.
Failed back surgery syndrome.
Pain Med. 2011;12(4):577–606Matsunaga S., Kabayama S., Yamamoto Y., Yone K., Sakou T., Nakanishi K.
Strain on Intervertebral discs after anterior cervical decompression and fusion.
Spine. 1999;24(7):670–675Hillibrand A., Carlson G., Palumbo M., Jones P., Bohlman H.
Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis.
J Bone Joint Surg Am. 1999;81(4):519–528Lord S., Barnsley L., Wallis B., Bogduk N.
Chronic Cervical Zygapophysial Joint Pain After Whiplash: A Placebo–Controlled Prevalence Study
Spine (Phila Pa 1976) 1996 (Aug 1); 21 (15): 1737–1744Laslett M., McDonald B., Aprill C., Tropp H., Oberg B.
Clinical predictors of screening lumbar zygapophyseal joint blocks: development of clinical prediction rules.
Spine J. 2006;6(4):370–379Laslett M., Young S., Aprill C., McDonald B.
Diagnosing painful sacroiliac joints: a validity study of a McKenzie evaluation and sacroiliac provocation test.
Aust J Physiother. 2003;49:89–97
Return to SPINAL PAIN MANAGEMENT
Since 9-12-2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |