

In Vitro Biomechanical Evaluation of Single Impulse
and Repetitive Mechanical Shockwave Devices
Utilized for Spinal Manipulative TherapyThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Annals of Biomedical Engineering 2014 (Dec); 42 (12): 2524–2536 ~ FULL TEXT
OPEN ACCESS Michael A. K. Liebschner, Kwonsoo Chun, Namhoon Kim, And Bruce Ehni
Department of Neurosurgery,
Baylor College of Medicine,
Houston, TX, USA,
Mechanical shockwave therapy devices have been in clinical use for almost 40 years. While most often used to treat back pain, our understanding of their biomechanical performance is very limited. From biomechanical studies we know that biological tissue is viscoelastic and preferably excited around its resonance frequency. Targeting these frequencies has been the focus in extracorporeal shock wave lithotripsy, but these concepts are relatively new in orthopedic and rehabilitation therapies. The exact mechanism by which shockwave therapy acts is not known.
Knowledge of the performance characteristics of these devices, correlated with clinical outcome studies, may lead to better patient selection, improvement of device functionality, and knowledge of the underlying working principals of therapy. The objectives of this study were to determine the ability of several commercial shockwave devices to achieve a desired thrust profile in a benchtop setting, determine the thrust profile in a clinical analog, and determine the influence of operator experience level on device performance.
We conducted two different types of testing: (1) bench testing to evaluate the devices themselves, and (2) clinical equivalent testing to determine the influence of the operator. The results indicated a significant dependence of thrust output on the compliance of the test media.
The Activator V-E device matched the ideal half-sine thrust profile to 94%, followed by the Impulse device (84%), the Activator IV/FS (74%), and the Activator II (48%). While most devices deviated from the ideal profile on the return path, the Impulse device exhibited a secondary peak. Moreover, the Activator V-E device provided evidence that the device performs consistently despite operator experience level. This has been a major concern in manual spinal manipulation. Based on our results, a hyper-flexible spine would receive a lower peak thrust force than a hypo-flexible spine at the same power setting.
Furthermore, a hand-held operation further reduced the peak thrust force as it increased the system compliance. However, that influence was dissimilar for the different devices. Although controlled clinical trials are needed to determine the correlation between thrust profile and clinical outcome, already ongoing clinical studies indicate an improved patient satisfaction due to reduced treatment pain when devices are used with a thrust characteristic closer to an ideal sine wave.
From the FULL TEXT Article:
INTRODUCTION
Mechanical shockwave therapy is widely used in orthopedics, rehabilitation medicine, and chiropractic practice. [3, 16, 18–20, 25, 33, 36, 38, 42] One of its widest known medical applications is lithotripsy, the destruction of kidney stones, and extracorporeal shockwave therapy for the treatment of multiple tendonopathies. Lowenergy shockwave devices, such as chiropractic instruments, have been around far longer and are generally utilized for instrumented spinal manipulation (SM). [12, 16]
A shockwave differs from an acoustic wave in that an acoustic wave generally consists of periodic oscillation whereas a shockwave is a single pulse. [11, 43] The shockwave is a mechanical pressure pulse that expands as a half sine wave within the human body. Its propagation capabilities and tissue penetration depth depends on the energy of the shockwave but also on the tissue damping effect. [17] Viscoelastic damping of the shockwave is minimized at or around the natural frequency of the tissue. [24] For the human spine, that resonance frequency is around 30–50 Hz, and is minimally influenced by pathology. [8, 15, 21, 23] It is therefore conceivable that high transmissibility is achieved at tissue resonance while at the same time reducing the energy requirement of the shockwave generator and diminishing side effects caused by the overstimulation of surrounding tissue. [6, 23, 35]
Although many studies have been conducted to assess the efficacy of shockwave therapy, the primary effect by which shockwave therapy acts to treat pathology is unknown. [2, 4, 18, 34] For extracorporeal shockwave therapy, the leading hypothesis is based on the inflammatory healing response. It is believed that the shockwave causes microtrauma to the affected tissue. This may results in inflammation, which allows the body to repair the affected site and increase the blood flow. [13, 41] For spinal mechanical shockwave therapy, the shockwaves are believed to trigger a gate response (Gate Theory) at or near the dorsal root ganglia (DRG) for pain modulation [28, 30, 36, 37, 39] and stimulate mechanoreceptors, which in turn trigger other body responses. [10]
Instrumented SM has to a large extent captured the field of spinal manipulative therapy. [26, 29] Instead of manually maneuvering a person’s body, these high velocity, low amplitude (HVLA) mechanical shockwave therapy devices are placed at the anatomic site of interest and triggered. These chiropractic instruments deliver a force–time profile lower in amplitude, shorter in duration and with a faster force rate compared with a manually applied HVLA-SM. [35] Nevertheless, there have been no performance standards promulgated by the FDA for these types of manipulation devices. Furthermore, previously published performance characteristics of such devices were conducted on highly idealized test structures consisting of a rectangular steel beam with a static bending stiffness similar to that of the human thoracolumbar spine. [22] During these tests, the dynamic thrust was applied at the mid length of the beam, perpendicular to its long axis. A force transducer measured the magnitude of the thrust while the signal of an accelerometer was used to calculate the beam deflection. A significant limitation was that due to the setup the transducers had to be attached to the device rather than to the beam. This setup drastically alters the dynamics of the system and limits its usage in predicting the thrust magnitude and duration that patients are experiencing during therapy. Specifically, the force profile of dynamic load cells is drastically obscured through dynamic movement of the load cell itself.
Although the error can be approximated if the acceleration of the load cell is known, it still is just an approximation. Furthermore, this test did not allow a bench test approach to eliminate the operator variability as the experiment had to be executed by hand. A follow-up publication six years later compared two devices [Activator II and the Harrison Adjusting Instrument (HAI)] in a shuttlecock experiment and four devices (Activator II & IV; HAI, Impulse) in a standard bench-type force calibration test. [7] The authors noted the lack of a linear correlation between bench-test parameters and shuttlecock experiment results. The deviation was contributed to drag on the shuttlecock during flight and experimental alignment issues. During the bench test, the operator pressed the devices directly against a load cell and executed a device thrust. This setup does include the compliance of operator as variable within the thrust line of action. Furthermore, the authors noted that a difference in stiffness response can be expected when this device is tested on patients; however, they were not able to simulate the stiffness of the human spine.
With the device development history in mind and existing limitations in properly determined device performance, the objectives of our studies were to:(1) determine the ability of several currently available SMT mechanical shockwave devices to achieve a desired thrust profile;
(2) determine the shockwave profile of the devices in a clinical analog setting; and
(3) determine the influence of device operator experience level on device performance.
MATERIALS AND METHODS
We investigated a combination of four different mechanical shockwave therapy devices used mainly for chiropractic SM, two experimental settings, and four operators to evaluate the performance characteristic of the devices. Outcome variables obtained from each set of experiments were statistically analyzed for significance.
Instrumentation

Table 1

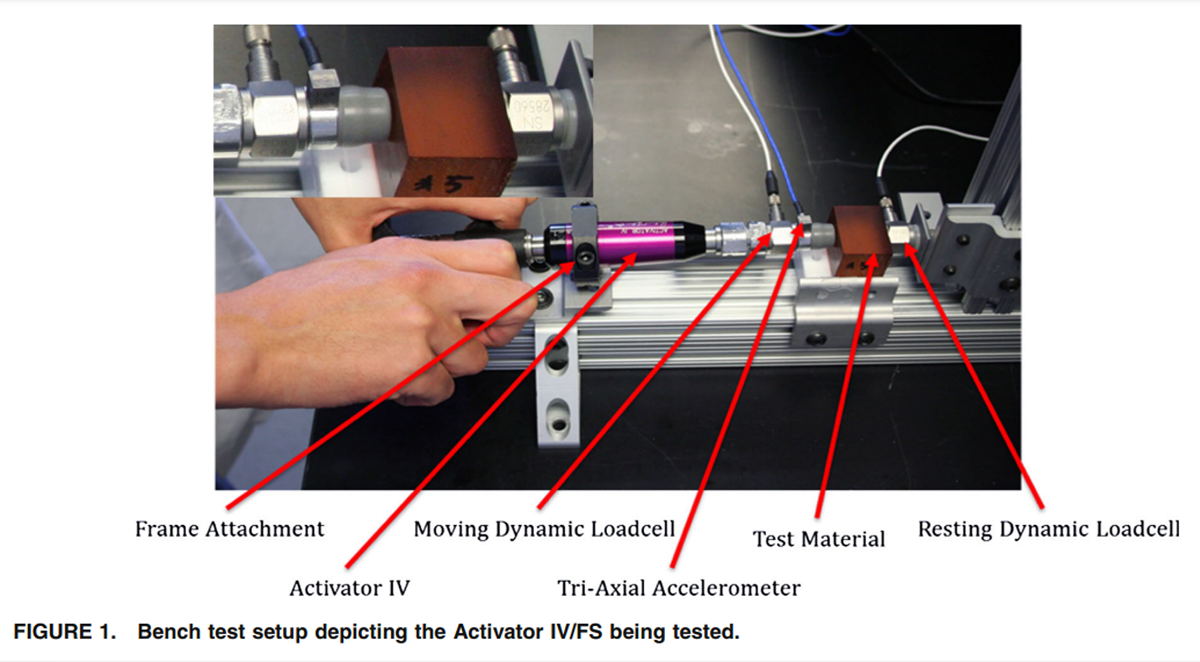
Figure 1 To achieve our objectives, we obtained four different mechanical shockwave devices; two devices were manually operated (spring loaded hammer): Activator II & Activator IV/FS (Activator Methods Int. Ltd., Phoenix, AZ) while the two other devices were electrically powered (electromagnetic solenoid): Impulse (Neuromechanical Innovations LLC, Chandler, AZ), and Activator V-E (Activator Methods Int. Ltd., Phoenix, AZ). The provided manufacturer’s specifications are listed in Table 1.
All devices were tested in a standardized fashion: one component of the device housing was affixed to the testing frame through a machined screw-on collar. The collar prevented a relative motion of the device with respect to the test frame. The rubber cap (TRP60) of the devices was removed and an impedance head attached instead. The rubber cap was then placed on the front of the impedance head. The impedance head included a dynamic load cell (Model 208C04; PCB, NY) and a triaxial accelerometer (Model 356A01; PCB, NY). See Figure 1 for details on the setup on one of the devices.
In front of the device were homogeneous polymer blocks (tissue analogs) and a second dynamic load cell. The polymer blocks were affixed to the load cell, which was rigidly mounted to the frame. The polymer blocks represented ranges of human tissue compliance values that might be seen in the clinic, plus additional extreme cases (see ‘‘Spinal Tissue Analog’’ section). This method is loosely based on vascular tissue modeling [9] and represents a significant improvement from previously highly idealized beam structures. [22]
During device testing, the mechanical shock wave propagates from the release mechanism through the impedance head, the rubber cap, the polymer blocks to the front plate of the resting dynamic load cell (see Fig. 1). The most compliant component within that line of action was the rubber cap, which was the commercial rubber cap used by Activator Methods Inc. on their devices. Our rationale for retaining the rubber cap was to keep the testing setup as close to the actual device application as possible.
The Activator IV/FS, Activator V-E and the Impulse device were pre-loaded based on the manufacturer’s recommendation. The Activator IV/FS and the Activator V-E required the tip to be completely retracted for pre-load while the Impulse device provided an indicator light to suggest when a pre-load of approximately 20 N was achieved. For the Activator II device, a pre-set gap distance between the device tip and the tissue analog was determined for each thrust magnitude setting and the device locked in that position. Because of the functionality of the Activator II device, a pre-load force per se could not be applied.

Table 2 After pre-loading, the Activator IV/FS and the Activator V-E devices were set to one of their four thrust settings. The four possible settings were selected in random fashion in order to eliminate systematic errors. The same procedure was repeated for the three possible settings of the Impulse device. For the Activator II device, a fraction of the full scale range was selected to represent intermediate values (see Table 2 for details).
The test run for each combination of device thrust magnitude and human tissue analog compliance was repeated ten times. For two of the devices, we used three different thrust magnitude settings (Activator II and Impulse), for the other two devices we used four different thrust settings (Activator IV/FS and Activator V-E). The repetitions were performed at a rate of approximately one per minute. This testing rate prevented overheating of the electromagnetic devices and heat dissipation, which may have an effect on the device performance. The electronic signals obtained from the force transducer and accelerometer were recorded through a data acquisition system at a rate of 12,800 samples per second per channel and stored in a binary file format on a PC using LabView (National Instruments, Austin, TX). Although slower sampling rates have been suggested as being sufficient, [7, 14] we were also collecting data for the plunger acceleration, which typically have a faster rise time than the load profile. Using a Matlab script, the binary data were converted into an ASCI format and graphed in the time domain. All transducers and data acquisition devices were within their calibration interval.
Spinal Tissue Analog
To simulate spinal kinematics when subjected to a mechanical shockwave, we developed a test setup that would mimic human physiology. We utilized tissue analogs that span the reported range of human spinal flexibility; including extreme hyper-flexible and hypoflexible spinal biomechanics. [27, 40] The biological tissue was approximated with a tissue analog that is built from standardized homogeneous polymer blocks. An indentation test was conducted on a material testing frame ElectroForce 3200 (Bose Corp., Eden Prairie, MS) to relate tabulated hardness values (Shore A) to indentation stiffness. The experiment was conducted in quasi-static loading conditions (>10 s per loading cycle) at room temperature. Eight intermediate positions during the indentation process were recorded. A mathematical best-fit regression line through the collected datasets was used to determine the indentation rigidity of the polymer blocks in units of N/mm. The choice of polymer blocks was made based on the compliance of the human spine measured in a patient trial. [5] Polymer blocks with stiffness values ranging from 30.22 to 258.07 N/mm were selected, spanning spinal flexibility of hyper-flexible to hypo-flexible patients. Accounting for the two extreme tissue analog cases, we conducted 80 experiments for the Activator IV/FS and Activator V-E devices, and 60 experiments for the Activator II and the Impulse devices in the fixed frame setup.
Clinical Equivalent Conditions

Figure 2 In order to compare if bench testing is able to replicate a clinical scenario, an additional test series with hand-held operations of one operator was performed. This test series was utilized to determine the maximum velocity of the plunger during operation in a clinical equivalent setup. Only the Activator V-E device and the Impulse device were tested in this configuration (Figure 2). The rationale for this selection was the similar internal working mechanism to generate a thrust and similar hand position during execution of a thrust. The influence of a hand-held device operation was investigated for the equivalent of a highly compliant human spine and a very stiff human spine and all possible device settings, with 10 repetitions each. Overall, 80 experiments were conducted with the Activator V-E and 60 experiments with the Impulse device for the clinical equivalent setup. The influence of the operator’s arm stiffness, as manifested in operator experience level, was investigated in the subsequent phase.
Experience Level of Device Operator
Acknowledging that experience level of the device operator may play a role in overall thrust output, we recruited two highly experience device operators and two novices. The two experienced operators had a combined working experience with mechanical shockwave therapy devices of more than 50 years while the novices received a brief introduction on proper handling of the device. All four operators followed manufacturer’s recommendations when operating the device. Only the Activator V-E device was selected for this part of the study as it required the least amount of training for the novices to operate. This was justified since the goal was to evaluate the device performance in the hands of a novice and not the learning curve that it would take to operate the device. The experimental setup followed the clinical equivalent conditions mentioned above, however, with only one tissue analog (equivalent to average spinal compliance) and two device settings (lowest and highest).
Shockwave Output Profile
As the treatment effectiveness depends significantly on the mechanical shockwave to propagate into the body, it is desirable for the shockwave to come as close to a half sine wave as possible. [6, 23, 35] Human tissue is considered to be a viscoelastic material with an eigenfrequency between 30 and 50 Hz at the spinal column. [8, 15, 21, 23] Vibration damping can be minimized if the shockwave is a pure sine wave at or near the eigenfrequency. [24] We therefore characterized the shockwave profile in terms of its crest factor and shape approximation of a half sine wave, with the deviation expressed in percent. Shape approximation was calculated as the ratio from a best fit area under the curve of a half sine wave when adjusted for pulse width and amplitude vs. the area under the curve for an ideal sine wave with the same parameters.
Several additional parameters were extracted and calculated from the recorded thrust output profiles of the four difference devices. Mainly, the peak thrust force in Newton, the peak thrust acceleration in m/s2 , the thrust duration or pulse width in Milliseconds, the plunger displacement in Millimeters. While force, acceleration, and time were directly recorded, plunger displacement was calculated through double time integration of the accelerometer signal. The data were tabulated and the mean and standard deviation calculated for each series (N = 10). This process was repeated for each device and setting.
Statistical Methods
Sample size of 10 repetitions per test was selected based on a previously measured variance of 5.86 N for a 90% detectability (Type II error) and a 0.05 level of significance (Type I error) to predict a minimal difference of 6.51 N. This value is well within the uncertainty of the experimental setup and below 3% of the expected maximum thrust (see Table 1 for details).
Due to the similar profiles of the four device types, a fixed-effects statistical model comparison was performed. Major focus was placed on statistical comparison of the peak output force (Newton), the force pulse duration (ms), the plunger displacement during thrust execution (mm) and the thrust velocity (m/s). Since the similar power settings were utilized for all devices, a multi-factorial analysis of variance (ANOVA) for device type, pulse width, plunger travel and thrust velocity was performed on the mean values of those parameters for all devices. Paired two-tailed T tests were conducted on the main effects and interactions between devices and parameters. 95% confidence interval was used for all analyses, with a type one error of 5%. Type II error was assumed to be 0.1.
RESULTS
Force Magnitude Range

Figure 3
page 6All four tested devices were substantially equivalent in their thrust force output. Due to its four different settings, the Activator V-E was able to span the largest variable range of thrust values. The device with the least range was the Activator IV/FS. Although the Activator II has an infinite number of adjustment capabilities between its maximum thrust and zero, only three settings were evaluated. The Impulse device achieved a range of thrust values between the Activator IV/FS and the Activator V-E devices. The overall thrust force comparison is depicted in Figure 3 for all devices tested against the stiff 258.07 N/mm polymer block (M#4) and the compliant 30.22 N/mm polymer block (M#1).
Influence of Spinal Flexibility
All four devices showed a progressive increase in generated peak force with increased power setting. Furthermore, all four devices showed a reduced capability to generate a consistent thrust force when the tissue analog compliance increased. This is depicted in Fig. 3, comparing the peak thrust against a hypo-flexible spine (Material M#4) and a hyper-flexible spine (Material M#1). In principle, the softer the tissue analog, the lower the force output of the devices. As the devices have a limited travel distance of their tip against the tissue analog during a thrust, a softer tissue analog needs to be deformed more to result in the same resistive force as a stiffer tissue analog.
Overall, the maximum thrust peak force for all commercially available devices is below the values published by the manufacturers (Table 1). The Impulse device, even though rated at a maximum force of 255 Newton, was only able to generate a peak force of around 130 Newton with the setup utilized. The Activator IV/FS device was comparable to the Impulse device and achieved a maximum force output of 109 Newton. The maximum thrust measured for the Activator II devices was 165 Newton. In comparison, the maximum value measured for the new Activator V-E was measured around 189 Newton for the stiff tissue analog (see Fig. 3 for comparison).
Shockwave Output Profile

Table 3
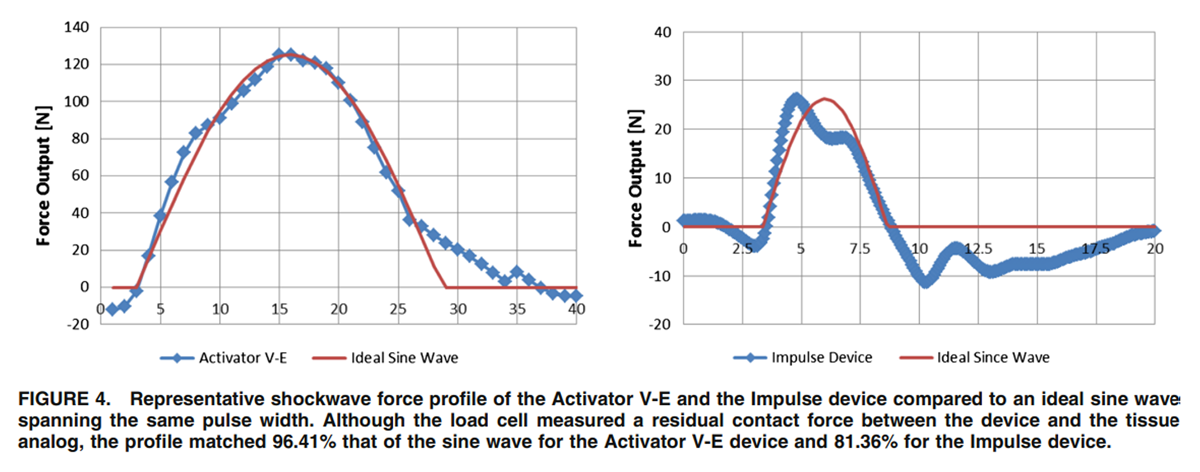
Figure 4 The shockwave profile differed significantly between devices and power settings. In general, the pulse width increased with increased compliance of the material and higher power settings. For most devices, the pulse width was between 3 and 7 ms. The exception was the Activator II, which had a pulse width of around 12 ms (Table 3). Considering this pulse width as part of a half sine wave, the driving frequency of the Activator II device was around 43 Hz, while for the remaining devices had a driving frequency between 72 and 126 Hz. The driving frequency for the Activator V device was 89 Hz, for the Activator IV 93 Hz, and for the Impulse 126 Hz.
The approximation of a half sine wave with the thrust curves was less consistent with the spring-loaded devices (Activator II and IV/FS) compared to the more programmable electromagnetically powered devices (Activator V-E and Impulse). On average, the Activator II device captures 48% (±6.1%) of the half sine wave profile, the Activator IV/FS 74% (±8.3%), the Impulse 83% (±3.9%), and the Activator V-E 94% (±3.5%). The sine wave approximation was consistent for each device across settings and tissue compliance. Two representative graphs are depicted in Figure 4. Note that these representative graphs show the two devices at different settings. Shortcomings of the sine wave approximations were mainly that secondary peaks that followed the primary peak or a delayed return to a minimum force threshold after the initial peak was reached were not captured. Therefore, the quality of the signal was not captured but rather the overall shape approximation. This finding, however, was also reflected in the Crest factor, which was 1.13 ± 0.21 for the Activator II device, 1.28 ± 0.16 for the Impulse device, 1.32 ± 0.18 for the Activator IV/FS device, and 1.43 ± 0.16 for the Activator V-E device. A Crest factor of 1.4142 indicates a perfect since wave, a factor above that value indicates a shape that is too pointy while a value below indicates a shape that is too wide.
Similar to pulse duration, the measured thrust velocity (maximum velocity of the plunger during the force generation phase) was less dependent on the compliance of the tissue analog than on the device power setting. This dependency was evident for all four tested devices (Table 3). The more compliant tissue analog (#M1) required a larger deformation to generate the measured output force compared to the more stiff tissue analog (#M4). Since the pulse width is reasonably constant, a higher velocity is needed to deform a softer material compared to a stiffer one.
Clinical Equivalent Conditions

Figure 5
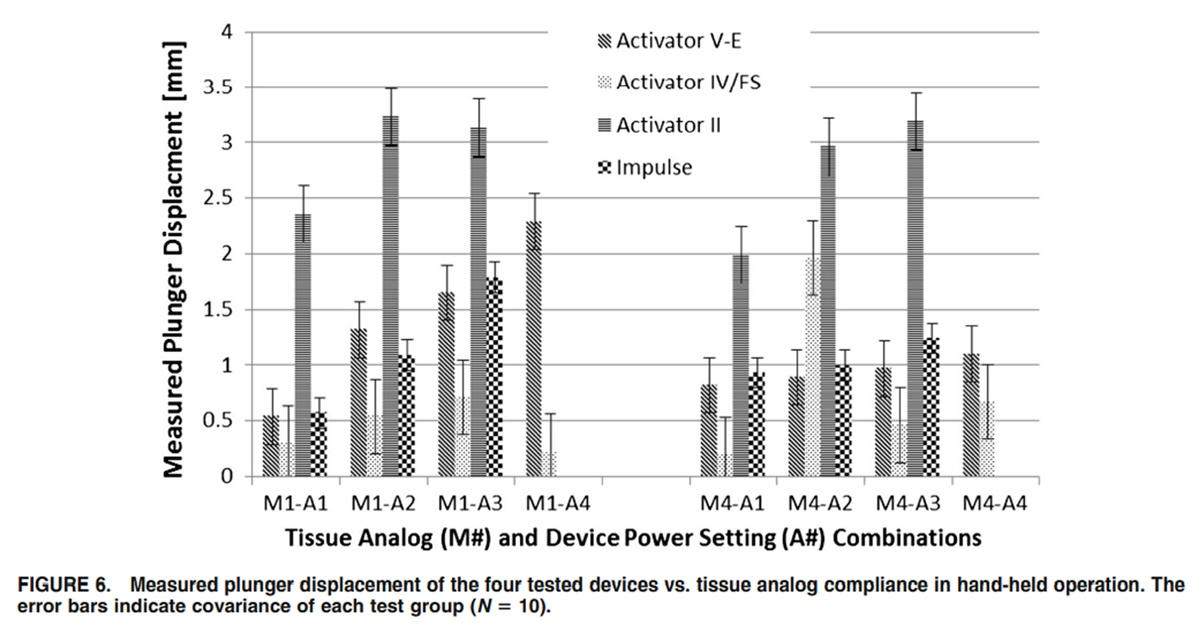
Figure 6 In general, the measured peak output force was reduced in hand-held operation compared to the fixedframe test setup. This is expected as the bench test features a rigid setup while the hand-held operation of the devices takes into account the compliance of the wrist and arm of the operator. Even though both devices utilize a solenoid to generate the mechanical shockwave, the Activator V-E device depicted no significant reduction in peak output force (p = 0.07) switching from bench test to hand-held operation. In contrast, the increase in system compliance in switching from bench test to hand-held operation caused a statistically significant drop in peak output force (p <0.01) for the Impulse device. Statistical probability was assessed with one-tailed paired T test for mean with a 95% confidence interval. No consistent difference between hand-held operation and fixed frame operation was detectable for the Activator V-E device (Figure 5). Furthermore, the standard error increased during hand-held operation for all devices. Interestingly enough, the pulse width was less sensitive to the compliance of the tissue analog than to the power settings. A higher power setting required a longer pulse width to complete the thrust.
Plunger displacement varied proportional with power settings for the stiff material (#M1) but less so for the softer material (#M4). The exception was the Activator II device, which showed a strong correlation (COE = 0.22) between power setting and plunger travel for both tissue analogs (Figure 6). The lowest correlation coefficient was found for the Activator V-E device (COE = 0.02).
Experience Level of Device Operator
To determine the role of operator experience on device output, we applied a two-factor ANOVA with repeated measures on the peak force value with operator experience level (expert vs. novice) and device setting (highest or lowest) as fixed effects. While device power setting had a significant influence on the shockwave amplitude (p <0.001), operator experience did not (p = 0.48). Individual F-tests for two samples for means confirmed the overall findings with probability values above 0.5. At the high power setting, the mean thrust output of the experienced operators was only 1.2% higher than the thrust output of the novices. That difference was reversed for the low power setting. Nevertheless, since the difference was well within the measurement error it can be neglected.
Statistical Analysis
For overall statistical comparison, we applied multifactorial ANOVA tests with repeated measures for the peak output force, the measured force pulse width, plunger velocity and plunger travel distance. The results indicated NO significant difference between devices (p = 0.64). A subsequent two-tailed paired T test for two samples for means was performed to statistically determine which combination of devices and settings differed statistically significant from each other. We included all 6 possible combination of paired analysis between the four tested devices for peak force, pulse width, plunger velocity and plunger travel.

Table 4 Limited two-factor ANOVA were statistically significant for devices (p<0.05) due to differences in peak force values for the different power settings. More specifically, paired T test analysis revealed differences in peak thrust values between the Impulse device and the Activator V-E (p = 0.004) and Activator IV/FS devices (p = 0.014), and significant differences between the Activator II and Activator IV/FS devices (p = 0.035). All other combinations were not statistically significant (see Table 4 for details).
The two-factor ANOVA for devices and pulse width revealed a statistically significant difference for both factors, p <0.001. The individual T tests showed significant difference for all device combinations, except for between the Activator IV/FS and Activator V-E devices (p = 0.804).
The two-factor ANOVA for devices and plunger velocity indicated a statistically significant difference between devices but not for power settings, p = 0.002 and p = 0.859 respectively. Individual T tests indicated statistically significant differences for the Activator II device to all other devices but no other device combination.
The two-factor ANOVA for devices and plunger travel distance indicated a statistically significant difference between devices but not between power settings. Individual T tests indicated statistically significant differences for the Activator II device compared to all other devices (p <0.001). No other device combination was significant.
Even though all devices operate in a similar force range and pulse width, the narrow standard deviation and small coefficient of variance (Figs. 3, 4, 5, 6; Table 3) resulted in a statistically significant difference between devices.
DISCUSSION
Our testing protocol focused on the in vitro comparison of mechanical shockwave therapy devices currently utilized for SM. Emphasis was placed on evaluating the shockwave profile in a standard bench test setting as well as in a clinical equivalent setting. The main investigated parameters were peak thrust force and pulse width of the force profile. The combination of force and time provides an approximation of the total impulse output generated by the devices. To further quantify the shape of the thrust curve, we evaluated the Crest factor and a percent approximation of an idealized half sine wave.
Since all four devices are hand-held instruments, the compliance of the operator’s upper extremities in addition to the device output have to be taken into account when predicting the clinical performance of the device. Although our results indicated that operator experience level does not play a significant role in the magnitude of the shockwave, the measured peak output force values in the bench setup (fixed frame) were slightly higher than the values measured for a hand-held operation (Fig. 5). Only one operator was used in this test protocol. Additional operators will increase the variance of the output forces. However, that increase is expected to be minor.
Although statistical differences between devices were found, these merely reflect difference found in the spread of the thrust magnitudes and plunger displacement (Activator II). The thrust pulse width was significantly the longest for the Activator II device and the shortest for the Impulse device, with the remaining two devices filling the gap. The conducted experiments were highly repeatable with an average coefficient of variance of less than 5%.
Although environmental conditions may influence the performance of the evaluated devices, temperature and humidity were not recorded as all devices were exposed to the same conditions. Since the objective was to provide a comparison of the biomechanical performance of the devices, the influence of the environmental conditions were considered as part of a systematic error in obtaining the measurements. In contrast, warm-up of the electromechanical devices was considered a significant source of error. Therefore, the devices were test fired several times before the actual recordings took place.
The compliance of the human tissue analog played a significant role in the generated peak force of each device. In principle, a softer tissue resulted in lower peak forces and vice versa. Using tissue analogs instead of a steel frame resulted in all devices generating substantially lower peak forces than previously published by the manufacturer and in the literature. [22] Furthermore, hand-held operation reduced the generated peak thrust force even more, although that difference varies between devices. While the maximum peak force measured for the Activator V-E device was less dependent on the test setup (reduction of 16% from fixed frame to hand-held), the Impulse device showed a reduction of 42% (Fig. 5).
The limitations of our study include the lack of information on ideal target parameters that would yield an optimal clinical outcome. Although all four investigated devices received regulatory approval, it is not clear what device characteristic will yield better clinical outcome than others. Based on the clinical objectives of the devices, as eluded to in "Introduction" section, the profile of the shockwave may play an important role for the propagation of the shockwave into the human tissue. Future studies should include in vivo or cadaveric studies determining what portion of the shockwave reaches the target area and if there is an optimal wave form.
CONCLUSION
The primary objective of this study was to create a baseline comparison of multiple commercially available devices currently utilized in mechanical shockwave therapy for SM. The devices we investigated were the Activator II device, the Full Spectrum Activator IV/FS device, the Activator V-E device (all from Activator Methods Int., Ltd.) and the Impulse device (Neuromechanical Innovations Inc., USA). The Activator V-E device depicted the largest range of thrust magnitude values compared to any of the other devices. The Activator II was able to generate the highest peak force while the Impulse device generated the lowest peak force of all tested devices. The peak forces generated by the Activator V-E devices were within ±15% of the other devices, with no difference found in pulse width, with the exception of the Activator II device, which had the longest pulse width. All four devices showed the same dependency of the generated peak thrust with the compliance of the tissue analogs. In general, a softer tissue analog (simulating a highly flexible human spine) resulted in lower thrust magnitudes and a harder tissue analog (simulating a very stiff human spine) resulted in a higher thrust magnitude for the same device setting. Although this behavior was expected, this is the first study to quantify that effect. Furthermore, the compliance of the tissue analogs resulted in lower peak thrust magnitudes then previously observed when the devices were tested against a rigid surface. [22] In addition, the maximum peak output thrust was further reduced when the test configuration was changed from a fixed frame setup (generally used for quality control) to hand-held operation (equivalent to clinical utilization) for all devices. The highly repeatable experimental setup resulted in statistically significant differences between the biomechanical behaviors of several devices, even though their overall behavior is substantially equivalent.
The secondary objective of this study was to develop a testing setup that closely simulates the compliance of the human spine while eliminating user dependency at the same time for the evaluation of mechanical shockwave therapy devices. We developed a setup where the devices were rigidly attached to a frame. This step eliminated the user dependency of the generated thrust magnitude. Furthermore, we simulated the flexibility of the human spine through tissue analogs. These tissue analogs were homogeneous polymer blocks of different compression stiffness. The softest polymer block had a stiffness of 30 N/mm, which was considered below values measured for the human spine. [5] The second polymer block had a stiffness of 258 N/mm, which was within the range of values measured for the human spine. The third testing condition did not include a tissue analog but rather had the devices in direct contact with the load cell. This test condition replicated the previous experimental condition published by Colloca et al. [7] compared to the results with the tissue analogs, placing the devices in direct contact with the load-cell seem to overestimate the peak thrust forces a patient would experience. In contrast to previous testing methods, the load cell was placed behind the tissue analogs in order to measure the transmitted force of the devices through the tissue. As in previous studies, the accelerometer was attached to the device tip. This novel testing setup was able to establish a device dependency on tissue compliance, a phenomenon not previously identified during device evaluation. [22] Additionally, by removing the variability of the user operating the device, the coefficient of variance was measured to be around 5% of all experiments combined. This value is close to half of what has been reported previously for a single user. [22, 31]
As complementary and alternative medicine will be more and more integrated into mainstream medicine, it will provide a tremendous opportunity for the field of mechanical shockwave therapy for better patient selection, evidence based treatment planning, and further optimization of the devices. The currently available devices significantly improved with each version and are approaching close to 100% of the ideal waveform. Based on our current knowledge, an ideal waveform is a half sine wave of a frequency specific to the target tissue. The more pure the signal the less side effects can be expected. The transition from purely mechanical to electromechanical devices is already a significant step in making the devices highly versatile.
ACKNOWLEDGMENTS We would like to acknowledge Activator Methods International LLC for providing us with the test instruments.
CONFLICT OF INTEREST
The authors have no financial conflict related to any aspect of this study
References:
Activator Methods International, Ltd.,
Phoenix, AZ, 2013.Anderson, R., et al.
A meta-analysis of clinical trials of spinal manipulation.
J. Manipulative Physiol. Ther. 15(3):181–194, 1992.Chow, D. H., et al.
Extracorporeal shockwave therapy for treatment of delayed
tendon-bone insertion healing in a rabbit model:
a dose-response study.
Am. J. Sports Med. 40(12):2862–2871, 2012.Colloca, C. J., and T. S. Keller.
Electromyographic reflex responses to mechanical force,
manually assisted spinal manipulative therapy.
Spine (Phila Pa 1976) 26(10):1117–1124, 2001.Colloca, C. J., and T. S. Keller.
Stiffness and neuromuscular reflex response of the human spine to
posteroanterior manipulative thrusts in patients with low back pain.
J. Manipulative Physiol. Ther. 24(8):489–500, 2001.Colloca CJ, Keller TS, Gunzburg R.
Neuromechanical Characterization Of In Vivo Lumbar Spinal Manipulation.
Part II. Neurophysiological Response
J Manipulative Physiol Ther. 2003 (Nov); 26 (9): 579–591Colloca CJ, Keller TS, Black P, Normand MC, Harrison DE, Harrison DD:
Comparison of Mechanical Force of Manually Assisted
Chiropractic Adjusting Instruments
J Manipulative Physiol Ther 2005 (Jul); 28 (6): 414–422
Colloca, C. J., et al.
Intervertebral disc degeneration reduces vertebral motion responses.
Spine (Phila Pa 1976) 32(19):E544–E550, 2007.Corbett, T. J., et al.
Engineering silicone rubbers for in vitro studies:
creating AAA models and ILT analogues
with physiological properties.
J. Biomech. Eng. 132(1):011008, 2010.Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ.
Changes in Pain Sensitivity Following Spinal Manipulation:
A Systematic Review and Meta-analysis
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 752–767Delius, M., et al.
Biological effects of shock waves: in vivo effect of high energy pulses on rabbit bone.
Ultrasound Med. Biol. 21(9):1219–1225, 1995.Fuhr, A. W., and D. B. Smith.
Accuracy of piezoelectric accelerometers measuring
displacement of a spinal adjusting instrument.
J. Manipulative Physiol. Ther. 9(1):15–21, 1986.Gruenwald, I., et al.
Shockwave treatment of erectile dysfunction.
Ther. Adv. Urol. 5(2):95–99, 2013.Gudavalli, M. R., et al.
Effect of sampling rates on the quantification of forces, durations,
and rates of loading of simulated side posture high-velocity,
low-amplitude lumbar spine manipulation.
J. Manipulative Physiol. Ther. 36(5):261–266, 2013.Guzik, D. C., et al.
A biomechanical model of the lumbar spine during upright
isometric flexion, extension, and lateral bending.
Spine (Phila Pa 1976) 21(4):427–433, 1996.Haas, M., et al.
Muscle testing response to provocative vertebral challenge and
spinal manipulation: a randomized controlled trial
of construct validity.
J. Manipulative Physiol. Ther. 17(3):141–148, 1994.Hatiboglu, G., et al.
Prognostic variables for shockwave lithotripsy (SWL) treatment success:
no impact of body mass index (BMI) using a third generation lithotripter.
BJU Int. 108(7):1192–1197, 2011.Hsu, R. W., et al.
Enhancing mechanical strength during early fracture healing
via shockwave treatment: an animal study.
Clin. Biomech. (Bristol, Avon) 18(6):33–39, 2003.Huang, C., et al.
Mechanotherapy: revisiting physical therapy and
recruiting mechanobiology for a new era in medicine.
Trends Mol. Med. 19(10):586–593, 2013.Keller TS, Colloca CJ:
Mechanical Force Spinal Manipulation Increases Trunk Muscle Strength
Assessed By Electromyography: A Comparative Clinical Trial
J Manipulative Physiol Ther. 2000 (Nov); 23 (9): 585–595Keller, T. S., and C. J. Colloca.
A rigid body model of the dynamic posteroanterior
motion response of the human lumbar spine.
J. Manipulative Physiol. Ther. 25(8):485–496, 2002.Keller, T. S., C. J. Colloca, and A. W. Fuhr.
Validation of the force and frequency characteristics of the
activator adjusting instrument: effectiveness as a
mechanical impedance measurement tool.
J. Manipulative Physiol. Ther. 22(2):75–86, 1999.Keller, T. S., C. J. Colloca, and A. W. Fuhr.
In vivo transient vibration assessment of the normal human thoracolumbar spine.
J. Manipulative Physiol. Ther. 23(8):521–530, 2000.Keller ST, Colloca CJ, Moore RJ, Gunzburg R, Harrison DE, Harrison DD.
Three-dimensional Vertebral Motions Produced by
Mechanical Force Spinal Manipulation
Journal of Manipulative and Physiological Therapeutics 2006 (Jul); 29 (6): 425–436Konczak, C. R.
Ulnar nerve neuropraxia after extracorporeal shock wave lithotripsy:
a case report.
J. Can. Chiropr. Assoc. 49(1):40–45, 2005.Lawrence, DJ and Meeker, WC.
Chiropractic and CAM Utilization: A Descriptive Review
Chiropractic & Osteopathy 2007 (Jan 22); 15: 2Lee, S. W., et al.
Development and validation of a new technique for assessing lumbar spine motion.
Spine (Phila Pa 1976) 27(8):E215–E220, 2002.Linderoth, B., and R. D. Foreman.
Physiology of spinal cord stimulation: review and update.
Neuromodulation 2(3):150–164, 1999.Meeker W, Haldeman S.
Chiropractic: A Profession at the Crossroads
of Mainstream and Alternative Medicine
Annals of Internal Medicine 2002 (Feb 5); 136 (3): 216–227Meyerson, B. A., and B. Linderoth.
Mechanisms of spinal cord stimulation in neuropathic pain.
Neurol. Res. 22(3):285–292, 2000.Nathan, M., and T. S. Keller. Measurement and analysis of the in vivo posteroanterior
impulse response of the human thoracolumbar spine:
a feasibility study.
J. Manipulative Physiol. Ther. 17(7):431–441, 1994.Neuromechanical Innovations, L.
http://www.neuromechanical.com/
2013.Notarnicola, A., and B. Moretti.
The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue.
Muscles Ligaments Tendons J. 2(1):33–37, 2012.Pickar, J. G., and Y. M. Kang.
Paraspinal muscle spindle responses to the duration of a
spinal manipulation under force control.
J. Manipulative Physiol. Ther. 29(1):22–31, 2006.Rodola, F., et al.
Anaesthesia for shock wave therapy in musculoskeletal disorders:
a preliminary report.
Eur. Rev. Med. Pharmacol. Sci. 6(6):133–138, 2002.Song, XJ, Gan, Q, Cao, J-L, Wang, Z-B, and Rupert, RL.
Spinal Manipulation Reduces Pain and Hyperalgesia After
Lumbar Intervertebral Foramen Inflammation in the Rat
J Manipulative Physiol Ther. 2006 (Jan); 29 (1): 5–13Stojanovic, M. P.
Stimulation methods for neuropathic pain control.
Curr. Pain Headache Rep. 5(2):130–137, 2001.Torrance, D. A., and C. Degraauw.
Treatment of posttraumatic myositis ossificans of the
anterior thigh with extracorporeal shock wave therapy.
J. Can. Chiropr. Assoc. 55(4):240–246, 2011.Waxman, S. G., et al.
Voltage-gated sodium channels and the molecular pathogenesis of pain: a review.
J. Rehabil. Res. Dev. 37(5):517–528, 2000.Wong, K. W., et al.
The flexion-extension profile of lumbar spine in 100 healthy volunteers.
Spine (Phila Pa 1976) 29(15):1636–1641, 2004.Yan, X., et al.
Improvement of blood flow, expression of nitric oxide, and vascular
endothelial growth factor by lowenergy shockwave therapy
in random-pattern skin flap model.
Ann. Plast. Surg. 61(6):646–653, 2008.Yoo, S. D., et al.
Effects of extracorporeal shockwave therapy on nanostructural and
biomechanical responses in the collagenase-induced
Achilles tendinitis animal model.
Lasers Med. Sci. 27(6):1195–1204, 2012.Zhong, P., and G. M. Preminger.
Mechanisms of differing stone fragility in
extracorporeal shockwave lithotripsy.
J. Endourol. 8(4):263–268, 1994.
Return to INSTRUMENT ADJUSTING
Since 5-30-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |