

Relationships Between Active Myofascial Trigger Points
and Depressive Symptoms and Physical and Clinical
Characteristics of Individuals With Shoulder Pain:
A Cross-sectional StudyThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Chiropractic Medicine 2022 (Dec); 21 (4): 249–259 ~ FULL TEXT
OPEN ACCESS José Diego Sales Do Nascimento PhD, Francisco Alburquerque-Sendín PhD,
Liziane Mafra Vale de Souza BS(PT), Catarina de Oliveira Sousa PhD
Physiotherapy Department,
Federal University of Rio Grande Do Norte,
Natal, Rio Grande Do Norte, Brazil.
Objective: The purpose of this study was to evaluate relationships between the presence and number of active myofascial trigger points (MTPs) in shoulder muscles and physical and demographic characteristics, depressive symptoms, pain and function, range of motion (ROM), and strength in individuals with shoulder pain.
Methods: Fifty-eight individuals were assessed for physical and demographic characteristics, depressive symptoms, shoulder pain and function, MTPs (upper and lower trapezius, infraspinatus, and supraspinatus), shoulder ROM and strength test, and pain during ROM and strength test. Relationships were verified using point-biserial (rpb), Spearman correlation test, and multiple linear regression analysis.
Results: We found weak to moderate (P < .05) correlations between presence and number of MTPs and depressive symptoms (rpb, 0.28–0.32), pain during ROM (rpb, 0.36–0.40), pain during strength test (rpb, 0.29–0.38), and shoulder function (rpb, –0.29 to 0.33) and strength (rpb, 0.26–0.34). MTPs in the infraspinatus contributed 10% (R² = 0.10; P < .05) to depressive symptoms; in the upper and lower trapezius contributed 27% (R² = 0.27; P < .05) to pain during internal rotation ROM; in the upper trapezius contributed 15% (R² = 0.15; P < .01) to pain during internal rotation strength test and 14% to pain during internal rotation ROM (R² = 0.14; P < .01); and in the supraspinatus contributed 17% (R² = 0.17; P < .01) to pain during external rotation ROM.
Conclusion: This study found that MTPs in individuals with shoulder pain contributed to depressive symptoms and pain during internal and external rotation ROM and internal rotation strength test.
Keywords: Depressive Disorder; Myofascial Pain Syndromes; Range of Motion, Articular; Shoulder Pain.
From the FULL TEXT Article:
Introduction
Myofascial trigger points (MTPs) are nodules in a taut band of muscle that are tender to palpation and that can generate referred pain spontaneously or by digital pressure. [1] MTPs may be present in symptomatic and asymptomatic individuals [2] and are classified as active (ie, pain recognized as familiar during digital pressure) [3] or latent (ie, pain evoked only during digital pressure).
Observational studies investigating the association between MTPs and clinical variables in individuals with shoulder pain suggested MTPs influence maximum isometric strength [4] and are weakly associated with mobility. [5] MTPs also were associated with changes in activation pattern of shoulder muscles, [6] fatigue, [7] and high rate of depression and anxiety. [8] However, these studies evaluated MTPs only in the upper trapezius and in asymptomatic individuals or with latent MTPs. [9, 10] Furthermore, individuals with pain in shoulder muscles have a high prevalence of MTPs in this region, [4, 6] contributing to reduced mobility, strength, and self-reported function, and increased pain perception and duration.
Other conditions may overlap nontraumatic shoulder pain. For instance, advanced age and obesity may be associated with low-grade systemic inflammation, contributing to tissue sensitization by increasing proinflammatory substances and decreasing anti-inflammatory action. [11] Depression also can be associated with pain sensitization [12]; however, active MTPs in shoulder muscles of depressive patients remains unknown.
Decreased anterior serratus and upper trapezius strength and increasing age are predictive factors for myofascial pain in the upper trapezius. [9] Despite that, there is no expert consensus suggests whether mobility restriction, pain during range of motion (ROM), or reduced strength are important criteria for diagnosing MTPs. [13] Understanding relationships between MTPs and physical, clinical, and psychological characteristics in individuals with shoulder pain is essential because active MTPs may be associated with clinical complaints of pain.
Thus, this study aimed to evaluate relationships between presence and number of active MTPs in the shoulder muscle complex and physical characteristics, depressive symptoms, and clinical variables of individuals with shoulder pain.
We hypothesized that(1) MTPs were not associated with physical characteristics and depressive symptoms; and
(2) the number of MTPs were negatively associated with ROM and strength.
Methods
This cross-sectional study was conducted between May 2018 and December 2019 in the Physical Therapy Department of Federal University of Rio Grande do Norte located in Natal, Brazil.
Ethics
The study was approved by the Research Ethics Committee of Federal University of Rio Grande do Norte (protocol number: CAAE 50199815.9.000.5537; approval number: 1.344.557) and performed according to the Declaration of Helsinki. All volunteers received verbal and written explanations about study objectives, methods, risks, and benefits, and signed the informed consent form.
Sample
Individuals with clinical diagnosis of unilateral shoulder pain were recruited through press and digital media. Sample size was calculated using 2 dependent Pearson tests (common index) and resulted in 58 individuals. The following parameters were used to perform this calculation: H1 ρ ac = –0.18; α = 0.05; power = 0.95; H0 ρ ab = 0.3; ρ ab = –0.3; and Cohen q effect size = 0.5. [14]
Inclusion criteria were age between 18 and 60 years, history of unilateral shoulder pain for at least 1 month, pain located in the anterolateral shoulder region [15] or C5 or C6 dermatome region, [16] and presence of at least 3 specialized tests for shoulder impingement syndrome (eg, Neer test, [17–19] Hawkins-Kennedy test, [18] Jobe test, [20] painful arc test, [21] external rotation strength test, [22] Gerber test, and Speed biceps test [23]).
Exclusion criteria were bilateral signs and symptoms of shoulder pain; primary adhesive capsulitis; history of symptom onset owing to displacement, glenohumeral subluxation, or trauma; history of surgical stabilization or rotator cuff repair; signs of complete rotator cuff tear [18, 24] evidenced by the positive drop test [25]; systemic disease involving joints (eg, rheumatoid arthritis, systemic lupus erythematosus, [26] and fibromyalgia) and affecting myofascial tissue; neurological pathologies; and use of analgesic and muscle relaxants or corticosteroid injection 72 hours and 3 months, respectively, before evaluation. [27]
Evaluation Protocol
Individuals were asked to complete(1) evaluation of depressive symptoms using Beck Depression Inventory, a 21–item instrument used to evaluate severity of depressive symptoms on a 4–point Likert scale ranging from 0 to 3 (maximum score of 63, considering values between 0 and 13 as “minimal depression” or “absence of depression,” 14 and 19 as “mild depression,” 20 and 28 as “moderate depression,” and above 28 as “severe depression”) [28];
(2) evaluation of pain and general function of the shoulder complex using the Brazilian version of the Penn Shoulder Score (Penn), a 100–point scale that includes pain, satisfaction, and function domains (maximum score indicates no pain, very satisfied, and good function) [29];
(3) evaluation of MTPs;
(4) shoulder mobility; and
(5) isometric strength of shoulder complex muscles.A physiotherapist with 8 years of experience performed all evaluations, and a physiotherapy student recorded measurements.
Evaluation of MTPs
Presence of MTPs was investigated in the supraspinatus, infraspinatus, and upper and lower trapezius. These muscles were chosen because they are associated with scapular stabilization and positioning (upper and lower trapezius), belong to the rotator cuff (supraspinatus and infraspinatus), and produce a pattern of referred shoulder pain. [30]
Individuals were asked about pain during palpation, and the following criteria were used to diagnose MTPs [31]: identification of a palpable taut band, palpable painful nodule, and local pain in a palpable nodule (located in a taut band) due to digital compression. MTPs were classified as active if individuals presented referred pain, referred pain beyond the site of palpation recognized as familiar. [30, 32] MTPs were evaluated in all muscles randomly and considered for analysis only if classified as active. The evaluation was performed only in the symptomatic side with individuals in supine (upper trapezius) and prone position (supraspinatus, infraspinatus, and lower trapezius).
Shoulder ROM Evaluation
Maximum ROM of arm elevation in the sagittal plane, arm elevation in the scapular plane, and shoulder internal and external rotation were evaluated using a digital inclinometer (model ACU001, Lafayette Instrument Company, Lafayette, Indiana). Arm elevation in the sagittal plane of the shoulder was measured with individuals seated, elbow extended, shoulder in neutral rotation, and thumb pointing upward. [33] Internal and external rotation ROM was measured with individuals in the supine position, shoulder abducted at 90° in frontal plane, humerus on the exam table, and elbow flexed at 90°. [34] Each test was performed twice, and the average was recorded and included for analysis. [34] This method showed good to excellent intrarater reliability (intraclass correlation coefficient, 0.83–0.95). [34] Pain during ROM evaluation was assessed using the Numerical Pain Rating Scale ranging from 0 (no pain) to 10 (worst pain).
Isometric Strength Evaluation
Muscle strength during arm elevation in the scapular plane and external and internal rotation of the shoulder was assessed with individuals seated and using a manual dynamometer (model 0116, Lafayette Instrument Company). Isometric strength during arm elevation was performed with arm positioned at 90° of elevation in the scapular plane (neutral rotation) with elbow extended. Isometric strength during external and internal rotation of the shoulder was assessed with individuals seated with arm alongside the body and elbow flexed at 90°. For both assessments, the dynamometer was fixed to the wall and adjusted for individuals to push it with the distal part of the forearm. [33, 34]
Individuals were instructed to perform maximum isometric contraction for 5 seconds. Each test was performed twice with an interval of 2 minutes in between, and the average value was calculated and included for analysis. This method showed high test-retest and interevaluator reliability (intraclass correlation coefficient, 0.97). [35] Individuals were asked about pain after tests using the Numerical Pain Rating Scale.
Strength data were normalized by body mass: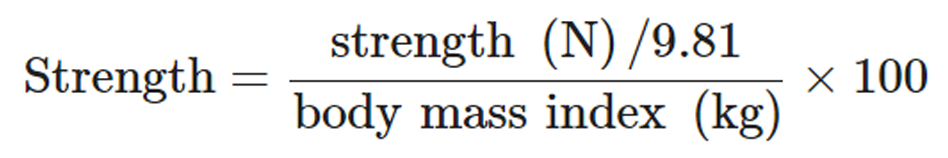
Interrater Reliability
A previous study of our research group showed that evaluation of MTPs in the same muscles of symptomatic individuals with shoulder pain was reliable (prevalence-adjusted bias-adjusted kappa or weighted kappa > 0.40) [36] and acceptable for clinical practice (agreement > 70%). [37] The same trained examiner performed reliability of ROM and strength variables in 20 asymptomatic individuals on the same day. Intraclass correlation coefficient was calculated (ICC2,1 for absolute agreement) for these variables and ranged between 0.91 and 0.96 (considered almost perfect). [38]
Statistical Analysis
Descriptive and inferential analyzes were performed using SPSS Statistics (IBM, Armonk, NY). A Kolmogorov-Smirnov test verified data normality. Data are shown as mean and standard deviation or median and 25% to 75% interquartile range.
Point-biserial correlation coefficient assessed relationships between presence of MTPs and self-reported clinical variables (pain duration and shoulder pain and function), physical examination (ROM, strength, and pain during ROM and strength tests), physical variables (age and body mass index), and depressive symptoms. Point-biserial correlation coefficient was used when 1 variable was dichotomous (eg, presence or absence of MTPs). [39] A Spearman correlation test assessed relationships between the number of MTPs in each muscle and aforementioned variables. Correlation coefficients (R) values were considered weak (below 0.40), moderate (between 0.40 and 0.60), strong (more than 0.60), or perfect (equal to 1). [40] A hierarchical linear regression model determined the effects of presence and the number of MTPs on variables with significant correlation in more than one muscle. [9]
Results

Figure 1

Table 1

Table 2

Table 3

Table 4 A total of 117 individuals were recruited; however, 59 individuals did not meet the inclusion criteria. Thus, 58 individuals participated in the study (Figure 1).
Table 1 shows demographic and clinical characterization of the sample. The sum of MTPs of each muscle from all individuals was calculated and resulted in a prevalence ranging from 28 (supraspinatus muscle) to 59 (infraspinatus muscle).
Table 2 shows correlations between presence of MTP and physical and clinical characteristics, physical examination, and depressive symptoms. MTPs in the upper trapezius correlated with pain during strength test (internal rotation) and ROM (internal and external rotation). In contrast, MTPs in the lower trapezius correlated with strength (external rotation), pain during strength test (scapular flexion and internal and external rotation), pain during ROM (sagittal flexion and internal rotation), and the Penn Shoulder Score. Although MTPs in the infraspinatus and supraspinatus correlated with depressive symptoms, only the former was correlated with ROM (sagittal flexion and external rotation).
We observed positive correlations between the number of MTPs in the upper trapezius and pain during ROM (internal and external rotation) and during strength test (internal rotation) (Table 3). The number of MTPs in the lower trapezius also correlated with pain during ROM (sagittal flexion and internal rotation) and strength test (internal and external rotation).
There was a negative correlation between the number of MTPs and function domain of Penn, and between the number of MTPs in the infraspinatus and external rotation ROM. Regarding strength, we found negative correlations between the number of MTPs in the lower trapezius and scapular flexion and external rotation strength.
Table 4 shows regression models for presence and the number of MTPs in shoulder muscles. Presence of MTPs contributed to depressive symptoms and pain during internal rotation ROM and strength test. MTPs in the infraspinatus contributed 10% to depressive symptoms compared with the model without predictors. The model developed for pain during internal rotation ROM showed MTPs in the upper and lower trapezius contributed 18% and 27% to pain, respectively. Likewise, MTPs in the upper trapezius contributed approximately 15% to the model built for pain during internal rotation strength.
We developed 4 regression models for the number of MTPs (Table 4). The number of MTPs in the upper trapezius contributed 14% to pain during internal rotation ROM and 13% to pain during internal rotation strength test. Finally, the number of MTPs in the supraspinatus contributed 16% to external rotation ROM.
Discussion
Our study was the first to assess relationships between presence and number of active MTPs in shoulder muscles and physical and clinical characteristics and depressive symptoms of symptomatic individuals with unilateral shoulder pain. We found correlations between presence of MTPs and depressive symptoms, pain during ROM, and maximum isometric strength and between number of active MTPs and shoulder function, mobility, and strength. MTPs also contributed to depressive symptoms and pain during ROM and strength.
Spontaneous referred pain is the most prominent clinical manifestation of active MTPs, with pain reference area being able to topographically add to the nontraumatic painful shoulder complaint. [32, 41] However, the persistent nociceptive stimulus could lead to regional or central sensitization and be influenced by MTPs. [42] Algogenic substances in active MTPs also may explain local sensitization, [43, 44] generating pain responsible for perpetuating myofascial pain. Although symptom duration was greater than 12 months in our sample, it was not correlated with the number of MTPs.
The presence or number of MTPs also was not correlated with age and body mass index. However, our sample consisted of young individuals with mean age of 30 years and normal body mass index (mean, 25 kg/m2). Even though these variables were not limited in our eligibility criteria, both showed little dispersion.
Relationship between depressive symptoms and chronic pain remains unclear. [45] One hypothesis is that somatosensory processing areas communicate with emotional processing areas (eg, insular, prefrontal, and cingulate cortex). [12, 46] Thus, depression could influence pain modulation (eg, persistence of pain) and cause chronic pain syndrome and depression. [17] One study identified more pronounced depressive symptoms in women with myofascial pain than the general population. [48] In our study, weak correlations were observed between MTPs in the infraspinatus and supraspinatus muscles and depressive symptoms, with only the infraspinatus being 10% predictive for the variable, regardless of the number of MTPs. Our sample showed values below the cutoff point for diagnosis of depression, which may have limited the significance in our study. [49]
Pain sensitization caused by MTPs could lead to decreased ROM and strength, [50] probably owing to mechanical stress to shortened sarcomeres in the tense band of the muscle with MTP during stretching or contraction. [51] Our findings seem to corroborate the hypothesis proposed by Simons, who suggested MTPs could cause local sensitization and functional repercussions. We found correlations between presence and number of MTPs and pain during ROM and strength test (especially internal rotation) in upper and lower trapezius muscles. In addition, regardless of the number, the presence of MTPs in these muscles could explain 15% to 27% of pain during assessments, whereas the number of MTPs in the upper trapezius and supraspinatus contributed to pain during internal rotation strength and external rotation ROM, respectively. We also found weak correlations between self-reported function and number of MTPs in the lower trapezius, infraspinatus, and supraspinatus muscles. However, we highlight that the evaluated individuals presented good functionality. [52, 53]
Individuals with latent MTPs present altered neuromuscular activation patterns. [6, 7, 52] Thus, changes in neuromuscular activation pattern in the presence of MTPs could be involved in irritation and activation of MTPs and regional muscle function, which may be more pronounced in individuals with spontaneous pain. Celik and Yeldan [4] found individuals with latent MTPs had less deltoid strength and flexion in the scapular plane test than individuals without MTPs. In addition, Ge et al [52] observed fatigue and early overload of motor units during isometric abduction. Low isometric strength also was observed in symptomatic individuals with myofascial pain in the upper trapezius during arm elevation in the scapular plane. [16] In contrast, despite correlations with ROM, we did not find correlations between supraspinatus MTPs and scapular flexion strength. The relationship between MTPs in supraspinatus and scapular and sagittal flexion ROM was possibly associated with regional sensitization caused by a movement above 90° ROM but not the strength generated during contraction.
We believe that implications for ROM and strength of individuals with shoulder pain were owing to sensitization generated by MTPs [42] rather than mechanical stress caused by stretching, muscle contraction, or both. Lucas et al [6] showed the presence of latent MTPs might alter periscapular muscle contraction pattern, which could increase fatigue [7] and peripheral sensitization [42] and alter muscle function and segment mobility.
Limitations
The relatively low number of individuals may not be representative of the general population. We could not perform a blind evaluation therefore this may have influenced our findings. The study design did not allow us to verify if relationships found were clinically meaningful. Other muscles involved in the shoulder complex (eg, anterior serratus, pectoralis minor, and medium trapezius) were not evaluated but could have also contributed to the parameters studied.
Clinical Applicability
Our results suggest that treatment of active MTPs in shoulder muscle could improve some painful symptoms during movement by reducing regional irritability and helping manage pain in the shoulder. The treatment for managing pain due to MTPs during ROM and strength also may contribute to tolerance in therapeutic exercises. Thus, future studies may investigate the long-term effects of treatments for MTPs in physical and pain variables.
Conclusion
We observed a weak to moderate correlation between active MTPs and depressive symptoms, pain during ROM and strength test, ROM, strength, and shoulder function. However, in the participants included in this study, we did not find correlations between active MTPs and physical variables in symptomatic individuals with unilateral shoulder pain. Our findings suggest that the presence of MTPs may contribute to depressive symptoms, pain during internal rotation ROM and strength test, whereas the number of MTPs may contribute to pain during internal rotation ROM and strength test, and external rotation ROM.
Practical Applications
The management of myofascial trigger points were considered in the manifestation of pain during ROM and strength to improve tolerance in therapeutic exercises.
We evaluated relationships between the presence and number of active myofascial trigger points in shoulder muscles and depressive symptoms, pain and function, range of motion (ROM), and strength in individuals with shoulder pain.
We found weak-to-moderate correlations between the presence and number of myofascial trigger points and depressive symptoms, pain during ROM, pain during strength test, and shoulder function and strength.
These findings suggest that active myofascial trigger points may contribute to depression symptoms and pain during ROM and force in individuals with shoulder pain.
Funding Sources and Conflicts of Interest
This study was partly financed by CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education) within the Ministry of Education of Brazil (Finance Code 001) (C.O.S. and J.D.S.N.). No conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): J.D.S.N., C.O.S.
Design (planned the methods to generate the results): J.D.S.N., C.O.S., F.A.S.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): J.D.S.N., C.O.S.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): J.D.S.N., L.M.V.S.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): J.D.S.N., C.O.S.
Literature search (performed the literature search): J.D.S.N., C.O.S.
Writing (responsible for writing a substantive part of the manuscript): J.D.S.N., C.O.S., F.A.S.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): C.O.S., F.A.S.
References:
Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L.
Myofascial trigger points then and now:
a historical and scientific perspective.
PM&R. 2015;7(7):746–761.Simons DG.
Review of enigmatic MTrPs as a common cause of
enigmatic musculoskeletal pain and dysfunction.
J Electromyogr Kinesiol. 2004;14(1):95–107.Ge H-Y, Arendt-Nielsen L.
Latent myofascial trigger points.
Curr Pain Headache Rep. 2011;15(5):386–392.Celik D, Yeldan I.
The relationship between latent trigger point and muscle
strength in healthy subjects: a double-blind study.
J Back Musculoskelet Rehabil. 2011;24(4):251–256.Fernández-de-las-Peñas C, Cuadrado ML, Pareja JA.
Myofascial Trigger Points, Neck Mobility, and Forward
Head Posture in Episodic Tension-Type Headache
Headache 2007 (May); 47 (5): 662—672Lucas KR, Rich PA, Polus BI.
Muscle activation patterns in the scapular positioning muscles
during loaded scapular plane elevation: the effects of
Latent Myofascial Trigger Points.
Clin Biomech (Bristol, Avon) 2010;25(8):765–770.Ge H-Y, Arendt-Nielsen L, Madeleine P.
Accelerated muscle fatigability of latent myofascial trigger points in humans.
Pain Med. 2012;13(7):957–964.Do TP, Heldarskard GF, Kolding LT, Hvedstrup J, Schytz HW.
Myofascial trigger points in migraine and tension-type headache.
J Headache Pain. 2018;19(1):84.Hwang U-J, Kwon O-Y, Yi C-H, Jeon H-S, Weon J-H, Ha S-M.
Predictors of upper trapezius pain with myofascial trigger points
in food service workers: The STROBE study.
Medicine (Baltimore) 2017;96(26):e7252.Kim HA, Hwang UJ, Jung SH, Ahn SH, Kim JH, Kwon OY.
Comparison of shoulder strength in males with and without
myofascial trigger points in the upper trapezius.
Clin Biomech (Bristol, Avon) 2017;49:134–138.Özkuk K, Ates Z.
The effect of obesity on pain and disability in chronic shoulder pain patients.
J Back Musculoskelet Rehabil. 2020;33(1):73–79.Sheng J, Liu S, Wang Y, Cui R, Zhang X.
The link between depression and chronic pain: neural mechanisms in the brain.
Neural Plast. 2017;2017Fernández-de-Las-Peñas C, Dommerholt J.
International consensus on diagnostic criteria and clinical considerations
of myofascial trigger points: a delphi study.
Pain Med. 2018;19(1):142–150.Faul F, Erdfelder E, Buchner A, Lang A-G.
Statistical power analyses using G*Power 3.1:
tests for correlation and regression analyses.
Behav Res Methods. 2009;41(4):1149–1160.Lin J, Chen W-H, Chen P-Q, Tsauo J-Y.
Alteration in shoulder kinematics and associated muscle activity
in people with idiopathic scoliosis.
Spine (Phila Pa 1976) 2010;35(11):1151–1157.McClure PW, Michener LA, Karduna AR.
Shoulder function and 3-dimensional scapular kinematics in
people with and without shoulder impingement syndrome.
Phys Ther. 2006;86(8):1075–1090.Zanca GG, Saccol MF, Oliveira AB, Mattiello SM.
Shoulder internal and external rotations torque steadiness
in overhead athletes with and without impingement symptoms.
J Sci Med Sport. 2013;16(5):433–437.Hung C-J, Jan M-H, Lin Y-F, Wang T-Q, Lin J-J.
Scapular kinematics and impairment features for classifying
patients with subacromial impingement syndrome.
Man Ther. 2010;15(6):547–551.Neer CS.
Anterior acromioplasty for the chronic impingement syndrome
in the shoulder: a preliminary report.
J Bone Joint Surg Am. 1972;54(1):41–50.Jobe FW, Jobe CM.
Painful athletic injuries of the shoulder.
Clin Orthop Relat Res. 1983;(173):117–124.Michener LA, Walsworth MK, Doukas WC, Murphy KP.
Reliability and diagnostic accuracy of 5 physical examination tests
and combination of tests for subacromial impingement.
Arch Phys Med Rehabil. 2009;90(11):1898–1903.Diercks R, Bron C, Dorrestijn O, et al.
Guideline for diagnosis and treatment of subacromial pain syndrome:
a multidisciplinary review by the Dutch Orthopaedic Association.
Acta Orthop. 2014;85(3):314–322.Camargo PR, Ávila MA, Asso NA, Salvini TF.
Muscle performance during isokinetic concentric and eccentric abduction
in subjects with subacromial impingement syndrome.
Eur J Appl Physiol. 2010;109(3):389–395.Struyf F, Cagnie B, Cools A, et al.
Scapulothoracic muscle activity and recruitment timing in patients
with shoulder impingement symptoms and glenohumeral instability.
J Electromyogr Kinesiol. 2014;24(2):277–284.Magee D. 4th ed.
Manole; São Paulo: 2005.
AvaliaçãoMusculoesquelética.Santamato A, Solfrizzi V, Panza F, et al.
Short-term effects of high-intensity laser therapy versus ultrasound
therapy in the treatment of people with subacromial impingement syndrome:
A randomized clinical trial.
Phys Ther. 2009;89(7):643–652.Alburquerque-Sendín F, Camargo PR, Vieira A, Salvini TF.
Bilateral myofascial trigger points and pressure pain thresholds in the
shoulder muscles in patients with unilateral shoulder
impingement syndrome: a blinded, controlled study.
Clin J Pain. 2013;29(6):478–486.Gomes-Oliveira MH, Gorenstein C, Neto FL, Andrade LH, Wang YP.
Validation of the Brazilian Portuguese version of the
Beck Depression Inventory-II in a community sample.
Rev Bras Psiquiatr. 2012;34(4):389–394.Napoles BV, Hoffman CB, Martins J, De Oliveira AS.
Tradução e adaptação cultural do Penn Shoulder Score para a Língua Portuguesa: PSS-Brasil.
Rev Bras Reumatol. 2010;50(4):389–397.Simons DG, Travell JG, Simons LS.
2nd ed. Vol. 1. Lippincott Williams & Wilkins; Baltimore, MD: 1999.
(Myofascial Pain and Dysfunction: The Trigger Point Manual).Bailón-Cerezo J, Torres-Lacomba M.
Presencia de puntos gatillo miofasciales y discinesia escapular en nadadores
de competición con y sin dolor de hombro: estudio piloto transversal
[Presence of myofascial trigger points and scapular dyskinesis in
competitive swimmers with and without shoulder pain:
a cross-sectional pilot study]
Fisioterapia. 2014;36(6):266–273. [in Spanish]Hidalgo-Lozano A, Fernández-de-las-Peñas C, Alonso-Blanco C, Ge H-Y.
Muscle trigger points and pressure pain hyperalgesia in the shoulder
muscles in patients with unilateral shoulder impingement:
a blinded, controlled study.
Exp Brain Res. 2010;202(4):915–925.Michener LA, Sharma S, Cools AM, Timmons MK.
Relative scapular muscle activity ratios are altered
in subacromial pain syndrome.
J Shoulder Elb Surg. 2016;25(11):1861–1867.Kolber MJ, Vega F, Widmayer K, Cheng M-SS.
The reliability and minimal detectable change of shoulder
mobility measurements using a digital inclinometer.
Physiother Theory Pract. 2011;27(2):176–184.Kolber MJ, Beekhuizen K, Cheng MSS, Fiebert IM.
The reliability of hand-held dynamometry in measuring isometric strength
of the shoulder internal and external rotator musculature
using a stabilization device.
Physiother Theory Pract. 2007;23(2):119–124.Nascimento JDS, Alburquerque-Sendín F, Vigolvino LP, Oliveira WF de, Sousa C de O.
Inter- and intraexaminer reliability in identifying and classifying
myofascial trigger points in shoulder muscles.
Arch Phys Med Rehabil. 2018;99(1):49–56.Bron C, Franssen J, Wensing M, Oostendorp RAB.
Interrater reliability of palpation of myofascial trigger points
in three shoulder muscles.
J Man Manip Ther. 2007;15(4):203–215.Koo TK, Li MY.
A guideline of selecting and reporting intraclass
correlation coefficients for reliability research.
J Chiropr Med. 2016;15(2):155–163.Lira SA, Neto AC.
Coeficientes de correlação para variáveis ordinais e
dicotômicas derivados do coeficiente linear de Pearson
[Correlation coefficient derived from Pearson linear
coefficient for ordinal and dichotomic variables]
Cienc y Eng Sci Eng J. 2006;15:45–53. [in Spanish]Dancey CP, Reidy J. 5th ed. Prentice-Hall; Harlow: 2011.
Statistics Without Maths for Psychology.Ge H-Y, Fernández-de-Las-Peñas C, Madeleine P, Arendt-Nielsen L.
Topographical mapping and mechanical pain sensitivity of
myofascial trigger points in the infraspinatus muscle.
Eur J Pain. 2008;12(7):859–865.Fernández-de-las-Peñas C, Dommerholt J.
Myofascial trigger points: peripheral or central phenomenon?
Curr Rheumatol Rep. 2014;16(1):395.Gerwin RD, Dommerholt J, Shah JP.
An expansion of Simons’ integrated hypothesis
of trigger point formation.
Curr Pain Headache Rep. 2004;8(6):468–475.Shah JP, Gilliams EA.
Uncovering the biochemical milieu of myofascial trigger points using
in vivo microdialysis: an application of muscle pain concepts
to myofascial pain syndrome.
J Bodyw Mov Ther. 2008;12(4):371–384.Altindag O, Gur A, Altindag A.
The relationship between clinical parameters and depression level
in patients with myofascial pain syndrome.
Pain Med. 2008;9(2):161–165.Meerwijk EL, Ford JM, Weiss SJ.
Brain regions associated with psychological pain:
implications for a neural network and its relationship to physical pain.
Brain Imaging Behav. 2013;7(1):1–14.Sergienko S, Kalichman L.
Myofascial origin of shoulder pain: a literature review.
J Bodyw Mov Ther. 2015;19(1):91–101.Giannakopoulos NN, Keller L, Rammelsberg P, Kronmüller KT.
Anxiety and depression in patients with chronic
temporomandibular pain and in controls.
J Dent. 2010;38(5):369–376.Furlanetto LM, Mendlowicz MV, Romildo Bueno J.
The validity of the Beck Depression Inventory-Short Form as a screening
and diagnostic instrument for moderate and severe
depression in medical inpatients.
J Affect Disord. 2005;86(1):87–91.Zhuang X, Tan S, Huang Q.
Understanding of myofascial trigger points.
Chin Med J (Engl) 2014;127(24):4271–4277.Gerwin RD.
Diagnosis of myofascial pain syndrome.
Phys Med Rehabil Clin N Am. 2014;25(2):341–355.Ge H-Y, Monterde S, Graven-Nielsen T, Arendt-Nielsen L.
Latent myofascial trigger points are associated with an increased
intramuscular electromyographic activity during synergistic muscle activation.
J Pain. 2014;15(2):181–187.Cook KF, Gartsman GM, Roddey TS, Olson SL.
The measurement level and trait-specific reliability of
4 scales of shoulder functioning: an empiric investigation.
Arch Phys Med Rehabil. 2001;82(11):1558–1565.
Return to SHOULDER
Since 6-10-2023


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |