

Prediction of Pain Outcomes in a Randomized Controlled
Trial of Dose-response of Spinal Manipulation for the
Care of Chronic Low Back PainThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: BMC Musculoskelet Disord. 2015 (Aug 19); 16: 205 ~ FULL TEXT
OPEN ACCESS Darcy Vavrek, Mitchell Haas, Moni Blazej Neradilek, and Nayak Polissar
University of Western States,
2900 NE 132nd Ave,
Portland, OR, 97230, USA.
dvavrek@uws.edu
BACKGROUND: No previous studies have created and validated prediction models for outcomes in patients receiving spinal manipulation for care of chronic low back pain (cLBP). We therefore conducted a secondary analysis alongside a dose-response, randomized controlled trial of spinal manipulation.
METHODS: We investigated dose, pain and disability, sociodemographics, general health, psychosocial measures, and objective exam findings as potential predictors of pain outcomes utilizing 400 participants from a randomized controlled trial. Participants received 18 sessions of treatment over 6-weeks and were followed for a year. Spinal manipulation was performed by a chiropractor at 0, 6, 12, or 18 visits (dose), with a light-massage control at all remaining visits. Pain intensity was evaluated with the modified von Korff pain scale (0-100). Predictor variables evaluated came from several domains: condition-specific pain and disability, sociodemographics, general health status, psychosocial, and objective physical measures. Three-quarters of cases (training-set) were used to develop 4 longitudinal models with forward selection to predict individual "responders" (≥50% improvement from baseline) and future pain intensity using either pretreatment characteristics or post-treatment variables collected shortly after completion of care. The internal validity of the predictor models were then evaluated on the remaining 25% of cases (test-set) using area under the receiver operating curve (AUC), R(2), and root mean squared error (RMSE).
RESULTS: The pretreatment responder model performed no better than chance in identifying participants who became responders (AUC = 0.479). Similarly, the pretreatment pain intensity model predicted future pain intensity poorly with low proportion of variance explained (R(2) = .065). The post-treatment predictor models performed better with AUC = 0.665 for the responder model and R(2) = 0.261 for the future pain model. Post-treatment pain alone actually predicted future pain better than the full post-treatment predictor model (R(2) = 0.350). The prediction errors (RMSE) were large (19.4 and 17.5 for the pre- and post-treatment predictor models, respectively).
CONCLUSIONS: Internal validation of prediction models showed that participant characteristics preceding the start of care were poor predictors of at least 50% improvement and the individual's future pain intensity. Pain collected shortly after completion of 6 weeks of study intervention predicted future pain the best.
From the FULL TEXT Article:
Background
The most common cause of disability is low back pain with an estimated 1099 years of life lost to disability each year per 100,000 people, worldwide, in 2010 [1]. The prevalence of chronic low back pain (cLBP) is approximately 10 % [1, 2]. An effective approach to treating low back pain can include spinal manipulative therapy (SMT) [35]. Advantageously, treatment of cLBP with spinal manipulative therapy does not appear to increase the cost of treatment plus lost productivity [6]. The question remains though, about what kind of patient has a greater chance of benefit with efficacious conservative therapies such as SMT [79], mechanical lumbar traction [10], and a stabilization exercise program [11]. Our study is a step in this direction and to our knowledge, this is the only study to date that has sought to create prediction models of prognosis in individuals receiving a dose of SMT for the care of cLBP. This scientific inquiry is of great societal interest given todays environment of prevention of opiate deaths in chronic pain management [1216].
There have been few studies evaluating determinants of outcomes in patients receiving SMT for the care of cLBP. Leboeuf-Yde et al., [17] looked at predictors in a cohort of chiropractic patients with persistent low back pain. They found sex, social benefit, severity of pain, duration of continuous pain at first consultation, and additional neck pain predictive of failure to recover in the short term. Most notable was being pain-free at the fourth visit was a strong predictor of recovery at 3 and 12 months. Dougherty et al. [18] modified the clinical prediction rule developed by Flynn et al. [7] so it could be tested in a randomized trial in a chronic patient population. The modified rule was not successful, a cautionary tale against using a prediction rule in individuals for which it was not specifically developed. A large practice-based observational study reported in Nyiendo et al. [8] and Haas et al. [9] found the following to be predictive of outcomes: baseline pain and disability, age, history of low back pain, duration of baseline LBP episode, pain below the knee, provider type, income, smoking, comorbidity, and chronic depression.
The aim of this secondary analysis was to build and attempt to internally validate prediction models for pain outcomes in cLBP patients treated with SMT in a randomized controlled trial. The purpose was prognosis related to a course of care, rather than development of a clinical prediction rule for selecting from among alternative interventions. Models were created separately using data from the two most natural time points for discussing prognosis with a patient in clinical practice: immediately prior to care (pre-treatment predictor models) and following completion of care (post-treatment predictor models). The two outcomes that were predicted by the models were the continuous pain score and a dichotomous responder indicator (50 % or greater improvement in pain score from the baseline). Our approach was unique for studies of SMT in that the sample was partitioned to both create and test the strength of the prediction models in one study.
Methods
Design
Data were obtained through a randomized clinical trial looking for the dose effect of spinal manipulative therapy for cLBP [3, 6]. Four hundred participants were randomized to 4 dose groups, 100 patients per group. Participants were treated 3 times per week for 6 weeks. They received 0, 1, 2, or 3 SMT sessions per week (0, 6, 12, or 18 total SMT visits) with control visits, consisting of light massage, on non-manipulation visits. All care was provided in the Portland metro area. Outcomes were collected at baseline and 6, 12, 18, 24, 39, and 52 weeks after randomization on. Randomization coincided with the first treatment visit.
Participants
All patients provided informed consent for participation in this study which was approved of by University of Western States Institutional Review Board. Patients were recruited through craigslist, mailers, and newspaper advertisements in the Portland Metro area to participate in our study. Details on subject enrollment, inclusion/exclusion criteria, design, and analysis are reported elsewhere [3]. Details on cost effectiveness and the doctor/patient interaction have also been reported [6, 19].
Patients were considered chronic if their low back pain was at least 3 months in duration and they had at least 30 days of low back pain in the last 6 weeks. A minimum pain score of 25 (0100 scale) was required in order to prevent floor effects. Participants were excluded for contraindications to SMT, such as active cancer, spine pathology, inflammatory arthropathies, autoimmune disorders, and anti-coagulant conditions. Also excluded were those with potential confounders to pain and disability improvement; including neurodegenerative diseases, pain radiating below the knee, organic referred pain, and disability compensation [3].
Intervention
The intervention was SMT consisting of manual high velocity, low amplitude, thrust spinal manipulation [20]. The control appointments consisted of a brief light massage, shorter and lighter than what would be considered appropriate in a therapeutic massage practice [21, 22]. In addition to SMT or light massage, at each visit, all participants received 5 min of hot pack treatment to relax the back musculature and 5 min of very low intensity pulsed ultrasound (0.5 watts/cm2) to enhance credibility of care. This was deemed necessary for fidelity of care in patients receiving only or mostly a brief light massage.
Pain and disability scales
We assessed cLBP with the modified Von Korff pain and disability scales, validated by Underwood et al. [23]. The pain scale consisted of asking patients to rate their pain today, average pain over the last four weeks, and worst pain over the past 4 weeks each on a 010 pain scale with no pain and as bad as pain could be as the anchors. The disability scale consisted of asking patients to rate how much their low back pain interfered with their daily activities; changed their ability to take part in recreational, social, and family activities; and changed their ability to work (including house and yard work) with no interference/change and extreme interference/change as the anchors. For both pain and disability, the 3 010 scales were averaged and then multiplied by 10 to create the two modified Von Korff scores on 0 to 100 scales.
Outcome measures
Pain intensity was measured by the Modified Von Korff pain scale described above [23]. Pain outcomes were analyzed either as a continuous score (future pain intensity) or as a binary indicator for a responder. Choosing a dichotomous responder indicator is recommended by the NIH Task Force for Research Standards on Chronic Low Back Pain [24]. For a given follow-up visit, a responder was defined as having at least 50 % improvement relative to the baseline pain intensity [24, 25]. Fifty percent improvement in back pain is considered substantial [26]. Note that the same participant could be classified as a responder during one visit and not at another. The pretreatment predictor models were used to predict the outcomes at the 6, 12, 18, 24, 39 and 52-week follow-ups and the post-treatment predictor models were used to predict the outcomes at the 12, 18, 24, 39 and 52-week follow-ups.
Predictive measures

Table 1 A more extensive discussion of the outcomes used in this study is provided in our paper on main results from our dose response study [3]. Potential pretreatment predictors were collected at baseline by questionnaire (Table 1). Demographics included age, gender, education, race, and ethnicity. Validated low back pain characteristics were the primary outcomes, pain intensity and disability [23], as well as pain unpleasantness [27]. Other common condition-specific variables we have used in the past were days with pain, days with disability, duration, and previous treatment [28, 29]. General Health status measures were comorbidity, smoking, and the validated EuroQol-5D [3032] and SF-12 [33, 34]. Psychosocial variables were the Fear Avoidance Beliefs Questionnaire [35] and previously used confidence in the success of SMT and light massage [28, 29]. Dose was determined during randomization at baseline and coded as 0, 1, 2, or 3 representing the multiple of 6 SMT sessions each study group received. Follow-up time point was used as a continuous variable measured in weeks from randomization.
Objective physical exam measures included lumbar range of motion, segmental hypomobility, and pain pressure threshold. Lumbar range of motion was measured in flexion , extension, and lateral bending [3638]; reliability has been established by Keeley et al. [39]. The accompanying self-reported pain was evaluated using the validated 0 to 10 numeric pain rating scale [40]. The sum of these pain scores, the difference between right and left lateral bending pain scores, and maximum pain score were also of interest for prediction model development. A modified Schober test [41, 42] was conducted on each patient; test reliability has been established [4345]. Segmental hypomobility from L1 through L5 was determined by manual motion palpation [46, 47] and defined for this study as restriction of motion in any plane of motion. The total number of hypomobile joints were also recorded [46, 47]. Stochkendahl et al. [47] recently observed that global assessment of hypomobility has clinically acceptable reproducibility. Pain pressure thresholds (PPT) were assessed from L1 through S1 using a validated pressure algometer [48, 49].
Potential post-treatment predictors were collected several days after the end of treatment (6 weeks after randomization) and included pain intensity, disability, LBP unpleasantness, days with LBP, days with disability due to LBP, satisfaction with care [8, 19, 50, 51] and objective physical exam measures, as well as dose and time (follow-up time points listed under outcome measures).
Analysis
The data set was randomly split into two sets. The training set included all data for 75 % of the participants and the test set included all data for the remaining 25 % of the participants. The multivariate prediction models were developed on the training-set and the ability of the developed models to predict the outcomes was evaluated on the test set. This random split enabled internal validation of the developed model in the same study and addressed the potential issue of over-optimism (over-fitting) of the model in the training set.
For each of the 2 outcomes, we developed a pretreatment and a post-treatment predictor model using the variables identified above. The outcomes were modeled by logistic regression (responder outcome) and linear regression (future pain intensity outcome) using generalized estimating equations (with the AR1 correlation structure) to account for the repeated measures for the same subject [5254]. Analysis included all time points in aggregate (as repeated measures) to improve the stability and power of the estimated model and to avoid the complexity of reporting and interpreting results from separate predictor models for each time point. A sensitivity analysis (not shown) of models for individual time points showed results that were consistent with and warranting the repeated-measures analysis.
Model development
The prediction models were developed in 2 steps. First, a univariate analysis was performed on the set of all potential predictors with treatment dose forced into all models (to adjust for the primary purpose of the trial which was to evaluate the effect of dose). Second, statistically significant variables (p < .05) in the univariate analysis were then considered for inclusion into the multivariate models. The multivariate predictor models used a forward stepwise variable selection procedure with p < .05 required for entry into the model [55]. Observations with missing data were dropped.
For the responder models (binary data outcome), the odds ratio, 95 % confidence intervals, and p-values are reported. The odds ratio gives the increase in the odds of being a responder after adjusting for the other variables in the model. For the future pain intensity models (continuous data outcome), linear regression coefficients (β), 95 % confidence intervals, and p-values are reported. The coefficient β estimates the change in mean future pain intensity score expected for a unit change of the predictor variable after controlling for the other variables in the predictor model.
Model evaluation
The predictive ability of the models in the training and test sets are expressed by the area under receiver operating characteristics curve (AUC) for the binary responder models [56] and by both root mean square error (RMSE) and R2 for the continuous future pain intensity models [57]. The AUC measures the ability to predict a binary outcome on a 0 to 1 scale with AUC = 0.50 representing prediction expected by chance alone and AUC = 1 representing perfect prediction. The RMSE estimates the standard deviation of the prediction error, which is defined as the difference between the predicted and observed future pain scores. R2 is the proportion of the variation in the continuous outcome explained by the model.
Of 400 participants, the 391 who provided follow-up data were included in the analysis for the pretreatment models, while 385 with follow-up data were included in the post-treatment models. Follow-up missing data were imputed for these intention-to-treat analyses using linear interpolation and last point carried forward.
Analyses were conducted with Stata 11.0 (StataCorp, College Station, TX) and R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided and p < 0.05 was used to denote statistical significance. Multiple testing adjustments were not made because this was an exploratory analysis aimed at evaluating the feasibility of prediction.
Results
Pretreatment variables
Table 1 shows the descriptive statistics for the baseline variables that were considered for entry into pretreatment predictor models. On average, these patients were 41 years old, equally split in gender, and white non-Hispanic; they had moderate pain (52/100) and disability (45/100), chronic back pain of about 12 years in duration, moderate baseline health, previous care for their back problems, and confidence in success of study care [3]. Mean global lumbar range of motion was 43° flexion, 15° extension, and 19° lateral bending. Overall, reported pain with lumbar motion was mild to moderate (3/10). Pain pressure thresholds were reported to be about 6 kg/cm2 from L1 through L5, on average. Table 1 also notes the statistically significant variables in the univariate analyses (p < .05) that were considered for inclusion into the multivariate models.
There were no missing values in the variables considered in the responder multivariate model and <2 % missing values for variables considered in the multivariate model for future pain intensity. Less than <3 % of values were missing for the remaining variables. In aggregate, only 2.6 % observations had to be dropped due to missing values in variables for building the multivariate model for future pain intensity. Pretreatment multivariate predictor models

Table 2

Figure 1
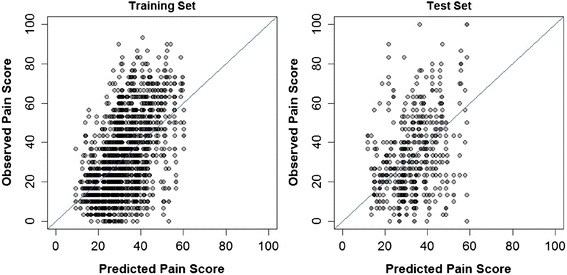
Figure 2

Table 3 The predictive ability of the pretreatment responder model in the training and test sets were quantified by the AUC statistic and illustrated visually by the receiver operating characteristics (ROC) curves (Table 2 and Figure 1). While the model appeared to predict some risk of being a responder in the training set (AUC = 0.624), in the test set the model performed similarly to chance only (AUC = 0.479 vs. 0.500 for chance). Among the variables selected into the model, greater odds of 50 % improvement in pain were associated with greater dose of SMT, while poorer odds were associated with comorbidity, less tendency towards self-care, and greater pain with lumbar motion.
The ability to predict future pain intensity was also poor (Table 2 and Figure 2). The model R2 was 0.268 for the training set and 0.065 for the test set. Hence, the model could account for only a trivial percentage of the variability in future pain. The prediction error (RMSE) was large for both the training and test set data (RMSE = 17.4 and 19.4 points, respectively). Predictors in the model associated with greater future pain were greater baseline pain intensity and unpleasantness, as well as greater pain with lumbar motion; less future pain was related to greater dose of SMT and greater general health evaluated with the EuroQol visual analogue scale.
Post-treatment variables
Patient characteristics evaluated within several days after completion of care (Table 3) show that, on average, patients were in mild to moderate pain (31/100) and disability (28/100). Patients generally agreed or strongly agreed, on average, that they were satisfied with care. Patient perception of provider confidence in treatment was more neutral because the study doctors were trained to show equipoise with regards to study interventions. Lumbar global range of motion increased only about 2°, on average, in each direction. Overall, patients had greater pain pressure thresholds than at baseline.
There were no missing values in most variables considered in the post-treatment multivariate models and for those with missing values 8 % or fewer were missing. In aggregate a total of 13.8 and 9.2 % of observations had to be dropped due to missing values for building the multivariate model for responders and for future pain intensity, respectively.
Post-treatment multivariate predictor models

Table 4

Figure 3
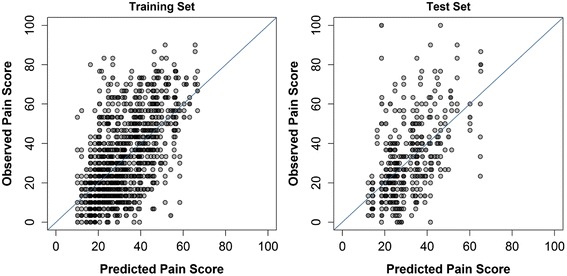
Figure 4 For responder prediction, AUC was 0.750 in the training set and 0.665 for the test set (Table 4, Figure 3). Greater odds of 50 % improvement in pain intensity were associated with greater dose of SMT and the passage of time. Poorer odds were associated with greater pain intensity, days with pain, and difference in pain with left and right lateral bending.
The ability to predict future pain in the study population can be considered moderate with an R2 of 0.366 for the training set and 0.261 for the test set. (Table 4, Figure 4). However, the RMSE was fairly large, 16.3 points in the training set and 17.5 points in the test set. Greater future pain intensity was related to greater 6-week pain intensity and pain in right lateral bending.
In addition, we would like to point out that the 6-week pain intensity predictor alone had better ability to predict future pain score (R2 = 0.350 in the test set) compared to the full multivariate predictor model (R2 = 0.261 in the test set). The RMSE for the 6-week pain intensity only model was 16.5 points for the training set and 17.6 points for the test set, almost identical to the RMSE for the full multivariate model.
Discussion
Our analysis demonstrates the importance of full internal validation using an independent data set. The differences between the training set and test set in AUC and R2 clearly illustrate the problem of over-optimism (i.e., over-fitting) during model development (Tables 2 and 4) [58]. Whereas the performance of the pretreatment model to predict responders and future pain intensity might be considered promising in the training data set, the internal validation of the model in the test set showed failure to identify responders better than chance and failure to explain variation in pain intensity after baseline. For the post-treatment predictor models, performance during internal validation was not as strong as during model development, but still demonstrated some ability to predict both responders and future pain intensity.
A desirable feature for clinical practice is the simplicity of a prediction model. In our models, post-treatment pain intensity alone predicted future pain intensity as well as or better than the post-treatment model built using many more predictor variables. The predictive ability of the single variable model also did not decrease as drastically between the training and test sets as the predictive ability of the multivariate model. This reflects the fact that fitting a model to a single variable does not result in as much over-optimism (over-fitting) as building a multivariate model using a large number of predictors. The sustained predictive ability of the post-treatment, pain-intensity-only model suggests that this variable is very likely a true predictor of future pain and would very likely be predictive of pain intensity in further studies carried out in similar populations.
In our study, participants were treated for 18 visits over six weeks. Timelines of treatment vary across different predictive studies. For example, the Nordic Back Pain study only looked at patients for the first four visits over an unspecified period of time [59, 60]. They also looked at patients with and without sciatica while we excluded people with pain below the knee; and they looked at daily versus intermittent pain which we did not. Of the predictors in their initial model, only increased pain also predicted a worse prognosis in our model. LeBoeuf-Yde et al. [17] found that early recovery was a strong predictor for 12 month recovery. This matches our findings that those with less pain at the end of care were more likely to have successful treatment over the rest of the year. However, Leboeuf-Yde et al. had no test set and it is difficult to tell if their model would hold in a similar population.
Limitations
The goal in developing the multivariate predictor models was to predict pain outcome for individuals. This is a different objective from the development of a model that seeks to identify specific predictors that impact the outcomes. While our models do identify potential predictors of pain (in an exploratory sense), they cannot reliably claim that variables selected into the models are determinants of pain and variables not selected into the models are not. For example, functional disability might in general be expected to predict pain outcomes. We found that among single predictors other than pain, this variable had the strongest correlation with future pain intensity but it was not included in the multivariate model because of its correlation with the pain predictors that were included. In general, the effort of building a good predictive model does not necessarily lead to reliable identification of specific risk factors that truly impact the outcome. The ability to identify these variables is compromised by the large number of the predictors and by the correlation among them.
A related issue is the use of p-values for selection of the specific variables in our results. The univariate p-values should be interpreted as indicators of variable importance to guide variable selection, not as traditional hypothesis tests of statistical significance. The confidence intervals and p-values for the coefficients and odds ratios in the multivariate regression models must also be viewed as exploratory because both tend to be over-optimistic in forward variable selection. We considered only variables with univariate p?
We also would like to emphasize that we did not endeavor to create a clinical prediction rule. We did not have a meaningful clinical comparator such as another efficacious treatment or no treatment at all. We also did not have the power to evaluate potential effect modifiers of intervention including the interaction effect between treatment alternatives and the final prediction-rule recommendation [61]. However, the results from our study data do suggest that it may be difficult to develop a prediction rule to identify patients who might benefit from SMT for cLBP before they receive treatment.
The principal limitation was that the study was a randomized controlled trial with treatment mostly limited to SMT of the low back. The baseline models included a rigorously controlled treatment environment for the first six weeks. Even though the post-treatment models are based on the clinical course of cLBP, the influence of other practice characteristics on patient outcomes not included in the prediction models cannot be ruled out, such as full-spine manipulation, physical modalities, supervised exercise, supplements, and advice on posture, diet, stress management, and other lifestyle and self-care measures [62]. The observation that decreased future pain is associated with decreased pain with right lateral bending, and not left, is questionable and may simply be an artifact of assessing so many predictors. Pain with some form of lumbar motion does, however, appear in all models. External validation in common clinical practice applied to a variety of patient population is required to evaluate the robustness of applicability of our models.
Conclusion
Internal validation of prediction models showed that participant characteristics preceding the start of care were poor predictors of responders (at least 50 % improvement in pain intensity) and future pain intensity as well. Variables collected shortly after completion of 6 weeks of study intervention predicted future pain the best. The findings from this exercise of model development remind us that creating prediction models is difficult. We are also reminded of the importance of validating models. Our results suggest that the simplest model and the best predictor may be post-treatment pain.
Acknowledgments
The study, including all authors, was funded by the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (U01 AT001908). NCCIH approved all study protocols. The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the official views of NCCIH.
Abbreviations
AUC = Area under the receiver operating curve
β = Regression coefficient
cLBP = Chronic low back pain
CI = Confidence interval
OR = Odds ratio
R2 = Coefficient of determination
ROC = Receiver operating characteristics curve
SD = Standard deviation
RMSE = Root mean square error
SMT = Spinal manipulative therapy
VAS = Visual analogue scale
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors contributions
DV was a co-investigator on the study, participated in managing and coordinating the study, contributed to analysis and interpretation of the data, and drafted the manuscript revising critically for important intellectual content. MH was the principal investigator on the study, conceived the study design, supervised over all aspects of study implementation, contributed to analysis and interpretation of the data, and revised the manuscript critically for important intellectual content. MN performed the statistical analysis and revised the manuscript critically for important intellectual content. NP supervised all aspects of the statistical analysis and revised the manuscript critically for important intellectual content.
References:
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, et al.:
Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990-2010:
A Systematic Analysis for the Global Burden of Disease Study 2010
Lancet. 2012 (Dec 15); 380 (9859): 21632196Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al.
The rising prevalence of chronic low back pain.
Arch Intern Med. 2009;169(3):2518Haas M, Vavrek D, Peterson D, Polissar N, Neradilek MB.
Dose-response and Efficacy of Spinal Manipulation for Care of Chronic Low Back Pain:
A Randomized Controlled Trial
Spine J. 2014 (Jul 1); 14 (7): 11061116Bronfort G, Haas M, Evans R, Leiniger B, Triano J.
Effectiveness of Manual Therapies: The UK Evidence Report
Chiropractic & Osteopathy 2010 (Feb 25); 18 (1): 3Goertz CM, Pohlman KA, Vining RV, Brantingham JW, Long CR.
Patient-centered Outcomes of High-velocity, Low-amplitude
Spinal Manipulation for Low Back Pain: A Systematic Review
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 670691Vavrek DA, Sharma R, Haas M.
Cost analysis related to doseresponse of spinal manipulative therapy for chronic low back pain: outcomes from a randomized controlled trial.
J Manipulative Physiol Ther. 2014;37(5):30011Flynn T, Fritz J, Whitman J, Wainner R, Magel J, Rendeiro D. et al.
A Clinical Prediction Rule for Classifying Patients with Low Back Pain
who Demonstrate Short-term Improvement with Spinal Manipulation
Spine (Phila Pa 1976). 2002 (Dec 15); 27 (24): 28352843Nyiendo J, Haas M, Goldberg B, Sexton G.
Pain, disability and satisfaction outcomes and predictors of outcomes: a practice-based study of chronic low back pain patients attending primary care and chiropractic physicians.
J Manipulative Physiol Ther. 2001;24(7):4339Haas M, Goldberg B, Aickin M, Ganger B, Attwood M.
A Practice-Based Study of Patients With Acute and Chronic Low Back Pain Attending
Primary Care and Chiropractic Physicians: Two-Week to 48-Month Follow-up
J Manipulative Physiol Ther 2004 (Mar); 27 (3): 160169Cai C, Pua YH, Lim KC.
A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with mechanical lumbar traction.
Eur Spine J. 2009;18(4):55461Hicks GE, Fritz JM, Delitto A, McGill SM.
Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program.
Arch Phys Med Rehabil. 2005;86(9):175362Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al.
Patterns of abuse among unintentional pharmaceutical overdose fatalities.
JAMA. 2008;300(22):261320Graham NA, Gold MS, Goldberger BA.
Prescription opioids and overdose deaths.
JAMA. 2009;301(17):1766Painkillers fuel growth in drug addiction. Opioid overdoses now kill more people than cocaine or heroin.
Harv Ment Health Lett. 2011, 27(7):45Kuehn BM.
Methadone overdose deaths rise with increased prescribing for pain.
JAMA. 2012;308(8):74950Centers for Disease Control and Prevention (CDC)
Vital signs: risk for overdose from methadone used for pain relief - United States, 19992010.
MMWR Morb Mortal Wkly Rep. 2012;61(26):4937Leboeuf-Yde C, Gronstvedt A, Borge JA, Lothe J, Magnesen E, Nilsson O, et al.
The Nordic Back Pain Subpopulation Program: Demographic and Clinical Predictors for Outcome in Patients Receiving Chiropractic Treatment for Persistent LowBack Pain
J Manipulative Physiol Ther. 2004 (Oct); 27 (8): 493502Dougherty, PE, Karuza, J, Savino, D, and Katz, P.
Evaluation of a Modified Clinical Prediction Rule For
Use With Spinal Manipulative Therapy in Patients
With Chronic Low Back Pain:
A Randomized Clinical Trial
Chiropractic & Manual Therapies 2014 (Nov 18); 22 (1): 41Haas M, Vavrek D, Neradilek MB, Polissar N.
A Path Analysis of the Effects of the Doctor-patient Encounter and Expectancy in an Open-label
Randomized Trial of Spinal Manipulation for the Care of Low Back Pain
BMC Complement Altern Med. 2014 (Jan 13); 14: 16Peterson DH, Bergmann TF.
Chiropractic technique: principles and practice.
St. Louis: Mosby; 2002Furlan AD, Brosseau L, Imamura M, Irvin E.
Massage for low back pain: a systematic review within the framework of the Cochrane collaboration back review group.
Spine. 2002;27:1896910Cherkin DC, Deyo RA, Sherman KJ, Hart G, Street JH, Hrbek A, et al.
Characteristics of licensed acupuncturists, chiropractors, massage therapists and naturopathic physicians.
J Am Board Fam Pract. 2002;15:46372Underwood MR, Barnett AG, Vickers MR.
Evaluation of two time-specific back pain outcome measures.
Spine. 1999;24(11):110412R.A. Deyo, S.F. Dworkin, D. Amtmann, G. Andersson, et al.,
Report of the NIH Task Force on Research Standards for Chronic Low Back Pain
Journal of Pain 2014 (Jun); 15 (6): 569585McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD.
Interpreting patient-reported outcome results: US FDA guidance and emerging methods.
Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):1639Fritz JM, Hebert J, Koppenhaver S, Parent E.
Beyond minimally important change: defining a successful outcome of physical therapy for patients with low back pain.
Spine (Phila Pa 1976) 2009;34(25):28039Price DD.
Psychological mechanisms of pain and analgesia.
Seattle, WA: IASP Press; 1999Haas M, Spegman A et al. (2010)
Dose Response and Efficacy of Spinal Manipulation for Chronic Cervicogenic Headache:
A Pilot Randomized Controlled Trial
Spine J. 2010 (Feb); 10 (2): 117128Haas, M., Groupp, E., and Kraemer, D.F.
Dose-response for Chiropractic Care of Chronic Low Back Pain
Spine J 2004 (Sep); 4 (5): 574583Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ.
Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index.
Med Care. 2005;43(00257079; 11):107886van Agt HM, Essink-Bot ML, Krabbe PF, Bonsel GJ.
Test-retest reliability of health state valuations collected with the EuroQol questionnaire.
Soc Sci Med. 1994;39(11):153744Soer R, Reneman MF, Speijer BL, Coppes MH, Vroomen PC.
Clinimetric properties of the EuroQol-5D in patients with chronic low back pain.
Spine J. 2012;12(11):10359Ware JE, Kosinski M, Keller SD.
A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity.
Med Care. 1996;34(3):22033Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, et al.
Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain.
Spine. 2003;28(15):173945Waddell G, Newton M, Henderson I, Somerville D, Main CJ.
A fear-avoidance beliefs questionnaire (FABQ) and the role of fear avoidance beliefs in chronic low back pain and disability.
Pain. 1993;52(2):15768Mayer TG, Tencer AF, Kristoferson S, Mooney V.
Use of noninvasive techniques for quantification of spinal range-of-motion in normal subjects and chronic low-back dysfunction patients.
Spine (Phila Pa 1976) 1984;9(6):58895Loebl WY.
Measurement of spinal posture and range of spinal movement.
Ann Phys Med. 1967;9(3):10310Troup JD, Hood CA, Chapman AE.
Measurements of the sagittal mobility of the lumbar spine and hips.
Ann Phys Med. 1968;9(8):30821Keeley J, Mayer TG, Cox R, Gatchel RJ, Smith J, Mooney V.
Quantification of lumbar function. Part 5: Reliability of range-of-motion measures in the sagittal plane and an in vivo torso rotation measurement technique.
Spine (Phila Pa 1976) 1986;11(1):315Jensen MP, Karoly P, Braver S.
The measurement of clinical pain intensity: a comparison of six methods.
Pain. 1986;27:11726Macrae IF, Wright V.
Measurement of back movement.
Ann Rheum Dis. 1969;28(6):5849Moll JM, Wright V.
Normal range of spinal mobility. An objective clinical study.
Ann Rheum Dis. 1971;30(4):3816Gill K, Krag M, Johnson G, Haugh L, Pope M.
Repeatability of four clinical methods for assessment of lumbar spinal motion.
Spine. 1988;13(1):503Williams R, Binkley J, Bloch R, Goldsmith CH, Minuk T.
Reliability of the modified-modified Schφber and double inclinometer methods for measuring lumbar flexion and extension.
Phys Ther. 1993;73:2637Robinson HS, Mengshoel AM.
Assessments of lumbar flexion range of motion: intertester reliability and concurrent validity of 2 commonly used clinical tests.
Spine (Phila Pa 1976) 2014;39(4):E2705Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al.
A Clinical Prediction Rule To Identify Patients With Low Back Pain Most Likely To Benefit
from Spinal Manipulation: A Validation Study
Annals of Internal Medicine 2004 (Dec 21); 141 (12): 920928Stochkendahl MJ, Christensen HW, Hartvigsen J, Vach W, Haas M, Hestbaek L, et al.
Manual Examination of the Spine: A Systematic Critical
Literature Review of Reproducibility
J Manipulative Physiol Ther 2006 (Jul); 29 (6): 475485Fischer AA.
Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold.
Pain. 1987;30:11526Ohrback R, Gale EN.
Pressure pain thresholds, clinical assessment and differential diagnosis: reliability and validity in patients with myogenic pain.
Pain. 1989;39:15762Cherkin, D.C. and MacCornack, F.A.
Patient Evaluations of Low Back Pain Care From
Family Physicians and Chiropractors
Western Journal of Medicine 1989 (Mar); 150 (3): 351355Cherkin D, Deyo RA, Berg AO.
Evaluation of a physician education intervention to improve primary care for low-back pain: II. Impact on patients.
Spine. 1991;16(10):11738Liang KY, Zeger SL.
Longitudinal data analysis using generalized linear models.
Biometrika. 1986;71(1):13Zeger SL, Liang KY.
Longitudinal data analysis for discrete and continuous outcomes.
Biometrics. 1986;42(1):12130Diggle PJ, Heagerty PJ, Liang KY, Zeger SL.
Analysis of Longitudinal Data. 2.
New York: Oxford University Press; 2002Hocking RR.
A biometrics invited paper. The analysis and selection of variables in linear regression.
Biometrics. 1976;32(1):149Proceedings of the Proceedings of Graphics Interface 2005:
Victoria, British Columbia. School of Computer Science,
University of Waterloo, Waterloo, Ontario, Canada:
Canadian Human-Computer Communications Society; 2005Weisberg S.
Applied linear regression. 3.
Hoboken, N.J.: Wiley-Interscience; 2005Kuhn M, Johnson K.
Applied predictive modeling.
New York: Springer; 2013Axen I, Rosenbaum A, Robech R, Larsen K, Leboeuf-Yde C.
The Nordic Back Pain Subpopulation Program: Can Patient Reactions
to the First Chiropractic Treatment Predict Early Favorable
Treatment Outcome in Nonpersistent Low Back Pain?
J Manipulative Physiol Ther. 2005 (Mar); 28 (3): 153158Axen I, Jones JJ, Rosenbaum A, Lovgren PW, Halasz L, Larsen K, et al.
The Nordic Back Pain Subpopulation Program: Validation and Improvement of a Predictive Model
for Treatment Outcome in Patients With Low Back Pain Receiving Chiropractic Treatment
J Manipulative Physiol Ther. 2005 (Jul); 28 (6): 381385Stanton TR, Hancock MJ, Maher CG, Koes BW.
Critical appraisal of clinical prediction rules that aim to optimize treatment selection for musculoskeletal conditions.
Phys Ther. 2010;90(6):84354Christensen MG, Kollasch MW, Hyland JK.
Practice analysis of chiropractic 2010.
Greeley Colorado: National Board of Chiropractic Examiners; 2010.

Return to LOW BACK PAIN
Return to CLINICAL PREDICTION RULE
Since 11-20-2015


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |