Antioxidants in Cancer Therapy:
Their Actions and Interactions
With Oncologic TherapiesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Alternative Medicine Review 1999 (Oct); 4 (5): 304–329 ~ FULL TEXT
Davis W. Lamson, MS, ND and Matthew S. Brignall, NDAbstract
There is a concern that antioxidants might reduce oxidizing free radicals created by radiotherapy and some forms of chemotherapy, and thereby decrease the effectiveness of the therapy. The question has arisen whether concurrent administration of oral antioxidants is contraindicated during cancer therapeutics. Evidence reviewed here demonstrates exogenous antioxidants alone produce beneficial effects in various cancers, and except for a few specific cases, animal and human studies demonstrate no reduction of efficacy of chemotherapy or radiation when given with antioxidants. In fact, considerable data exists showing increased effectiveness of many cancer therapeutic agents, as well as a decrease in adverse effects, when given concurrently with antioxidants.
Introduction
Dietary and endogenous antioxidants prevent cellular damage by reacting with and eliminating oxidizing free radicals. However, in cancer treatment, a mode of action of certain chemotherapeutic agents involves the generation of free radicals to cause cellular damage and necrosis of malignant cells. So a concern has logically developed as to whether exogenous antioxidant compounds taken concurrently during chemotherapy could reduce the beneficial effect of chemotherapy on malignant cells. The importance of this concern is underlined by a recent study which estimates 23 percent of cancer patients take antioxidants. [1]
The study of antioxidant use in cancer treatment is a rapidly evolving area. Antioxidants have been extensively studied for their ability to prevent cancer in humans. [2] This paper reviews the use of antioxidants as a therapeutic intervention in cancer patients, and their potential interactions with radiation and chemotherapy. There has been significant investigation of this area, with promising findings which indicate continuing investigation is warranted. For further discussion of the use of antioxidants as sole cancer therapy, refer to the review article by Prasad published earlier this year. [3] A number of reports show a reduction in adverse effects of chemotherapy when given concurrently with antioxidants. These data are more completely summarized by Weijl et al. [4]
Conflicting Views of Antioxidant Use in Cancer Therapy
It was suggested in a recent publication that no supplementary antioxidants be given concurrently with chemotherapy agents which employ a free radical mechanism. [5] The paper must be commended for pointing out that the combination of antioxidants and chemotherapy agents needs more investigation, and should serve as a wake-up call regarding how much we need further definition of the actions of specific antioxidants with chemotherapeutic agents. However, it should not serve as scientific closure on an adjunctive treatment of possible great promise in cancer therapy.
The present authors are by no means recommending any lack of caution about use of antioxidants. On the contrary, published research indicates the cautious and judicious use of a number of antioxidants can be helpful in the treatment of cancer; as sole agents and as adjuncts to standard radiation and chemotherapy protocols.
It was suggested that antioxidants might interfere with the oxidative mechanisms of alkylating agents. [5] These drugs create substantial DNA damage, resulting in cell necrosis. However, recent evidence indicates a sizeable amount of chemotherapy damage is by other mechanisms, which trigger apoptosis. [6] Antioxidants have been shown to increase cell death by this mechanism. [7,8] Given this, any argument that antioxidants are likely to interfere with most chemotherapy is too simplistic and probably untrue.
Numerous animal studies have been published demonstrating decreased tumor size and/or increased longevity with the combination of chemotherapy and antioxidants. [7,9-16] A recent study was conducted on small-cell lung cancer in humans using combination chemotherapy of cyclophosphamide, Adriamycin (doxorubicin), and vincristine with radiation and a combination of antioxidants, vitamins, trace elements, and fatty acids. The conclusion was "antioxidant treatment, in combination with chemotherapy and irradiation, prolonged the survival time of patients" compared to expected outcome without the composite oral therapy. [17] Two human studies found melatonin plus chemotherapy to induce greater tumor response than chemotherapy alone. [18,19] The treatments producing these positive results would have been advised against by those advocating no antioxidant use during chemotherapy. These studies will be discussed in more detail below.
It is the opinion of the authors of this paper that interactions between antioxidants and chemotherapeutics cannot be predicted solely on the basis of presumed mechanism of action. The fact remains that physicians must be aware of the available research to help their patients take advantage of positive interactions existing between antioxidants and chemotherapy or radiation.
Additionally, physicians need to remain aware of the large body of evidence showing a positive effect of antioxidants in the period following chemotherapy administration. The general protocol with standard oncologic therapies is to follow a watch-and-wait strategy after therapeutic administration is concluded. This is a period when supplemental therapies are highly indicated and have been demonstrated to result in a higher percentage of successful outcomes. [20,21]
Overview of Cancer Therapeutic Agents
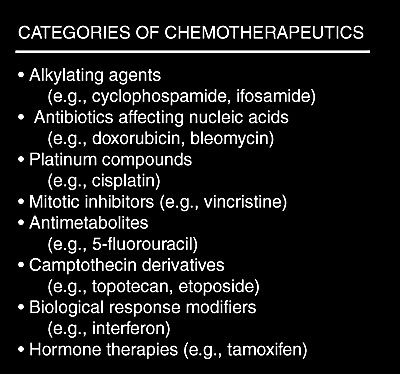
Chemotherapy agents can be divided into several categories: alkylating agents (e.g., cyclophosphamide, ifosfamide), antibiotics which affect nucleic acids (e.g., doxorubicin, bleomycin), platinum compounds (e.g., cisplatin), mitotic inhibitors (e.g., vincristine), antimetabolites (e.g., 5-fluorouracil), camptothecin derivatives (e.g., topotecan), biological response modifiers (e.g., interferon), and hormone therapies (e.g., tamoxifen).The agents most noted for creating cellular damage by initiating free radical oxidants are the alkylating agents, the tumor antibiotics, and the platinum compounds. The agents in these categories demand definition concerning interactions with antioxidants which might reduce effectiveness of chemotherapy. There is also the possibility of adverse interaction between antioxidant treatment and agents that do not act via an oxidative mechanism (e.g., 5-fluorouracil or tamoxifen).
In addition to the idea that chemotherapy must create a lethal injury to DNA to produce malignant cell death is the mechanism of apoptosis. A dose of chemotherapy which does not produce necrosis can trigger apoptosis, either immediate or delayed. Additionally, anti-apoptotic mutations can result in drug resistance in human tumors. At least one antioxidant (quercetin) has been demonstrated to overcome such an anti-apoptotic blockage. [22]
Radiotherapy uses ionizing radiation to produce cell death through free radical formation. Two mechanisms are involved. The apoptosis mechanism results in cell death within a few hours of radiation. The second mechanism is radiation-induced failure of mitosis and the inhibition of cellular proliferation, which kills cancer cells. Currently, the principal target of radiation is considered to be cellular DNA. However, studies show the signal for apoptosis can be generated by the effect of radiation on cell membranes, apparently through lipid peroxidation. This suggests an alternate mechanism to the hypothesis that DNA damage is required for cell death. [23]
Categories of Chemotherapeutics
Vitamin A and Carotenoids as Cancer Treatment
Many research reports on the anti-cancer properties of vitamin A and the related retinoids have been published over the last 20 years. Most of these studies examined all-trans retinoic acid (RA). RA is formed in human tissues from beta-carotene and retinol, does not accumulate in the liver, thus it is not associated with significant hepatotoxicity. [24] Treatment with RA is associated with many side effects, including headache, lethargy, anorexia, vomiting, and visual disturbance. [24] Another retinoid used in cancer treatment is 13-Cis-retinoic acid (cRA), also known as isotretinoin. [25]
RA in vitro demonstrates growth inhibitory activity against at least 14 types of human cancers. [24] Acute promyelocytic leukemia (APL) has been shown to respond well to RA, but not to cRA. [26] In one study, nine of 11 patients with APL entered complete remission after treatment with 45 mg/m2 daily oral dose of RA. [27] Similar results are reported elsewhere, [28,29] and have been confirmed in vitro. [30]
Local application of an RA-containing cream demonstrated low toxicity and some histological improvement of cervical intraepithelial neoplasia II (CIN II) in a phase I study. [31]In a phase III trial, RA led to complete regression of CIN II in 42 percent of women compared with 27 percent in the placebo group. [32] No significant effect was noted in severe cervical dysplasia. [32] After remission induced by conventional therapy, treatment with cRA is associated with fewer second primary tumors in head and neck squamous-cell carcinoma. [33]
Retinoic acid decreased the growth rate and increased differentiation of human small cell lung cancer lines in vitro. [34 ]Daily oral administration of 300,000 IU vitamin A as retinol palmitate led to a significant reduction in second primary tumors and an increase in disease-free survival post-surgery in stage I lung cancer. [35] However, a small trial of cRA at 200 mg/day found no appreciable benefit in the treatment of advanced non-small cell lung cancer. Of 23 patients evaluated in this trial, only one achieved a partial response to treatment. [36]
A trial of oral vitamin A at 100,000 IU/day in patients with resected malignant melanoma found no survival benefit compared with those taking placebo. [37] In a trial of oral RA for hormone-refractory prostate cancer, dosed 45 mg/m2 daily, only a 15-percent response rate was seen. [38 ]It is clear from these data that the effects of the retinoids as sole therapeutic agents are limited, perhaps mainly to hematologic malignancies, which tend to develop RA resistance over time. [28 ]For further information on the use of retinoids in cancer therapy, refer to the review by MA Smith, et al. [24]
In contrast to the retinoids, comparatively little is known about the use of carotenoids as anti-cancer agents in vivo. The interest in carotenoids mainly stems from the extensive epidemiological evidence associating dietary intake with lower incidences of many cancer types. [39] Alpha- and beta-carotene have been examined for in vitro tumor inhibitory activity against human neuroblastoma cell lines, and alpha-carotene was found to have 10 times the anti-tumor activity of beta-carotene. [40] Currently there is some concern regarding supplementation with carotenoids, [41] as beta-carotene has been associated with higher risk of lung cancer in smokers, but not in the general population. [42] Aside from this concern, high doses of beta-carotene, even over long periods of time, are not associated with serious toxicity. [39] There are also promising data showing chemopreventative activity of the carotenoid lycopene against prostate cancer. [43] In vitro work suggests lycopene can induce differentiation, with vitamin D3, in human leukemia cells. [44] One study showed lycopene to be a stronger inhibitor of human cancer cell proliferation in vitro than alpha- or beta-carotene. [45] As yet, human trials are lacking on the use of lycopene.
Vitamin A and Carotenoids with Radiation
Evidence exists to support the use of retinoids concomitantly with radiotherapy. In vitro studies have shown retinoic acid (RA) causes radiosensitization in human tumor cell lines at concentrations which do not cause cellular toxicity. This effect was reversible with removal of RA. [46] In mice bearing human breast adenocarcinoma tumor lines, the effect of local radiation was enhanced by supplemental vitamin A (150,000 IU) and beta-carotene (90 mg/kg) given during treatment. The beneficial effect of the supplemental treatment was noted as decreased tumor size and increased survival time. Supplemental vitamin A and beta-carotene plus radiation had significantly greater anti-tumor effect than radiation or supplementation alone. The effect of vitamin A was not significantly different from beta-carotene. [9]
In a randomized trial of oral vitamin A (1.5 million IU/day) plus radiotherapy for advanced cervical cancer, vitamin A plus radiotherapy significantly increased T-cell response and non-significantly reduced relapse rates compared with those undergoing radiotherapy only. [46] A pilot human study of cis-retinoic acid (cRA) with radiotherapy and interferon-a2a on locally advanced cervical cancer noted a 47-percent tumor response and 33-percent complete remission rate, with no grade 3 or 4 toxicity noted. Historical controls without cRA treatment had a 42-percent tumor response rate and only 17-percent complete remissions. [47] The ability of vitamin A to increase tumor response to radiation while reducing toxicity has been theorized to be due to the stimulation of immune response to tumor tissue. [48]
In a human study, beta-carotene at 75 mg daily during radiation treatment for advanced squamous cell carcinoma of the mouth significantly reduced the incidence of severe mucositis reactions without causing noticeable side effects. The remission rate was unchanged by beta-carotene treatment.49 In vitro evidence suggests synthetic beta-carotene does not have the radioprotective effect noted with the natural form. [50] The meaning of this finding is as yet unclear.
Vitamin A and Carotenoids with Chemotherapy
Perhaps more than any other antioxidant treatment, retinoids are increasingly being pursued as adjunctive treatment to standard chemotherapeutics. Most evidence suggests an increased cytotoxic effect with reduced toxicity. In vitro studies using human small cell lung cancer lines demonstrated that incubation with retinoic acid (RA) led to an increased sensitivity to etoposide, but more resistance to doxorubicin. [51] Human synovial sarcoma cells exposed to RA in vitro were found to have enhanced response to doxorubicin, vincristine, and especially cisplatin. [52] Although the potential adverse interaction with doxorubicin was not confirmed in the latter study, this is an area that merits further definition.
In studies of mice with transplanted human breast tumor tissue, concurrent treatment of either vitamin A or beta-carotene with cyclophosphamide led to a significantly greater tumor response and survival time compared to cyclophosphamide treatment alone. The effect of beta-carotene was roughly equivalent to that of vitamin A.9 Also in mice, co-administration of vitamin A with methotrexate ameliorated intestinal damage, without inhibiting its in vivo anti-tumor activity. [53 ]
In a phase I human trial of cisplatin with 13-cis-retinoic acid (cRA), the two agents were noted to have strong synergism against head and neck squamous cell carcinoma. Of 10 evaluable patients, all had complete tumor response at the primary site. Dosages of 20 mg/day cRA were well tolerated, but severe toxicities were seen at 40 mg/day.54 Extremely high oral doses of RA (150 mg/m2 daily) showed no inhibitory effect on the activity of cisplatin and etoposide on small cell lung carcinoma in humans. This dose also was not associated with any therapeutic benefit, and needed to be discontinued in a majority of patients due to side effects . [55] Vitamin A palmitate at an oral dose of 50,000 IU twice daily, plus b-interferon and combined chemotherapy (epirubicin, mitomycin C, and 5-fluorouracil) prolonged symptom palliation in 35 percent of pancreatic cancer patients. This treatment was associated with severe toxicities in several systems, but only hepatotoxicity was thought to be associated with the addition of retinoids. [56] Sequential treatment of non-lymphocytic leukemia patients with conventional chemotherapy, followed by 16,000 IU/day of retinol palmitate led to a further induction of maturation in blast cells than seen with chemotherapy alone. In three of four patients undergoing this sequential therapy, complete remission resulted. [57] Addition of 400,000 IU/week vitamin A to a conventional chemotherapy regimen (doxorubicin, bleomycin, 5-fluorouracil, and methotrexate) led to improved survival with less than the expected severity of side effects compared with historical controls. [10]
Although the relative lack of toxicity compared to the retinoids makes it an attractive option, beta-carotene in combination with chemotherapy is a largely unexplored area. In mice, beta-carotene co-administration led to increased tumor growth delay with doxorubicin and etoposide, and increased tumor cell killing with cyclophosphamide in solid tumors. The co-administration of beta-carotene and 5-fluorouracil, however, reduced tumor growth delay in murine fibrosarcomas, but not in squamous cell carcinomas. [58] Data on other carotenoids is lacking.
Vitamin A Summary
Vitamin C as Cancer Treatment
The use of vitamin C in the treatment of cancer has been the source of many claims and controversies over the last 25 years. Initial reports from Drs. Pauling and Cameron were promising, and gained much notoriety. They reported 100 cases of terminal cancer, independently assessed and refractory to conventional treatment, who lived on average four times longer than 1000 age- and disease-matched controls. [59] The protocol included intravenous and oral administration, and is described in detail elsewhere. [60]Prospective randomized trials held at the Mayo Clinic were unable to replicate these results, finding negligible difference between treated patients and controls in survival time. [61,] [62 ]These results were criticized on a number of grounds, including the noticeable difference between the vitamin C and placebo, lack of intravenous administration, and termination of treatment with tumor progression. [60 ]Later in vitro and in vivo research, and well documented case reports, 63 suggest a vitamin C dose much higher than that used in the Pauling/Cameron studies can actually be cytotoxic to tumors without damaging normal cells. The required tissue concentrations are thought to only be reachable with intravenous doses over long periods of time. This research, as well as the proposed mechanism of action, is examined elsewhere. [64] Vitamin C is generally well-tolerated by healthy people, even in doses as high as 200 g/day IV. [64,] [65 ]Dr. Cameron has noted that a small percentage of cancer patients will respond to vitamin C with rapidly proliferating and disseminating tumors. [66] Other investigators have not noted this effect.
Vitamin C with Radiation
Quite surprisingly, no published studies have looked at the effect of doses over five grams of oral or intravenous vitamin C on radiotherapy in humans. It has been shown, however, that cancer patients have a significant elevation in plasma and leukocyte ascorbate levels after radiotherapy compared with pretreatment levels without any change in dietary intake. [67]
In mice, vitamin C (1 g/kg), given intraperitoneally with vitamin K3 (10 mg/kg), increased the therapeutic effect of radiation on solid tumors without causing any signs of toxicity due to the vitamins. [68] In another mouse study, a single intraperitoneal dose of 4.5 g/kg vitamin C was not cytotoxic to normal tissue and did not change the radiation effect on tumor tissue. The lethal dose of radiation increased and skin desquamation reaction was reduced by ascorbate treatment. It should be noted that these vitamin C doses are much greater than have been used historically in humans. [69] The radioprotection of healthy tissue and radiosensitizing effect in tumors with use of ascorbate were confirmed in two other mouse tumor models. [70,] [71]
A randomized trial with 50 human subjects looked at the effect of concurrent vitamin C (five daily doses of 1 g each) and radiotherapy on different tumor types. More complete responses to radiation were noted in the vitamin C group at one month (87% to 55%) and four months (63% to 45%) post treatment. Side effects tended to be fewer in the ascorbate-treated subjects as well. Plasma levels of ascorbate in the treatment group were greater than control subjects, but less than the mean of 20 healthy subjects tested. [72] It remains to be investigated whether continuing treatment beyond the end of radiotherapy or use of a higher dose would improve these results. A double-blind trial of topical vitamin C solution for the prevention of radiation dermatitis failed to find any beneficial effect. The trial did not examine the absorption of the aqueous preparation, although previous trials showed about 12 percent of the vitamin C penetrated into the epidermis. [73]
Vitamin C with Chemotherapy
Vitamin C has been extensively tested in vitro and in vivo for its ability to prevent the adverse effects of, decrease resistance to, and increase the effects of chemotherapeutic agents. Co-treatment with doxorubicin and vitamin C (2 mg/kg) led to a reduction in the toxicity seen with doxorubicin alone in mice and guinea pigs. The prevention of cardiomyopathy was confirmed by electron microscopy. Treatment with ascorbic acid was not associated with decreased effect of doxorubicin, and was associated with an increased life span compared with doxorubicin treatment alone. [11] In vitro experiments do suggest, however, that vitamin C does enhance doxorubicin resistance in human breast cancer cell lines already known to be resistant. It did not lead to resistance in cells which were doxorubicin-sensitive. [74] Vitamin C at non-cytotoxic concentrations (1 mM) increased the activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. This effect was particularly marked and synergistic with doxorubicin. The authors note that since vitamin C has already shown an ability to reduce the cardiotoxicity of doxorubicin, ascorbic acid and doxorubicin are an attractive future treatment for breast cancer. [75]
Vitamin C has been shown to increase the drug accumulation and decrease resistance to vincristine in human non-small-cell lung cancer cells in vitro. An ascorbic acid-sensitive uptake mechanism was theorized to explain these results. [76]
Combined intraperitoneal administration of vitamin C (1g/kg) and vitamin K (10 mg/kg) given prior to chemotherapy increased survival and the effect of several chemotherapeutic agents (cyclophosphamide, vinblastine, doxorubicin, 5-fluorouracil, procarbazine, and asparaginase) in a murine ascitic liver tumor model. The vitamin combination did not increase the toxicity of these agents to healthy tissue. Splenic and thymic weights of the vitamin-treated animals were higher than those receiving cytotoxic treatment alone, suggesting an immune-stimulating action of the vitamins.12 These results have yet to be confirmed in humans.
Vitamin C Summary
Vitamin E as Cancer Treatment
Vitamin E succinate (VES, alpha tocopherol succinate),has generated some interest as an adjunctive cancer therapy recently. VES demonstrated growth inhibition of human B-cell lymphoma77 and estrogen receptor-negative breast cancer78 cell lines in vitro. Vitamin E at 3 mM concentration arrested tumor cells in the G1 phase of the cell cycle, leading to apoptosis. [7] Recent research on human oral squamous carcinoma cells suggests the VES effect is biphasic; growth stimulatory at physiological concentrations, while pharmacological concentrations are inhibitory. [79] A phase I trial of intravenous vitamin E in treatment refractory neuroblastoma found mild toxicity (tendency toward increased bleeding time was noted) at doses below 2,300 mg/m2. Five of 13 patients experienced pain relief and/or tumor regression with treatment. No complete remissions resulted from treatment. [80] Vitamin E, 200 mg daily, given together with 18 g/day omega-3 fatty acids from fish oil, prolonged survival in patients with generalized malignancy in a randomized controlled trial. Improvement in T-helper/suppressor ratio was also noted with treatment. [81] Phase I clinical trials are being planned or are underway in patients with breast and prostate cancers. [82] Vitamin E and its derivatives are particularly attractive therapeutic agents due to their remarkable lack of toxicity in vivo. [83]
Vitamin E with Radiation
The picture here is unfortunately far from clear. An initial report showed mice treated with 1 g/kg of vitamin E had an increased in the lethal radiation dose (LD50). Unfortunately, squamous cell carcinoma cell lines treated in this study were less radiosensitive, with 35-percent cell survival versus 13 percent in controls. [84] A later experiment was able to replicate this finding in vitro in cells incubated for several weeks with vitamin E, but not those in which it was added immediately before irradiation. [85] The latest experiment to look at this issue actually found that some doses of vitamin E enhanced mouse sarcoma tumor cell kill. Intraperitoneal pretreatment with 50, 250, and 500 mg/kg, but not 1000 mg/kg, led to better tumor response than radiation alone. The authors also noted that intramuscular and oral tocopherol administration had a similar effect. [86] From these results it would appear vitamin E doses used in humans increase the effect of radiotherapy, and super-human doses (above 35,000 IU) may blunt the therapeutic efficacy of radiotherapy.
Radiation-induced fibrosis is a sequela to irradiation therapy which does not spontaneously regress. A combination of vitamin E (1000 IU/day) and pentoxifylline (800 mg/day) completely reversed a case of radiation-induced cervicothoracic fibrosis in a 67-year-old woman after an 18-month course of treatment. The findings were confirmed with CT scan. A phase II trial is currently underway to confirm these results. [87]
Vitamin E with Chemotherapy
There are a few interesting recent reports on the concurrent use of vitamin E with chemotherapy. Vitamin E, 750 mg/kg intraperitoneally, given with 5-fluorouracil had a greater anti-tumor effect in mice bearing human colon cancer lines than either agent alone; treatment led to complete cessation of tumor growth. The same investigators found in vitro addition of vitamin E to either 5-fluorouracil or doxorubicin enhances the effect of these agents on human colon cancer cells. [7] Another report showed pre-treatment with 85 mg (approximately 4000 mg/kg) alpha-tocopherol reduced the lethality of a single 15 mg/kg dose of doxorubicin from 85 percent to 10 percent in mice. This dose of tocopherol did not alter the suppression of tumor cell DNA synthesis by doxorubicin. The tumor-bearing mice pretreated with vitamin E lived longer on average than those treated with doxorubicin alone. The authors theorized the vitamin E blocked lipid peroxidation-mediated toxicity, while not impairing the anti-tumor property of doxorubicin.14 Both the toxicity prevention effect and the lack of inhibition of vitamin E toward doxorubicin were confirmed in a later experiment. [89] In vitro experiments showed VES can enhance the cytotoxic effect of doxorubicin on human prostate cancer cells at concentrations easily attained in human plasma (5 mg/ml). This inhibition was found to be dose-dependent. [89 ]Oral and intraperitoneal administration of vitamin E (20 mg/kg/day) enhanced the anti-tumor activity of cisplatin on neuroblastoma in mice. [90]
Vitamin E Summary
Selenium as Cancer Treatment
The use of selenium compounds as a cancer treatment predates most conventional treatments currently in use. [91] In spite of this, comparatively little is known regarding the use of selenium as a cancer therapy in living systems. Subcutaneous injection of 2 mcg/g selenium into tumor-bearing mice led to a 75-percent reduction in tumor mass compared to controls. [92] This inhibitory effect of selenium was confirmed in human breast cancer cells in vitro. [93] In an open trial of 32 patients with treatment refractory brain tumors, intravenous infusion of selenium (1000 mcg/day for 4-8 weeks) was associated with a slight to definite improvement in all participants. Symptomatic decrease was seen in nausea, emesis, headache, vertigo, and seizure activity. Although the results are largely credited to the selenium treatment, it should be noted these patients were concurrently receiving chemotherapy, oxygen therapy, vitamins E and A, dietary changes, and psychotherapy. [94] Unpublished research from the 1950s outlines the treatment of over 1000 malignancies with selenium compounds, reportedly with beneficial results. [95] Unfortunately, a study of this magnitude has yet to appear in the peer-reviewed literature.
Selenium with Radiation
Little is known about the interaction between selenium supplementation and radiotherapy. In the one human trial available, patients with advanced rectal cancer were given daily supplementation with 400 mcg of selenium after treatment. The selenium was well-tolerated, but the researchers presented no data regarding interaction between the two treatments. [96] An animal study suggests that selenium depletion reduces the lethal dose of radiation. [97] Until more is known regarding the effect of selenium on radiotherapy, pharmacological doses (above 400 mcg/day) cannot be advised.
Selenium with Chemotherapy
Interactions between selenium and platinum-containing chemotherapy agents have been extensively studied. In a mouse study, selenium decreased nephrotoxicity of cisplatin, while simultaneously increasing its anti-tumor activity. [15] Other animal studies confirmed these findings. [16,98] A randomized crossover trial in humans looked at the effect of selenium (4000 mcg/day from four days before until four days post-chemotherapy) on the toxicity of cisplatin. Selenium consumption was associated with a higher WBC count, even with less consumption of granulocyte stimulating factor. Nephrotoxicity, measured by urine enzymes, was also significantly less in patients taking selenium. No mention is made in this study of any effect of selenium intake on the therapeutic activity of cisplatin. [99]
One in vitro study suggests a selenium-containing antioxidant compound called Ebselen (2-phenyl-1,3-benzisoselenazol-3(2H)one) has a mild inhibitory effect on the anti-tumor effect of bleomycin. The authors did not speculate on whether dietary selenium would have an adverse effect on therapeutic use of bleomycin. [100] Perhaps until these results are followed up, it would be best to avoid this combination.
Selenium Summary
Coenzyme Q10 as Cancer Treatment
A series of case reports from the Institute for Biomedical Research at the University of Texas at Austin describe the therapeutic benefit of coenzyme Q10 (CoQ10) in cancer patients. These investigators have noted tumor regressions and long-term survival associated with oral CoQ10, at doses from 90 to 390 mg/day. [101,102] This same group used 90 mg CoQ10/day, combined with other antioxidants (vitamin C 2850 mg, vitamin E 2500 IU, b-carotene 32.5 IU, selenium 387 mcg) and 3.5 g omega-3 fatty acids, in an open trial in node-positive breast cancer patients. Patients also underwent conventional treatment. The investigators observed no distant metastasis in any patient, and partial remission in six of 32 patients. No patients died during the 18-month study period. The lack of a control group makes these data hard to interpret. [103]
CoQ10 with Radiation
A 1998 study warns that CoQ10 reduces the effect of radiotherapy on small-cell lung cancer in mice. This trial did indeed show a significant inhibition of radiation-induced cell growth delay at 40 mg/kg oral dose, and a borderline inhibition at 20 mg/kg. However, no inhibitory effect on radiotherapy was noted at 10 mg/kg CoQ10, a dose roughly equivalent to 700 mg in an adult human. [104] Based on this, the normal human dose of CoQ10 of 100-400 mg/day probably has little inhibitory effect on concurrent radiotherapy.
CoQ10 with Chemotherapy
A number of studies have looked at the capacity of CoQ10 to prevent the cardiac toxicity associated with doxorubicin. A small study in humans showed CoQ10 administration at 1 mg/kg led to an over 20-percent reduction in episodes of ECG change post-treatment compared with doxorubicin alone. Diarrhea and stomatitis were also significantly reduced. [105] A mouse study confirms the protective effect of CoQ10 treatment on the toxicity of doxorubicin. In this study, it was noted that CoQ10 did not reduce the anti-tumor effect of doxorubicin. Instead, a trend toward better tumor control was seen. [106] In a study of 20 leukemia patients undergoing treatment with the similar agent daunorubicin, 100 mg CoQ10 twice daily was able to significantly reduce adverse cardiac events as measured by echocardiography. No mention was made of the effect of CoQ10 treatment on the therapeutic benefit of daunorubicin chemotherapy. [107]
CoQ10 Summary
Melatonin as Cancer Treatment
Although it is not usually considered a standard antioxidant, melatonin, the hormone secreted by the pineal gland in response to cycles of light and dark, has exhibited potent free radical-scavenging properties against hydroxyl and peroxyl radicals. [108]
Melatonin has also been found to have some interesting anti-tumor properties in vitro. It increases p53 expression in breast cancer cells, and therefore significantly reduces cell proliferation.8 Impaired p53 expression is associated with many human cancers. [109] Melatonin is also known to modify many cytokines, including TNF, IL1, IL-2, IL-6, and gamma-interferon, in ways consistent with increased host defense against cancers. [110] Melatonin, perhaps through reduction of TNF secretion, has been shown to reduce cachexia in patients with metastatic solid tumors. Patients taking melatonin (20 mg/day) were found to have significantly less weight loss (3 kg vs. 16 kg) and disease progression (53% vs. 90%) than those treated with supportive care alone. [111]
In another study, 63 patients with non-small cell lung cancer refractory to cisplatin therapy were randomized to receive either 10 mg/day of melatonin or supportive care alone. Patients receiving melatonin lived longer on average than those receiving supportive care alone (6 vs. 3 months) and were more likely to survive for one year (8/31 survivors vs. 2/32). No drug-related toxicity was noted by the authors. [112] Treatment with melatonin (20 mg/day) was also associated with greater one-year survival than supportive care alone in patients with brain metastases. [113] Other studies have noted increased survival in malignant melanoma [114] and patients with metastatic disease. [115] The latter study stressed that based in its effects on the immune system, melatonin could be tested in association with other anti-tumor treatments. [115] The DiBella multitherapy of cancer, of which melatonin is a part (along with many other agents), was found not to have sufficient efficacy against advanced cancer to warrant further investigation. [116] Animal experiments suggest doses as high as 250 mg/kg are non-toxic. [117]
Melatonin with Radiation
In a randomized trial including 30 patients with glioblastoma, the effect of radiotherapy plus 20 mg/day of melatonin was compared to that of radiotherapy alone. At the end of one year, six of the 14 patients receiving melatonin were still living, compared to one of the 16 undergoing radiotherapy alone. The authors also noted fewer side effects from radiotherapy in patients taking melatonin. [118]
Melatonin with Chemotherapy
Melatonin has been studied a number of times as an adjunct to standard chemotherapy in humans. A phase II study used tamoxifen plus melatonin (20 mg/day) in the treatment of metastatic breast cancer which had progressed under treatment with tamoxifen alone. Four of the 14 patients tested had partial response to this combination, with a median of eight months before disease progression. Treatment was well-tolerated and relief of anxiety or depression was noted by many patients. [19] A similar study was conducted using the same combination of treatments in patients with metastatic solid tumors other than breast cancer which had not responded to previous chemotherapies. Partial response or stable disease was seen in 16/25 patients. One year survival was seen in 7/25 patients. [119]
In another phase II study, melatonin (20 mg/day) led to a normalization of platelet counts in nine of twelve breast cancer patients who acquired thrombocytopenia during epirubicin therapy. Objective tumor regression was noted in five of the 12 patients. [120] A randomized trial investigated the difference between melatonin (20 mg/day), cisplatin, and etoposide, and treatment with cisplatin and etoposide alone in advanced non-small cell lung cancer. One-year survival was significantly higher in patients receiving adjunctive melatonin compared to standard chemotherapy alone (15 of 34 vs. 7 of 36). There was a non-significant trend toward greater tumor response in melatonin-treated patients as well (11 of 34 vs. 6 of 36). Myelosuppression, neuropathy, and cachexia were noted less frequently in patients receiving melatonin than in those that were receiving only chemotherapy. [121 ]A double-blind trial was unable to replicate this protective effect of melatonin on the myelosuppression mediated by carboplatin and etoposide. This may reflect the effect higher doses of chemotherapeutic agents given in the second trial. The authors concluded that potentiation of the effect of chemotherapy by melatonin was unlikely. [122] Concomitant therapy with melatonin (40 mg/day) has been found to increase the effect of interleukin-2 against a variety of solid cancers. [123] The combination of melatonin (40 mg/day) and interleukin-2 has been found to be a more effective treatment than cisplatin and etoposide in non-small cell lung cancer. [124]
Melatonin Summary
N-acetylcysteine as Cancer Treatment
We were unable to locate research demonstrating N-acetylcysteine (NAC) as an anti-tumor agent. NAC is known to be safe in doses well above that used in most human trials. Diarrhea is the most commonly reported side effect of higher doses. [125]
N-acetylcysteine with Radiation
The effect of NAC on radiotherapy was observed in 10 patients with non-small cell lung cancer. NAC was administered by IV (100 mg/kg over 30 minutes) before the first radiation session, followed by 30 mg/kg IV over the next seven hours. They then inhaled a nebulized solution containing 600 mg NAC 30 minutes prior to and after each subsequent radiation treatment. Patients receiving NAC had tissue reactions and tumor responses from radiotherapy judged to be similar to a control group. Average survival time was similar between patients receiving NAC treatment and those who underwent radiation only. The authors concluded the treatment outcome did not justify the expense. [126]An in vitro experiment showed that NAC is not likely to block the tumor cell killing effect of radiation. [127 ]Application of gauze soaked in 10-percent NAC solution to the skin 15 minutes before radiotherapy was tested in an unblinded trial. Topical NAC appeared to be associated with more rapid healing and less use of analgesics compared with those in the control group. [128]
N-acetylcysteine with Chemotherapy NAC has been employed with a number of chemotherapy agents as a means of reducing toxicity. It has gained such recognition in this regard that it is often used in clinical trials as an adjunct to the therapy being tested. Animal studies have shown NAC protects against hematuria resulting from cyclophosphamide therapy without reducing its tumoricidal effect. [129-131] A phase I human trial found 6 g/day NAC completely protected against hematuria, which is a dose-limiting side effect of ifosfamide (an analogue of cyclophosphamide). [132] Another human study found similar results. [133]
Human trials with dosages as high as 140 mg/kg NAC were unable to show any prevention of cardiomyopathy due to treatment with doxorubicin. One of these trials also noted that NAC treatment was not associated with a reduction of the anti-tumor action of doxorubicin, [134,135] and a mouse study concurred. This study also noted prevention of cardiotoxicity, which as noted above, was not replicated in human studies. [136] Another animal study raises the possibility of a reduction of the anti-neoplastic action of doxorubicin by NAC. NAC did, however, lead to a significant reduction in cardiac toxicity. [137]As data on the subject of doxorubicin with NAC are currently conflicting, this combination might best be avoided at this time.
It has been shown in two separate in vitro studies that NAC inhibits the cytotoxic activity of cisplatin. [138,139] NAC may have a role, however, in the reversal of renal toxicity due to cisplatin. [140] Other than use in salvage therapy, the combination of cisplatin and NAC should also probably be avoided at this time.
N-acetylcysteine Summary
Glutathione as Cancer Treatment
Glutathione is a tri-peptide thiol (sulfhydryl-containing) compound which is the major intracellular antioxidant in the body. A human study suggests oral glutathione is poorly absorbed, with negligible plasma concentrations found after administration of a single 3 g oral dose. [141] This conclusion is contradicted by a rat study which found dietary glutathione was absorbed in a dose-dependent manner, and remained elevated in the plasma for three hours after administration. [142] Aerosol administration of glutathione is an effective means of delivery to the plasma [143], as is intravenous administration. [144] Glutathione is thought to be non-toxic to humans, [144] although one study found a 5 g oral daily dose was associated with GI irritation and sulfur odor. [145]
A case report from Japan in 1984 raised the possibility that glutathione might be an effective treatment for hepatocellular carcinoma. A trial of six hepatocarcinoma patients on 5 g oral glutathione daily found regression or stagnation of tumor growth in three patients. One patient also had a reduction in alpha-fetoprotein (a tumor marker) from 496 to 5. Two patients of the six survived for one year. These patients were both women, raising the possibility of a sex-dependent effect. [145] In a rat study, oral administration of glutathione caused regression of liver tumors, and increased survival of tumor-bearing animals. [146] The usefulness of glutathione as an anti-tumor agent may be limited to the liver, kidney, and peripheral neurons, as these are the only tissues believed to have sufficient transport enzymes for cellular uptake. [144] For further discussion of glutathione as an antioxidant, refer to the review article by Kidd. [147]
Glutathione with Radiation
A randomized pilot trial with 45 participants investigated the radioprotective effect of glutathione. Patients were administered 1200 mg glutathione or saline placebo intravenously 15 minutes prior to pelvic radiotherapy. Patients receiving glutathione suffered less from post-therapy diarrhea (28%, compared to 52% of controls) and were more likely to complete the treatment cycle (71% to 52%). Although the sample size was too small to show significance, the authors concluded glutathione was unlikely to interfere with the effect of radiation on neoplasms. [148] The argument was not based on patient outcome.
Glutathione with Chemotherapy
Increased cellular concentrations of glutathione have been associated with resistance to both anthracyclines and platinum agents. [149] Given the suggestion of the inability of most cell types to take up exogenous glutathione, [144] decreased chemotherapy efficacy due to glutathione administration may be limited to liver, kidney, and neurological tumors.
The use of cisplatin and glutathione concurrently has been studied in several small human trials. One human trial found 3 g/m2 intravenous glutathione given 20 minutes prior to cisplatin (100 mg/m2) led to a significant reduction in nephrotoxicity in patients with ovarian cancer compared with those receiving cisplatin alone. There was a trend toward greater tumor response in the glutathione group--73 percent, compared to 62 percent in the control group. [150] A similar trial using smaller doses of glutathione (2500 mg/m2) and cisplatin (50 - 75 mg/m2) did not find the reduction in nephrotoxicity reported above. However, the trend toward greater tumor response with glutathione treatment (72% response, compared to 52% in controls) was comparable. [151]
A double-blind trial studied the neuroprotective effect of intravenous glutathione (1500 mg/m2) during cisplatin treatment for gastric cancer. After nine weeks, no patient of the 24 receiving glutathione, but 16 of 18 patients receiving placebo, had developed neuropathy symptoms. Again, a trend toward greater tumor response (76%, compared to 52% in controls) was seen with glutathione treatment. [152]
An open trial with 79 ovarian cancer patients found i.v. administration of 2500 mg glutathione prior to treatment with a cisplatin / cyclophosphamide combination led to greater tumor response and reduced toxicity compared to that found in other trials using these chemotherapeutic agents. [153] Another trial using the same glutathione dose with the same combination chemotherapy found no cases of nephrotoxicity in 20 patients. The authors reported, based on their experience, that the effect of the chemotherapy was not interfered with, and may have been enhanced. [154] These results have not been followed up in controlled trials, however. The interactions between glutathione and chemotherapy agents other than cisplatin and cyclophosphamide have not been explored in human trials.
Glutathione Summary
Flavonoids as Cancer Treatment
Flavonoids are plant compounds known to have antioxidant properties in vitro and in vivo. Many of the thousands of flavonoids in nature have been studied for anti-cancer properties. Space does not permit a detailed discussion of this work. The most well characterized anti-tumor flavonoids are epigallocatechin gallate (from green tea), [155] genistein (from soy and red clover), [156,] [157 ]curcumin (from turmeric), [158] silibinin (from milk thistle), [159] and quercetin (from many yellow vegetables). The authors are presently preparing a review of the use of quercetin as cancer therapy.
Flavonoids with Radiation
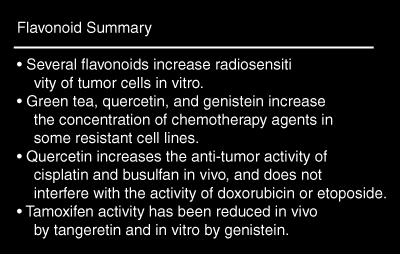
Little is known about the effects of flavonoids on radiotherapy. An in vitro experiment showed post-treatment application of quercetin caused greater cell death in radiation-treated hepatoma cells than radiation alone. In the same experiment, genistein was showed to be associated with increased cell death from radiation when applied during or after treatment. [160] Many different rutosides (flavonoids with similar structures to quercetin) were found to have neither a protective nor sensitizing effect on radiotherapy in experimental mouse tumors. [161] There is not enough evidence currently to support or argue against the use of therapeutic doses of flavonoids together with radiation.
Flavonoids with Chemotherapy
Recent research has focused on the ability of flavonoids to increase the concentration of chemotherapeutics in tumor cells. Resistance to many chemotherapy agents is thought to be due to reduced accumulation in tumor cells. [162] Oral administration of green tea in mice to increased the concentration of doxorubicin in two tumor types, but not in normal tissue. The anti-tumor activity of doxorubicin was enhanced 2.5 times. [163] Another report confirmed this action of green tea, finding the tumor inhibition of doxorubicin increased from negligible to 62 percent. This report, however, determined the activity of green tea to be due to an amino acid, theanine, rather than its flavonoid content. [164]
Quercetin has been shown in vitro to increase the concentration of doxorubicin in multidrug-resistant human breast cancer cells. [165] Conversely, quercetin decreased the concentration of doxorubicin in a resistant human colon cancer cell line. [166] Quercetin and genistein both increased the concentration of daunorubicin in some multidrug-resistant cell lines, but had no effect in others. [167] Genistein in vitro increased the concentration of cisplatin in resistant cell lines. [168] Other than the green tea studies, none of these studies analyzed cell death due to flavonoid administration. In mice with transplanted human tumors, quercetin (20 mg/kg) given with cisplatin reduced tumor growth to a greater degree than cisplatin alone. [169] In a separate experiment, quercetin enhanced the effect of cisplatin and busulfan in vitro and in vivo. No enhancement or reduction of the anti-tumor activity of doxorubicin or etoposide was seen. [170] An in vitro study found quercetin increased the effect of doxorubicin against resistant breast cancer cells. [165] It should be cautioned, however, that a recent study showed a potential adverse interaction. Tangeretin, a flavonoid found in citrus fruits, completely blocked the inhibitory effect of tamoxifen on mammary cancer in mice. [171] One in vitro study suggests attenuation of tamoxifen may be a concern with genistein as well. [172] Another in vitro study, however, shows tamoxifen and genistein synergistically inhibit the growth of estrogen receptor-negative breast cancer cells. [173] Until the flavonoid-tamoxifen interactions are investigated more completely, it may be best to avoid using therapeutic doses of flavonoid compounds in breast cancer treated with tamoxifen.
Flavonoid Summary
Combinations of Antioxidants
Given that many antioxidants have been shown to have anti-tumor properties, it is worth exploring their use in combination. A study in mice found co-administration of beta-carotene and alpha-tocopherol led to much greater tumor regression than either agent alone. The effect was synergistic, being much greater than the sum of the mild tumor inhibition of beta-carotene and alpha-tocopherol. [174] Other studies have shown multivitamin supplements were associated with fewer recurrences of solid tumors after remission following standard oncologic therapies. [20,21]
A small double-blind trial of a mixture of antioxidants, including 600 mg vitamin E, 1 g vitamin C, and 200 mg NAC taken only during treatment, looked at the potential of this mixture to prevent cardiotoxicity during chemo- and radiotherapy. No patient taking the antioxidant mixture had a fall in ejection fraction greater than 10 percent. In patients taking placebo, four of six patients undergoing radiotherapy and two of seven patients treated with chemotherapy had an ejection fraction reduction of 10 percent or more. Treatment outcomes in patients taking antioxidants versus placebo were not discussed. [175]
An open trial of combination antioxidant treatment along with chemotherapy and radiation in patients with small-cell lung cancer had encouraging results. Patients taking the supplement, which contained at least 15,000 IU vitamin A, 10,000 IU beta-carotene, 300 IU alpha-tocopherol, 2 g vitamin C, and 800 mcg selenium, were able to tolerate chemotherapy and radiation well. Their two-year survival rate was greater than that of historical controls (>33% to <15%), with 44 percent still alive at the end of the study (mean survival time for survivors = 32 months). No side effects from nutritional treatment were noted.17 Hopefully these promising results will be followed up with larger and more well-controlled studies.
Combinations of Antioxidants Summary
Current Attitudes and New Approaches to Treatment
Cancer therapy has been remarkably consistent for the last 50 years. Surgery, radiation, and chemotherapy have been the cornerstones of conventional treatment. Not surprisingly, the clinical success of these treatments has reached a plateau. [176 ]Some authors have even questioned the validity of chemotherapy as a treatment for most cancers. [177] Clearly, there is a need for new therapies which can increase the efficacy of cancer treatment. Careful application of antioxidants may be a means helping to raise cancer therapy to a new level of success. [4]
The attitude of many conventional practitioners toward antioxidant therapy for cancer has been hostile. [178] Others have raised the argument that antioxidants could blunt the effect of standard therapies, particularly alkylating, platinum, and tumor antibiotic agents, which are oxidative in nature. [5] While this appears a theoretical concern, the evidence reviewed here shows that this proposed interaction of anti- and pro-oxidant therapies is not generally of primary importance in vivo. It is time to put this argument in perspective.
Potential Mechanisms of Antioxidants in Cancer
Therapy How could antioxidant therapy protect normal cells against damage from cancer therapies, while often increasing their cytotoxic effect against malignant cells? While the answer to this question is not entirely mapped out, there are concepts which might help us understand. One is the recent evidence that radiation and chemotherapy often harm DNA to a relatively minor extent, which causes the cells to undergo apoptosis, rather than necrosis. [6] Since many antioxidant treatments stimulate apoptotic pathways, [7,8] the potential exists for a synergistic effect with radiation or chemotherapy with antioxidants.
A second concept is that the defensive mechanisms of many cancer cells are known to be impaired. This presumably makes tumor cells unable to use the extra antioxidants in a repair capacity; this has been illustrated in vitro. An experimental murine ascites tumor cell line was found to have 10 -100 times less catalase than comparable normal cells. This led to a build-up of hydrogen peroxide in the cells upon treatment with vitamin C, in turn leading to cell death. The cytotoxic effects of vitamin C were completely eliminated by addition of catalase to the cell culture. [179] Since publication of these findings, most human tumor cell lines studied have proved to be similarly low in catalase. [180]
Caveats When Considering Using Antioxidants in Cancer Treatment
We wish to emphasize three concerns regarding the use of antioxidants raised in this paper. One is the routine use of N-acetylcysteine with certain chemotherapeutic agents, namely cisplatinum and doxorubicin. Given the limited therapeutic benefits associated with NAC in cancer treatment, and the number of other antioxidants shown above to help reduce the toxicities of these two chemotherapeutic agents, there appears little reason to consider NAC a first-line adjunct with either agent. Since the potential for adverse interaction with chemotherapy appears to be greater with NAC, perhaps it should be used only in situations where it has clearly been shown to not interfere with other therapies.
The second concern we wish to reiterate is the interaction between tangeretin and tamoxifen. Except in cases where interactions with specific flavonoids are clearly defined, it seems prudent to avoid treatment with flavonoids in therapeutic doses concurrently with tamoxifen. It is unknown currently if there is any reduction in tamoxifen activity associated with dietary flavonoids, which are ubiquitous in the plant kingdom.
The third area of concern is the potential reduction of 5-fluorouracil (5-FU) activity by beta-carotene. [58]The nature of this interaction is not clear. Until this is clarified, the combination would best be avoided.
Conclusion
Frequently, the effects of using antioxidants concurrent with chemotherapy and radiation are synergistic. Except for three specific interactions outlined above (flavonoids with tamoxifen, NAC with doxorubicin, and beta-carotene with 5-fluorouracil), there is no evidence to date showing that natural antioxidants interfere with conventional cancer therapeutics in vivo. Studies have shown patients treated with antioxidants, with or without chemotherapy and radiation, have many benefits. Patients have been noted to tolerate standard treatment better, experience less weight loss, have a better quality of life, and most importantly, live longer than patients receiving no supplements. It is time to research the role of these agents in conventional oncologic treatment, rather than dismiss them as a class based on theoretical concerns.
The authors wish to thank the Smiling Dog Foundation for financial support of this project and to Bastyr University for its administration.

Return to ANTIOXIDANTS
Return to CANCER AND NUTRITION
Return to CHIROPRACTIC AND CANCER
Since 6-13-2003


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |