Spinal Manipulative Therapy-specific Changes in
Pain Sensitivity in Individuals with Low Back PainThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Pain 2014 (Feb); 15 (2): 136–148 ~ FULL TEXT
OPEN ACCESS Joel E Bialosky, PT, PhD, Steven Z George, PT, PhD, Maggie E Horn,
Donald D Price, PhD, Roland Staud, MD, and Michael E Robinson, PhD
Department of Physical Therapy,
Center for Pain Research and Behavioral Health,
University of Florida,
Gainesville, Florida.
bialosky@phhp.ufl.edu
Spinal manipulative therapy (SMT) is effective for some individuals experiencing low back pain; however, the mechanisms are not established regarding the role of placebo. SMT is associated with changes in pain sensitivity, suggesting related altered central nervous system response or processing of afferent nociceptive input. Placebo is also associated with changes in pain sensitivity, and the efficacy of SMT for changes in pain sensitivity beyond placebo has not been adequately considered. We randomly assigned 110 participants with low back pain to receive SMT, placebo SMT, placebo SMT with the instructional set "The manual therapy technique you will receive has been shown to significantly reduce low back pain in some people," or no intervention.
Participants receiving the SMT and placebo SMT received their assigned intervention 6 times over 2 weeks. Pain sensitivity was assessed prior to and immediately following the assigned intervention during the first session. Clinical outcomes were assessed at baseline and following 2 weeks of participation in the study. Immediate attenuation of suprathreshold heat response was greatest following SMT (P = .05, partial η2 = .07). Group-dependent differences were not observed for changes in pain intensity and disability at 2 weeks. Participant satisfaction was greatest following the enhanced placebo SMT.
This study was registered at www.clinicaltrials.gov under the identifier NCT01168999.
PERSPECTIVE: The results of this study indicate attenuation of pain sensitivity is greater in response to SMT than the expectation of receiving an SMT. These findings suggest a potential mechanism of SMT related to lessening of central sensitization and may indicate a preclinical effect beyond the expectations of receiving SMT.
KEYWORDS: Central sensitization; low back pain; manual therapy; placebo; spinal manipulation
From the FULL TEXT Article:
Introduction
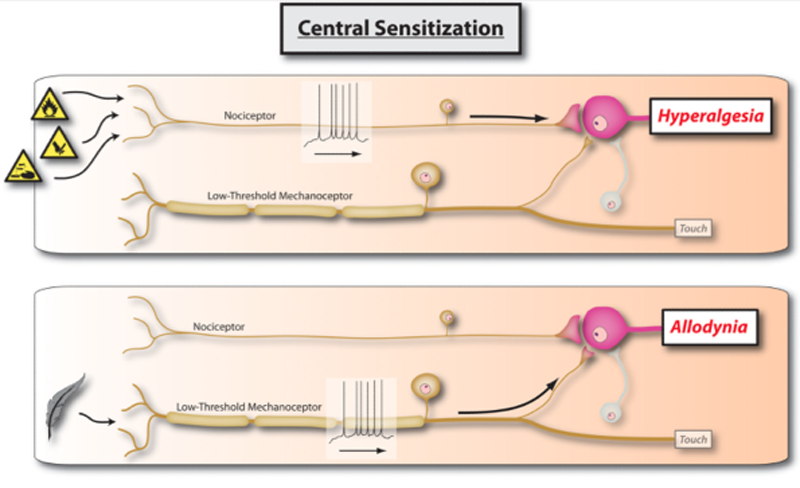
Low back pain (LBP) is a significant public health problem with lifetime incidence rates up to 90% [89] and loss of work production estimated at 7.4 billion dollars for workers in the United States between the ages of 40 and 65. [70] Chronic LBP, similar to other chronic pain conditions (e.g. fibromyalgia), is associated with altered pain processing [42, 63] suggesting a mechanism related to central sensitization of pain. [56, 76] Specifically, chronic LBP is associated with generalized pain sensitivity [42] and cortical responses to painful stimuli differing from those observed in healthy individuals. [2, 28] Central sensitization is considered a factor in the progression of acute pain to chronic pain and the maintenance of chronic pain. [71] Subsequently, attenuation of central sensitization may represent a treatment target. [81]
Spinal manipulative therapy (SMT) is an effective [13–15, 31] complementary and alternative medicine intervention for some individuals experiencing LBP. SMT is recommended by many LBP clinical practice guidelines; [21] however, not all clinical practice guidelines support SMT and variability exists between those which do suggesting a need for stronger evidence. [57] Improved understanding of the mechanisms of SMT could enhance clinical effectiveness and clarify the variability in the present literature. LBP is a heterogonous condition for which the anatomical basis is commonly unidentifiable. [26] Subsequently, a pathoanatomical diagnosis is generally not helpful for guiding treatment [24] and identifying subgroups of individuals with LBP most likely to benefit from a specific intervention is a research priority. [27] Clarifying the mechanisms of SMT could assist in identifying key features of individuals with LBP likely to respond to these interventions allowing more efficacious clinical application.
SMT is associated with changes in pain sensitivity [19, 61] suggesting a mechanism related to attenuation of central sensitization. [10] SMT results in increased mechanical pain thresholds in individuals with neck pain [23, 88] and lateral epicondylalgia [30] and attenuation of suprathreshold heat response. [8, 9, 38] Consequently, the clinical effectiveness of SMT could result from lessening of central sensitization.
Placebo is associated with robust analgesia [85] enhanced by expectation for pain relief. [66] For instance, saline is associated with analgesia in patients with fibromyalgia [69] and irritable bowel syndrome [86] believing they received a pain relieving drug. Clinical outcomes related to interventions for pain result from both intervention specific and placebo mechanisms. [91] This point is exemplified in open- hidden paradigm studies in which a known analgesic agent is provided in an open manner or through hidden infusion resulting in greater analgesia when openly administered. [5, 16] Expectation is also influential in outcomes related to complementary and alternative medicine interventions. For example, a study comparing the efficacy of massage and acupuncture for individuals with LBP observed a moderating effect of expectation. [54]
Participants expecting more relief with acupuncture demonstrated better outcomes when receiving acupuncture while those expecting more relief with massage demonstrated better results when receiving massage. [54] Furthermore, active acupuncture is associated with similar analgesic properties as placebo acupuncture in participants following dental surgery. [3] However, participants believing they received acupuncture reported significantly less pain than those believing they received the placebo acupuncture. [3] Collectively these studies suggest placebo mechanisms related to expectation are influential in clinical outcomes for complementary and alternative medicine interventions yet rigorous assessment in SMT is lacking.
The primary purpose of this mechanistic trial was to consider a potential mechanism of SMT by determining the efficacy of SMT upon pain sensitivity. We have observed immediate lessening of pain sensitivity in response to SMT [8, 9, 38] and the current study was designed to extend these findings by determining whether lessening of pain sensitivity is specific to SMT or the expectation of receiving SMT. As a secondary purpose, we considered the clinical efficacy of SMT and the influence of expectation upon these outcomes.
Materials and Methods
Participants
The study was approved by the Institutional Review Board of the University of Florida. A sample of convenience was recruited from the general community of the University of Florida campus and Health Science Center by posted flyers and electronic distribution. Participants between the ages of 18 and 60, currently experiencing mechanical LBP rated ≥ 4/10 at its worst over the past 24 hours on a numeric rating scale (NRS) (0 = no pain at all, 10 = worst pain imaginable) were included in the study. We based the diagnosis of LBP on clinical presentation related to pain in the lumbar region rather than on imaging abnormalities as an anatomical cause is not identifiable in the majority of cases of LBP. [26]
Participants were excluded for;1) pain or paresthesia below the knees;
2) potential non- musculoskeletal causes of LBP as indicated bya) unexplained weight loss of greater than 10 pounds,
b) fever corresponding to LBP,
c) non- mechanical pain,
d) bowel or bladder dysfunction;3) surgery to the low back within the past 6 months;
4) systemic illness known to affect sensation i.e. diabetes;
5) chronic pain condition unrelated to LBP;
6) fracture as the cause of LBP;
7) pregnancy.Duration of LBP was not a consideration for inclusion/ exclusion from the study because we wished to include a full range of individuals with LBP for ecological validity while anticipating individuals with LBP more chronic in nature would predominate due to our recruitment strategy. We felt our primary mechanistic aim related to central sensitization justified this approach and anticipated any influence of duration upon the outcomes would be negated by the parallel group design. All individuals meeting the criteria for participation and providing informed consent were enrolled in the study.
MeasuresDemographic and Clinical Characteristics Demographic information was obtained at baseline through a questionnaire specific to age, sex, years of education, and duration of LBP.
Psychological Questionnaires Psychological measures known to influence experimental pain39, 64 and LBP outcomes [33, 48, 74, 75] were assessed as we wished to control for these factors in the event our randomization process did not evenly distribute them across the groups. Psychological measures included the Fear Avoidance Belief Questionnaire (FABQ), [90] the Tampa Scale of Kinesiophobia (TSK), [92] and the Pain Catastrophizing Scale (PCS). [82]
NOTE: You may review the Pain Catastrophizing Scale at our Outcome Assessment Section
Assessment of Pain Sensitivity Measures of pain sensitivity served as primary outcomes reflective of SMT related changes in central sensitization and included:Mechanical Pain Sensitivity Mechanical pain sensitivity lessens in response to SMT [19] and we wished to determine if similar changes occurred in the current study. A pressure algometer (Pain Diagnostics & Treatment, Great Neck, NY) was used to determine suprathreshold mechanical pain sensitivity. Six kg of force was applied at a rate of 1 kg/second through a 1 cm2 application tip at the dominant side PSIS to determine local changes in pain sensitivity and the web space of the dominant foot to determine remote changes in pain sensitivity as SMT is associated with both local and remote changes in mechanical pain sensitivity. [19] Mechanical pain sensitivity was quantified through a 100 mm mechanical visual analog scale (MVAS) anchored with “No pain” and “The most intense pain sensation imaginable”. MVAS are commonly used in the assessment of pain and have demonstrated sound psychometric properties including the characteristics of a ratio scale. [65]
Thermal Pain Sensitivity Thermal pain sensitivity was assessed for suprathreshold heat response, and after sensations. Participants underwent thermal pain assessment using the Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel) with a hand-held, peltier-element-based stimulator.
Suprathreshold heat response Suprathreshold heat response assessment used previously established protocols for temporal summation [69, 80] at 51° C applied to the plantar surface of the dominant foot with an inter-stimulus interval of 0.33 seconds. A 101 point NRS anchored with “no pain” and “the most intense pain sensation imaginable” quantified the pain experienced with each heat pulse and participants were instructed to rate their “second pain”. We have previously observed moderate within session stability of this protocol in both healthy participants and those experiencing pain conditions. [1] The rating provided for the 5th pulse in this temporal summation protocol is considered primarily C- fiber mediated [67] and corresponds most highly to clinical pain. [84] We selected the rating provided for the 5th pulse as our measure of suprathreshold heat response based on its translational potential due to the established relationship to clinical pain.
After sensation Participants quantified pain they continued to feel 15 seconds following the tenth pulse in the suprathreshold heat response protocol using a NRS. [78] After sensation is considered primarily C- fiber mediated. [67, 69, 77] We elected to consider after sensation as a competing measure of C- fiber mediated pain and because of its relationship to clinical pain in other chronic pain conditions. [78–80] We have previously observed good within session reliability of the assessment of after sensation in both healthy participants and those experiencing pain conditions. [1]
Clinical OutcomesClinical Pain Intensity Clinical pain intensity was assessed for changes over the 2 weeks of the study using the NRS for “usual pain over the past week” from the PCOQ. NRSs are reliable and valid [37, 52] and a common measure of clinical pain intensity.
Low Back Pain Related Disability Low back pain related disability was assessed through the Oswestry Disability Index. The Oswestry Disability Index is a 10 item questionnaire specific to LBP. Each item contains a 6 point adjectival scale scored from 0 to 5. We doubled the total score as is commonly done [29] to provide a percentage with higher scores indicating greater perceived disability. The Oswestry Disability Index is a commonly used measure of disability in the study of LBP and has demonstrated strong reliability and validity. [12, 29, 34, 35]
Participant Satisfaction Satisfaction is related to expectation [4, 49] and unmet expectations may lead to dissatisfaction. [4] We included satisfaction as a secondary outcome measure to determine whether differing group related expectations were associated with differences in satisfaction separate from changes in clinical outcomes. We used two questions from the North American Spine Society Lumbar Spine Outcome Assessment [22] indicative of satisfaction. [40]
Participants were asked,1) “Would you have the same intervention you received in this study again for low back pain?”
Possible responses ranged from 1= definitely not to 5= definitely yes.
2) “How would you rate the overall results of the intervention you received in this study for low back pain?”
Possible responses ranged from 1= terrible to 6= excellent.Interventions (Figure 2)

Figure 2 All interventions were performed by a licensed physical therapist (JEB or MEH).
The SMT group received a SMT previously shown effective in the treatment of some individuals experiencing LBP. [13, 31] Furthermore, we have previously observed attenuation of suprathreshold heat response in response to the studied SMT. [8, 38] Similar to our prior studies, the SMT was performed 2x on each side. [8, 38] Participants receiving the SMT were instructed through the informed consent process they would receive either a studied SMT or a placebo intervention and were provided no additional information regarding which intervention they received.
The standard SMT placebo group received a placebo SMT. SMT interventions depend upon biomechanical approaches related to positioning and force application intended to isolate a vertebral segment or spinal region and impose motion. [41] The novel placebo was intended to mimic the studied SMT; however, differ biomechanically. Specifically, the placebo maintained the lumbar spine in a neutral position (as opposed to contralateral sidebending in the studied SMT). Participants were log rolled towards the examiner and then returned to a supine position (as opposed to maintained in rotation as in the studied SMT). A thrust of similar force to the studied SMT was then applied to the contralateral anterior superior iliac spine of the pelvis directly into the table. The placebo SMT was designed to apply a thrust to a neutral spine and directly into the table rather than thrusting into rotation in a spine positioned in sidebending and rotation as occurs in the studied SMT. We acknowledge load was applied to the spine with the placebo SMT; however, believe this necessary to provide a credible comparison as non- thrust placebo comparisons such as light touch are associated with lower treatment expectancies than SMT. [36] Additionally, the applied load was to a spine positioned vastly differently from typical clinical practice and not done with therapeutic intent. Similar to the studied SMT, the placebo SMT was performed 2x on each side. Participants receiving the Placebo SMT were instructed through the informed consent process they would receive either a studied SMT or a placebo intervention and were provided no additional information regarding which intervention they received.
The enhanced SMT placebo group received the same placebo as the standard placebo group. Participants receiving the enhanced SMT placebo were instructed through the informed consent process they would receive either a studied SMT or a placebo intervention; however, were told, “The manual therapy technique you will receive has been shown to significantly reduce low back pain in some people” immediately prior to the first intervention and subsequent intervention sessions. Similar instructional sets have been incorporated in mechanistic studies of placebo and are associated with enhanced placebo analgesia in subjects with irritable bowel syndrome. [68, 86] Similar to the SMT and the standard placebo group, the enhanced placebo SMT group received the placebo SMT 2x on each side.
The no treatment control group sat quietly for 5 minutes during the initial session.Procedures Individuals agreeing to participate signed an informed consent form approved by the University of Florida Institutional Review Board and then completed the intake demographic form, psychological questionnaires, the PCOQ, and the Oswestry Disability Index. Participants next underwent baseline pressure and thermal pain testing and were randomly assigned to receive either SMT, placebo SMT, enhanced placebo SMT, or no intervention. Randomization was computer generated with group assignment maintained in sealed, sequentially numbered, opaque envelopes. The envelopes were opened in sequential order based on entry in the study and after all baseline measures were completed for the participant.
We wished to ensure the appropriateness of the placebo SMT as indicated by the believability and resulting expectation for treatment effectiveness. Believability was assessed immediately following the application of the assigned intervention. Participants receiving the SMT, placebo, or enhanced placebo received the instruction, “in this study you received either a manual therapy intervention or a placebo. Please indicate whether you believe you received the manual therapy intervention or the placebo”. Participants were handed a form and asked to circle the intervention they believed they received (SMT or placebo). Expectation was also assessed immediately following the initial application of the assigned intervention.
Participants were handed a form with the options of1) more LBP,
2) less LBP,
3) the same amount of LBP and asked to circle the option most reflective of their expected level of LBP upon completion of the study.Next, participants underwent repeat mechanical and thermal pain sensitivity testing to consider an immediate, within session change in pain sensitivity. Participants receiving the SMT and both placebo groups were scheduled for 5 additional sessions during the next 2 weeks to receive their assigned intervention. Participants in the SMT and standard placebo group were provided no information regarding their assigned intervention during any of the intervention sessions. Following the 2 week period of the study, all participants were seen for a final session in which clinical outcomes for pain intensity, disability, and satisfaction were assessed. Upon completion of the study, participants were debriefed regarding their group assignment and the purpose of the study.
Data Analysis
Individual t-tests and chi square tests were used to assess for post-randomization group differences. Significance was set at 0.05 and all analyses were performed using the SPSS statistical package, version 21.0 (SPSS Inc, Chicago, IL)

Table 1 We determined the appropriateness of our placebo comparison prior to consideration of our primary and secondary purposes. Separate Chi- square analyses compared perceived group assignment (SMT, placebo SMT, placebo SMT with enhanced instructional set) to both actual assignment (SMT or placebo SMT) and to categorized expectation for results (more, less, or the same amount of LBP). Significant group related differences were observed in perception of group assignment immediately following the first intervention. χ2 (2, N = 81) = 10.02, p = 0.01) (Table 1A). More participants receiving the standard placebo SMT believed they received a placebo than did those receiving SMT (p = 0.03) or the enhanced placebo SMT (p < 0.01). Differences in perceived intervention were not found between participants receiving the SMT and the enhanced placebo SMT (p = 0.36). These findings suggest participants found the enhanced placebo SMT similarly believable as a rehabilitation intervention as the studied SMT.
Significant group related differences were observed in expected 2 week changes in LBP immediately following the first intervention. χ2 (6, N = 110) = 20.91, p < 0.01) (Table 1B). A larger percentage of participants receiving the SMT and enhanced placebo SMT expected less pain than those receiving the standard placebo SMT and the no treatment control group (p < 0.05). Expected LBP at 2 weeks in response to the intervention did not differ for participants receiving the SMT and enhanced placebo SMT (p = 0.67) or for participants in the no treatment control and the standard placebo SMT group (p = 0.23). These findings suggest the enhanced placebo SMT was associated with similar expectations for effectiveness as the studied SMT.
Pain sensitivity
Separate mixed- model ANOVAs were used to test for a group (SMT, placebo SMT, enhanced placebo SMT, control) × time (pre to immediately post intervention during the initial session) interaction for measures of mechanical and thermal pain sensitivity. In the event of a statistically significant group × time interaction, simple contrasts were performed to assess within group changes. Changes in aftersensation were assessed only in participants reporting continued pain at 15 seconds following the last pulse in the suprathreshold heat response protocol at baseline.
Clinical outcomes
Separate mixed- model ANOVAs were used to test for a group (SMT, placebo SMT, enhanced placebo SMT, control) × time (baseline to 2 weeks) interaction for clinical pain intensity and disability.
Participant satisfaction
Separate Chi-square analyses were used to compare group assignment (SMT, placebo SMT, enhanced placebo SMT, control) to the responses to the following questions.1) “Would you have the same intervention you received in this study again for low back pain?” Possible responses ranged from 1= definitely not to 5= definitely yes and were further categorized with individuals answering “definitely not”, “probably not”, and “completely uncertain” combined into one category and those answering, “probably yes” and “definitely yes” combined into a second category.
2) “How would you rate the overall results of the intervention you received in this study for low back pain?” Possible responses ranged from 1= terrible to 6= excellent and were further categorized with individuals answering, “terrible”, “poor”, and “fair” combined into one group and those responding “good”, very good”, or “excellent” grouped separately.Influence of expectation upon clinical outcomes
Participants were categorized by whether they expected more, less, or the same amount of LBP immediately following the initial intervention. Separate mixed- model ANOVAs were used to test for a group (expect more, less, or the same amount of LBP) × time (baseline to 2 weeks) interaction for clinical pain intensity and disability. In the event of a statistically significant group × time interaction, pairwise comparisons were performed to assess within group changes.Sample size determination Sample size was determined based on reduction in suprathreshold heat response by using effect sizes from our prior studies comparing the same technique to other common physical therapy interventions for LBP.8, 38 We used a conservative analgesic effect size of measures of suprathreshold heat response from our previous studies (eta2 = 0.17), a 2–tailed null hypothesis, and an alpha of 0.05 (to account for the multiple comparisons) to generate a conservative estimate of power. Twenty participants per treatment group were determined to provide greater than 95% power to detect a group × time interaction in the proposed ANOVA model. We oversampled to 28 subjects per treatment group to account for potential drop-outs and allow for extra power if smaller than anticipated effect sizes were observed.
Results

Figure 1

Table 2

Table 3

Figure 3 One hundred and twenty seven individuals were screened for the study and 110 signed the informed consent form and agreed to participate. (Figure 1) Seventy percent of participants were female and mean age was 31.68 (sd = 11.85) years. Baseline measures of the sample as a whole and by group assignment are presented in Table 2. Individual groups did not differ by baseline demographic measures, clinical measures, psychological measures, or pain sensitivity measures.
Pain sensitivity (Table 3)
Group by time (pre- first intervention to immediately post first intervention) differences were not observed in mechanical pain sensitivity assessed at the PSIS (F(3,104) = 1.14, p= 0.34, partial η2= 0.03) nor was a main effect for time (F(1,104) = 3.65, p= 0.06, partial η2= 0.03). Group by time (pre- first intervention to immediately post first intervention) differences were not observed in mechanical pain sensitivity assessed at the web space of the foot (F(3,104)= 0.93, p= 0.43, partial η2= 0.03) nor was a main effect for time (F(1,104)= 2.31, p= 0.13, partial η2= 0.02). Group by time (pre- first intervention to immediately post first intervention) differences were observed in suprathreshold heat response (F(3,106) = 2.63, p= 0.05, partial η2= 0.07). Statistically significant lessening of pain sensitivity was observed only in response to the SMT (p< 0.05). (Figure 3) Thirty eight of the 110 participants (34.5%) reported continued pain 15 seconds following the 10th pulse in the suprathreshold pain protocol and were considered in the analysis of aftersensation.
Eight of 28 (29%) participants in the SMT group, 9 of 27 (33%) of participants in the placebo SMT group, 8 of 27 (30%) in the enhanced placebo SMT group, and 13 of 28 (46%) participants in the no treatment group reported aftersensation. Group by time (pre- first intervention to immediately post first intervention) differences were not observed in aftersensation (F(3,34) = 1.42, p= 0.25, partial η2= 0.11) nor was a main effect for time (F(1,34) = 1.88, p = 0.18, partial η2= 0.05).
Clinical outcomes (Figure 4)

Figure 4 A group × time interaction was not observed for LBP over the two weeks of the study (F(3,103) =0.51, p=0.68, partial eta2= 0.02). Significant main effect for time was observed with LBP (F(1,103) =36.56, p<0.01, partial eta2= 0.26). A mean decrease in LBP of 10.27 (sd= 18.22) was observed across participants in the study regardless of group assignment. A group × time interaction was not observed for LBP related disability (F(3,102) =0.43, p=0.73, partial eta2= 0.01). Significant main effect for time was observed with disability (F(1,102) =13.86, p<0.01, partial eta2= 0.12). A mean decrease in LBP related disability of 2.93 (sd= 8.06) was observed across participants in the study regardless of group assignment.
Participant satisfaction (Table 4)

Table 4 Significant group related differences were observed in response to the question, “Would you have the same intervention you received in this study again for low back pain?” χ2 (3, N = 106) = 8.15, p = 0.04). Significantly more participants receiving the enhanced placebo SMT indicated “probably to definitely yes” than the other groups individually (p< 0.05). Significant group related differences were observed in response to the question, “How would you rate the overall results of the intervention you received in this study for low back pain?” Significantly more participants receiving the enhanced placebo SMT indicated “good to excellent” than participants receiving the standard placebo SMT or no treatment (p< 0.05). A significant difference was not observed between participants receiving the SMT and the enhanced placebo SMT (p=0.07).
Influence of expectation upon outcomes
A group (expect more LBP, less LBP, the same amount of LBP) × time (baseline to immediately following the first intervention) interaction was not observed for immediate change in suprathreshold heat response (F(2,107) =0.32, p=0.73, partial eta2= 0.01). A group (expect more LBP, less LBP, the same amount of LBP) × time (baseline to 2 weeks) interaction was not observed for change in LBP (F(2,104) =0.76, p=0.47, partial eta2 = 0.01) or LBP related disability (F(2,103) =2.19, p=0.12, partial eta2= 0.04) over the two weeks of the study.
Discussion
Efficacy of SMT on suprathreshold heat response
The present study extends our prior work related to the mechanisms of SMT. We have previously observed attenuation of suprathreshold heat response following SMT in both healthy participants [9, 38] and those experiencing LBP. [8] Furthermore, we have observed heightened suprathreshold heat response following SMT in healthy participants expecting to experience more pain [7] indicating an influence of expectation. The current study adds to these observations by indicating the lessening of pain sensitivity accompanying SMT is likely specific to1) SMT rather than the expectation of receiving SMT and
2) suprathreshold heat response and not other thermal or mechanical measures of pain sensitivity used in this study.Studies in anesthetized animals confirm wind up of neurons in the dorsal horn of the spinal cord in response to repeated C-fiber stimulation. [20, 43] Thus, we interpret our findings to reveal a mechanism of SMT related to modulation of dorsal horn excitability. Lessening of central sensitization as indicated by changes in suprathreshold heat response suggests a treatment target with potential relevance to clinical pain conditions. [81] Our SMT specific changes in suprathreshold heat response suggest the potential for a clinically beneficial intervention if these effects are lasting and associated with clinical pain reduction. Furthermore, the specificity of this finding to SMT and not placebo SMT suggests a mechanism beyond the expectation of receiving SMT.
Clinical Outcomes
We did not observe group related differences in clinical pain or disability over the two weeks of the study despite differences in blinding, expectation, and immediate within session changes in pain sensitivity. These findings contrast with systematic reviews suggesting SMT is an effective intervention for individuals with LBP. [14] LBP is a heterogeneous condition resulting in frequently small treatment effects in response to common interventions. [55] A more recent management approach advocates determining homogeneous groups of individuals with LBP likely to benefit from specific interventions. [27] Specific to SMT, a clinical cluster has been formulated [31] and validated [13] identifying individuals experiencing LBP likely to benefit from SMT. Additionally, SMT may be more effective for acute LBP [32] and when combined with exercise. [13, 15, 31] We did not base inclusion in our study on meeting the clinical cluster and included individuals with chronic LBP. Our primary purpose was mechanistic and specific to the efficacy of SMT on proxy measures of central sensitization of pain. Given that central sensitization is more likely to be prevalent in a chronic pain population, the inclusion of those with long standing pain was justified.
The results of this study will provide important foundational findings for future studies in more acute samples of individuals with LBP. Furthermore, we elected to only include SMT (rather than SMT and exercise) as we were interested in focusing on mechanisms specific to SMT. Clinical treatment effects may have been observed if we had powered the study to detect them, been more selective in our participant selection, or included an exercise intervention with SMT. Related to these limitations, our findings should not be interpreted as an indication of the efficacy of SMT but rather complimentary data to the more mechanistically inclined outcomes. Numerous studies have considered the immediate effects of manual therapy interventions upon neurophysiological responses such as changes in pain sensitivity. A methodological weakness of these studies is the failure to link the observed findings to clinical outcomes. [17] The clinical findings of the current study allow for interpretation of the clinical relevance of SMT related changes in pain sensitivity.
Our findings may be viewed as paradoxical as we observed SMT specific changes in pain sensitivity not reflected in changes in clinical outcomes over the two weeks of the study. We have parallel results in another manual therapy model (neurodynamic interventions) in individuals with signs and symptoms of chronic carpal tunnel syndrome. [6] Specifically, clinical outcomes did not correspond to changes in pain sensitivity observed over the 3 weeks of the study. [6] Suprathreshold heat response as obtained through the included protocol are believed to be a measure of neuroplastic changes in the nervous system in response to pain. One interpretation of these findings is neuroplastic changes in pain sensitivity may be a precursor to subsequent changes in clinical outcomes requiring more time to manifest. Manual therapy related within session changes in clinical pain are associated with longitudinal changes in clinical outcomes [18, 44] and immediate changes in pain sensitivity may provide similar predictive value given adequate follow up time. Another competing interpretation of these findings is favorable changes in pain sensitivity corresponding to SMT may not be directly linked to the studied clinical outcomes. Future studies with longer follow up times are necessary to determine whether immediate positive changes in suprathreshold heat response are a precursor to improved clinical outcomes.
Significantly more participants receiving the enhanced placebo indicated satisfaction with the intervention despite the lack of group dependent differences in clinical outcomes. Our findings are consistent with others who observed satisfaction as independent of clinical outcomes related to pain and function. For example, George and Hirsh found satisfaction for treatment delivery to differ from that of treatment effect [40] and Breen and Breen observed “overall improvement” to explain only 57% of the variance for satisfaction in individuals seeking chiropractic care due to LBP. [11] All participants in the current study were instructed they could receive either a studied SMT or a placebo during the consent process. Participants in the enhanced placebo SMT group were told they were receiving an effective intervention while those receiving the SMT and the standard placebo received no instruction as to which intervention they received and were left to their own conclusions. Participants receiving the enhanced placebo may have been more satisfied as the delivery met their expectations for treatment (i.e. they received a perceived active and potentially effective intervention). [83] SMT is associated with high satisfaction. [50] Our findings suggest SMT related satisfaction is influenced by the context of the intervention and not necessarily the intervention itself or corresponding outcomes.
Influence of expectation upon clinical outcomes
We did not find expectation to influence immediate changes in suprathreshold pain response. We have previously observed worsening of suprathreshold heat response in healthy participants told to expect more pain.7 Ethical considerations prevented us from providing an instructional set suggestive of worsening of LBP in the current study; however, suprathreshold heat response to SMT may be more susceptible to negative expectation. Additionally, our measure of expectation was specific to longitudinal changes in LBP and not suprathreshold heat response. Expectation related changes in suprathreshold heat response may have been observed had we manipulated and measured expectation specific to the experimental pain protocol. Our findings of a lack of expectation dependent change in clinical outcomes contrast prior findings of expectation as influential in outcomes related to musculoskeletal pain conditions [51, 62] and Complementary and Alternative Medicine interventions. [54, 59] Similar to the lack of treatment group dependent changes in clinical outcomes, two weeks may have provided insufficient time to observe expectation dependent changes in clinical outcomes in our sample.
A final finding of the study was the identification of a novel placebo comparison for SMT associated with similar believability and expectations for treatment effect as the studied SMT, but differing effects on pain sensitivity. A placebo control for SMT is inherently difficult as a consensus is lacking regarding the “active” agent of SMT and the appropriateness of prior SMT comparative placebo interventions questioned. [36, 45, 58] A valid placebo control should be indistinguishable from the studied intervention in a blinded design and create similar expectations for treatment effectiveness as the studied intervention. [47, 87] Prior manual therapy comparative placebos [25, 72] are associated with lower expectations or believability than comparative SMT. [36, 60] Our enhanced placebo SMT was effective in blinding participants and creating similar expectations as the studied SMT with different effects on pain sensitivity. Therefore, the placebo SMT used in this study may merit future investigation in clinical trials for those interested in distinguishing the non-specific effects of SMT.
Limitations
The current study has several limitations. First, we did not maintain blinding of the researcher providing the intervention and obtaining outcomes. While researcher/participant interactions were scripted for consistency, we cannot be certain the lack of blinding did not bias our findings. Second, participants in the study were responding to a study advertisement and may differ from individuals with LBP seeking medical care. In fact, baseline measures of clinical pain intensity and disability were significantly lower in our sample than in those reported in studies of SMT in participants seeking care. [13, 15, 31] Our inclusion criteria required participants rate their pain as ≥4/10 indicating moderate, more restrictive pain requiring treatment. [46, 53, 73] Subsequently, we believe our cohort is representative of individuals with chronic LBP who may seek SMT but did not recruit them from a health care environment.
A third limitation was the lack of a full balanced design. Specifically, we did not include a group receiving the SMT with an enhanced instructional set (“The manual therapy technique you will receive has been shown to significantly reduce low back pain in some people”). SMT is typically provided clinically by enthusiastic practitioners with instructional sets likely more similar to that provided to our participants receiving the enhanced placebo. We considered including an intervention group with SMT provided with the enhanced placebo instructional set; however, we elected against this due to the concern that group would essentially receive two interventions (SMT + enhanced expectations). Future studies should consider whether an additive effect occurs when SMT is provided with an instructional set known to enhance placebo analgesia. [86]
Conclusions
We observed SMT specific attenuation of suprathreshold heat response suggesting an effect beyond only the expectation of receiving an SMT.
Perspective
The results of this study indicate attenuation of pain sensitivity is greater in response to SMT than the expectation of receiving an SMT. These findings suggest a potential mechanism of SMT related to lessening of central sensitization and may indicate a pre- clinical effect beyond the expectations of receiving SMT.
Acknowledgments
This study was supported by the University of Florida Research Opportunity Fund. This manuscript was written while JEB received support from Rehabilitation Research Career Development Program (5K12HD055929-02) and MER and SZG received support from the National Center for Complementary and Alternative Medicine (5R01AT006334).
Disclosures
We acknowledge we have no conflict of interest or financial involvement with any commercial organization that has a direct interest in any matter included in this manuscript.
References:
Alappattu MJ, Bishop MD, Bialosky JE, George SZ, Robinson ME.
Stability of behavioral estimates of activity-dependent modulation of pain.
J Pain Res. 2011;4:151–157Baliki MN, Geha PY, Fields HL, Apkarian AV.
Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli
changes in the presence of chronic pain.
Neuron. 2010;66:149–160Bausell RB, Lao L, Bergman S, Lee WL, Berman BM.
Is acupuncture analgesia an expectancy effect? Preliminary evidence based on participants’
perceived assignments in two placebo-controlled trials.
Eval Health Prof. 2005;28:9–26Bell RA, Kravitz RL, Thom D, Krupat E, Azari R.
Unmet expectations for care and the patient-physician relationship.
J Gen Intern Med. 2002;17:817–824Benedetti F, Carlino E, Pollo A.
Hidden administration of drugs.
Clin Pharmacol Ther. 2011;90:651–661Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ.
A Randomized Sham-Controlled Trial of a Neurodynamic Technique in the Treatment of Carpal Tunnel Syndrome.
Journal of Orthopaedic & Sports Physical Therapy. 2009;39:709–723Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ.
The Influence of Expectation on Spinal Manipulation Induced Hypoalgesia:
An Experimental Study in Normal Subjects
BMC Musculoskelet Disord. 2008 (Feb 11); 9: 19Bialosky JE, Bishop MD, Robinson ME, Zeppieri G, Jr, George SZ.
Spinal Manipulative Therapy Has an Immediate Effect on Thermal Pain Sensitivity
in People With Low Back Pain: A Randomized Controlled Trial
Phys Ther. 2009 (Dec); 89 (12): 1292–1303Bishop MD, Beneciuk JM, George SZ.
Immediate reduction in temporal sensory summation after thoracic spinal manipulation.
Spine J. 2011;11:440–446Boal RW, Gillette RG.
Central neuronal plasticity, low back pain and spinal manipulative therapy.
J Manipulative Physiol Ther. 2004;27:314–326Breen A, Breen R.
Back Pain and Satisfaction with Chiropractic Treatment:
What Role Does the Physical Outcome Play?
The Clinical Journal of Pain 2003 (Jul); 19 (4): 263–268Changulani M, Shaju A.
Evaluation of responsiveness of Oswestry low back pain disability index.
Arch Orthop Trauma Surg. 2009;129:691–694Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al.
A Clinical Prediction Rule To Identify Patients With Low Back Pain Most Likely To Benefit
from Spinal Manipulation: A Validation Study
Annals of Internal Medicine 2004 (Dec 21); 141 (12): 920–928Chou R, Huffman LH; American Pain Society.
Nonpharmacologic Therapies for Acute and Chronic Low Back Pain:
A Review of the Evidence for an American Pain Society/
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 492–504Cleland JA, Fritz JM, Childs JD, Kulig K.
Comparison of the effectiveness of three manual physical therapy techniques in a subgroup
of patients with low back pain who satisfy a clinical prediction rule:
a randomized clinical trial.
Spine (Phila Pa 1976) 2009;34:2720–2729Colloca L, Lopiano L, Lanotte M, Benedetti F.
Overt versus covert treatment for pain, anxiety, and Parkinson's disease.
Lancet Neurol. 2004;3:679–684Cook C.
Immediate effects from manual therapy: much ado about nothing?
J Man Manip Ther. 2011;19:3–4Cook CE, Showalter C, Kabbaz V, O'Halloran B.
Can a within/between-session change in pain during reassessment predict outcome using a
manual therapy intervention in patients with mechanical low back pain?
Man Ther. 2012;17:325–329Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ.
Changes in Pain Sensitivity Following Spinal Manipulation:
A Systematic Review and Meta-analysis
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 752–767Cuellar JM, Dutton RC, Antognini JF, Carstens E.
Differential effects of halothane and isoflurane on lumbar dorsal horn neuronal windup and excitability.
Br J Anaesth. 2005;94:617–625Dagenais S, Tricco AC, Haldeman S.
Synthesis of Recommendations for the Assessment and Management
of Low Back Pain From Recent Clinical Practice Guidelines
Spine J. 2010 (Jun); 10 (6): 514–529Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH.
The North American spine society lumbar spine outcome assessment Instrument:
reliability and validity tests.
Spine (Phila Pa 1976) 1996;21:741–749de Camargo VM, Alburquerque-Sendin F, Berzin F, Stefanelli VC, de Souza DP.
Immediate effects on electromyographic activity and pressure pain thresholds after a
cervical manipulation in mechanical neck pain: a randomized controlled trial.
J Manipulative Physiol Ther. 2011;34:211–220Delitto A, Erhard RE, Bowling RW.
A treatment-based classification approach to low back syndrome: identifying and staging patients for conservative treatment.
Phys Ther. 1995;75:470–485Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC.
Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee.
A randomized, controlled trial.
Ann Intern Med. 2000;132:173–181Deyo RA.
Diagnostic evaluation of LBP: reaching a specific diagnosis is often impossible.
Arch Intern Med. 2002;162:1444–1447Deyo RA, Mirza SK, Turner JA, Martin BI.
Overtreating Chronic Back Pain: Time to Back Off?
J Am Board Fam Med. 2009 (Jan); 22 (1): 62–68Diers M, Koeppe C, Diesch E, Stolle AM, Holzl R, Schiltenwolf M, van Ackern K.
Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study.
J Clin Neurophysiol. 2007;24:76–83Fairbank JC, Pynsent PB.
The Oswestry Disability Index
Spine (Phila Pa 1976) 2000 (Nov 15); 25 (22): 2940–2952Fernandez-Carnero J, Fernandez-de-Las-Penas C, Cleland JA.
Immediate hypoalgesic and motor effects after a single cervical spine manipulation
in subjects with lateral epicondylalgia.
J Manipulative Physiol Ther. 2008;31:675–681Flynn T, Fritz J, Whitman J, Wainner R, Magel J, Rendeiro D. et al.
A Clinical Prediction Rule for Classifying Patients with Low Back Pain
who Demonstrate Short-term Improvement with Spinal Manipulation
Spine (Phila Pa 1976). 2002 (Dec 15); 27 (24): 2835–2843Fritz JM, Childs JD, Flynn TW.
Pragmatic Application of a Clinical Prediction Rule in Primary Care to Identify Patients with
Low Back Pain with a Good Prognosis Following a Brief Spinal Manipulation Intervention
BMC Fam Pract. 2005 (Jul 14); 6 (1): 29Fritz JM, George SZ, Delitto A.
The role of fear-avoidance beliefs in acute low back pain: relationships with
current and future disability and work status.
Pain. 2001;94:7–15Fritz JM, Irrgang JJ.
A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale.
Phys Ther. 2001;81:776–788Frost H, Lamb SE, Stewart-Brown S.
Responsiveness of a patient specific outcome measure compared with the Oswestry Disability Index v2.1
and Roland and Morris Disability Questionnaire for patients with subacute and chronic low back pain.
Spine. 2008;33:2450–2457Fulda KG, Slicho T, Stoll ST.
Patient expectations for placebo treatments commonly used in osteopathic manipulative treatment (OMT) clinical trials: a pilot study.
Osteopath Med Prim Care. 2007;1:3Gagliese L, Weizblit N, Ellis W, Chan VW.
The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients.
Pain. 2005;117:412–420George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr., Robinson ME.
Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study.
BMC Musculoskelet Disord. 2006;7:68George SZ, Dannecker EA, Robinson ME.
Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor
predicts tolerance or blood pressure reactivity: An experimental investigation in pain-free individuals.
Eur J Pain. 2005George SZ, Hirsh AT.
Distinguishing patient satisfaction with treatment delivery from treatment effect:
a preliminary investigation of patient satisfaction with symptoms after physical therapy
treatment of low back pain.
Arch Phys Med Rehabil. 2005;86:1338–1344Gibbons P, Tehan P.
Patient positioning and spinal locking for lumbar spine rotation manipulation.
Man Ther. 2001;6:130–138Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ.
Evidence of augmented central pain processing in idiopathic chronic low back pain.
Arthritis Rheum. 2004;50:613–623Guan Y, Borzan J, Meyer RA, Raja SN.
Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms.
J Neurosci. 2006;26:4298–4307Hahne AJ, Keating JL, Wilson SC.
Do within-session changes in pain intensity and range of motion predict between-session
changes in patients with low back pain?
Aust J Physiother. 2004;50:17–23Hancock MJ, Maher CG, Latimer J, McAuley JH.
Selecting an appropriate placebo for a trial of spinal manipulative therapy.
Aust J Physiother. 2006;52:135–138Hartrick CT, Kovan JP, Shapiro S.
The numeric rating scale for clinical pain measurement: a ratio measure?
Pain Pract. 2003;3:310–316Hawk C, Long CR, Rowell RM, Gudavalli MR, Jedlicka J.
A randomized trial investigating a chiropractic manual placebo: a novel design using
standardized forces in the delivery of active and control treatments.
J Altern Complement Med. 2005;11:109–117Hill JC, Dunn KM, Lewis M, et al.
A Primary Care Back Pain Screening Tool:
Identifying Patient Subgroups For Initial Treatment
(The STarT Back Screening Tool)
Arthritis and Rheumatism 2008 (May 15); 59 (5): 632–641Hirsh AT, Atchison JW, Berger JJ, Waxenberg LB, Lafayette-Lucey A.
Patient satisfaction with treatment for chronic pain: predictors and relationship to compliance.
Clin J Pain. 2005;21:302–310Hurwitz EL:
Epidemiology: Spinal Manipulation Utilization
J Electromyogr Kinesiol. 2012 (Oct); 22 (5): 648–654Iles RA, Davidson M, Taylor NF.
Psychosocial predictors of failure to return to work in non-chronic non-specific low back pain:
a systematic review.
Occup Environ Med. 2008;65:507–517Jensen MP, Karoly P, Braver S.
The measurement of clinical pain intensity: a comparison of six methods.
Pain. 1986;27:117–126Jensen MP, Smith DG, Ehde DM, Robinsin LR.
Pain site and the effects of amputation pain: further clarification of the meaning
of mild, moderate, and severe pain.
Pain. 2001;91:317–322Kalauokalani D, Cherkin DC, Sherman KJ, Koepsell TD, Deyo RA.
Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects.
Spine. 2001;26:1418–1424Keller A, Hayden J, Bombardier C, van TM.
Effect sizes of non-surgical treatments of non-specific low-back pain.
Eur Spine J. 2007;16:1776–1788Kindler LL, Bennett RM, Jones KD.
Central sensitivity syndromes: mounting pathophysiologic evidence to link fibromyalgia
with other common chronic pain disorders.
Pain Manag Nurs. 2011;12:15–24Koes BW, van Tulder M, Lin CW, Macedo LG, McAuley J, Maher C.
An Updated Overview of Clinical Guidelines for the Management of Non-specific Low Back Pain
in Primary Care
European Spine Journal 2010 (Dec); 19 (12): 2075–2094Licciardone JC, Russo DP.
Blinding protocols, treatment credibility, and expectancy: methodologic issues in
clinical trials of osteopathic manipulative treatment.
J Am Osteopath Assoc. 2006;106:457–463Linde K, Witt CM, Streng A, Weidenhammer W, Wagenpfeil S, Brinkhaus B.
The impact of patient expectations on outcomes in four randomized controlled trials
of acupuncture in patients with chronic pain.
Pain. 2007;128:264–271Michener LA, Kardouni JR, Lopes Albers AD, Ely JM.
Development of a sham comparator for thoracic spinal manipulative therapy for use with shoulder disorders.
Man Ther. 2013;18:60–64Millan M, Leboeuf-Yde C, Budgell B, Amorim MA.
The Effect of Spinal Manipulative Therapy on Experimentally Induced Pain:
A Systematic Literature Review
Chiropractic & Manual Therapies 2012 (Aug 10); 20 (1): 26Myers SS, Phillips RS, Davis RB, Cherkin DC, Legedza A,
Kaptchuk TJ, Hrbek A, Buring JE, Post D, Connelly MT, Eisenberg DM.
Patient Expectations as Predictors of Outcome in Patients with Acute Low Back Pain
Journal of General Internal Medicine 2008 (Feb); 23 (2): 148–153O'Neill S, Manniche C, Graven-Nielsen T, rendt-Nielsen L.
Generalized deep-tissue hyperalgesia in patients with chronic low-back pain.
Eur J Pain. 2007;11:415–420Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L.
The Pain Catastrophizing Scale: further psychometric evaluation with adult samples.
J Behav Med. 2000;23:351–365Price DD, Bush FM, Long S, Harkins SW.
A comparison of pain measurement characteristics of mechanical visual analogue and
simple numerical rating scales.
Pain. 1994;56:217–226Price DD, Finniss DG, Benedetti F.
A Comprehensive Review of the Placebo Effect: Recent Advances and Current Thought.
Annu Rev Psychol. 2007Price DD, Hu JW, Dubner R, Gracely RH.
Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses.
Pain. 1977;3:57–68Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS.
An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm.
Pain. 1999;83:147–156Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ.
Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients.
Pain. 2002;99:49–59Ricci JA, Stewart WF, Chee E, Leotta C, Foley K, Hochberg MC.
Back pain exacerbations and lost productive time costs in United States workers.
Spine. 2006;31:3052–3060Rygh LJ, Svendsen F, Fiska A, Haugan F, Hole K, Tjolsen A.
Long-term potentiation in spinal nociceptive systems--how acute pain may become chronic.
Psychoneuroendocrinology. 2005;30:959–964Santilli V, Beghi E, Finucci S.
Chiropractic Manipulation in the Treatment of Acute Back Pain and Sciatica
with Disc Protrusion: A Randomized Double-blind Clinical Trial
of Active and Simulated Spinal Manipulations
Spine J. 2006 (Mar); 6 (2): 131—137Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS.
When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function.
Pain. 1995;61:277–284Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA.
Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain.
J Pain. 2006;7:261–271Spinhoven P, Ter KM, Kole-Snijders AM, Hutten MM, Den Ouden DJ, Vlaeyen JW.
Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain.
Eur J Pain. 2004;8:211–219Staud R.
Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia.
Curr Rheumatol Rep. 2011;13:513–520Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr.
Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome.
Pain. 2003;102:87–95Staud R, Robinson ME, Price DD.
Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients.
J Pain. 2007;8:893–901Staud R, Robinson ME, Vierck CJ, Jr., Cannon RC, Mauderli AP, Price DD.
Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome.
Pain. 2003;105:215–222Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD.
Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome.
Pain. 2001;91:165–175Staud R, Weyl EE, Price DD, Robinson ME.
Mechanical and heat hyperalgesia highly predict clinical pain intensity in patients with chronic musculoskeletal pain syndromes.
J Pain. 2012;13:725–735Sullivan MJ, Bishop S, Pivik J.
{UNPRINTABLE FOREIGN TITLE}
Psychol Asess. 1995;4:524–532.Thompson AG, Sunol R.
Expectations as determinants of patient satisfaction: concepts, theory and evidence.
Int J Qual Health Care. 1995;7:127–141Valencia C, Fillingim RB, George SZ.
Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain.
J Pain. 2011;12:133–140Vase L, Petersen GL, Riley JL, III, Price DD.
Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007.
Pain. 2009;145:36–44Vase L, Robinson ME, Verne GN, Price DD.
The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation.
Pain. 2003;105:17–25Vernon H, MacAdam K, Marshall V, Pion M, Sadowska M.
Validation of a Sham Manipulative Procedure for the Cervical Spine for Use in Clinical Trials
J Manipulative Physiol Ther 2005 (Nov); 28 (9): 662–666Vernon HT, Aker P, Burns S, Viljakaanen S, Short L.
Pressure pain threshold evaluation of the effect of spinal manipulation in the treatment of chronic neck pain: a pilot study.
J Manipulative Physiol Ther. 1990;13:13–16Waddell G.
Low back pain: a twentieth century health care enigma.
Spine. 1996;21:2820–2825Waddell G, Newton M, Henderson I, Somerville D, Main CJ.
A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability.
Pain. 1993;52:157–168Witt CM, Martins F, Willich SN, Schutzler L.
Can I help you? Physicians’ expectations as predictor for treatment outcome.
Eur J Pain. 2012;16:1455–1466Woby SR, Roach NK, Urmston M, Watson PJ.
Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia.
Pain. 2005;117:137–144
Return to LOW BACK PAIN
Return to SPINAL PAIN MANAGEMENT
Return to CHIROPRACTIC SUBLUXATION
Since 5-25-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |