

A SMART Design to Determine the Optimal Treatment
of Chronic Pain Among Military PersonnelThis section was compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Contemp Clin Trials. 2018 (Oct); 73: 6874 ~ FULL TEXT
OPEN ACCESS Diane Flynn, Linda H. Eaton, Dale J. Langford, Nicholas Ieronimakis, Honor McQuinn, Richard O. Burney, Samuel L. Holmes, Ardith Z. Doorenbos
Madigan Army Medical Center,
9040 Jackson Ave,
Tacoma, WA 98431, USA.
diane.m.flynn4.civ@mail.mil
Chronic pain is a leading cause of disability among active duty service members in the U.S. armed forces. Standard rehabilitative care and complementary and integrative health therapies are used for chronic pain rehabilitation. However, the optimal sequence and duration of these therapies has yet to be determined. This article describes a sequential multiple assignment randomized trial (SMART) protocol being used to identify the optimal components and sequence of standard rehabilitative care and complementary and integrative health therapies for reducing pain impact and improving other patient outcomes. Active duty service members referred to Madigan Army Medical Center for treatment of chronic pain are being recruited to the Determinants of the Optimal Dose and Sequence of Functional Restoration and Integrative Therapies study.
Study participants are randomized to either standard rehabilitative care (physical and occupational therapy and psychoeducation) or complementary and integrative health therapies (chiropractic, acupuncture, yoga and psychoeducation).
Those participants who do not respond to the first 3 weeks of treatment are randomized to receive an additional 3 weeks of either(1) the alternative treatment or
(2) the first-stage treatment plus the alternative treatment.This study will also determine factors associated with treatment response that can support clinical decision making, such as baseline fitness, pain catastrophizing, kinesiophobia, post-traumatic stress, pain self-efficacy, and biological indicators. The information gained from this research will be applicable to all integrative chronic pain rehabilitation programs throughout the U.S. Department of Defense and the U.S. Department of Veterans Affairs, and the broader rehabilitation community.
There are more articles like this @ our: SPINAL PAIN MANAGEMENT Page KEYWORDS: Chronic pain; Complementary therapies; Integrative health therapies; Military; Rehabilitation; SMART design
TRIAL REGISTRY: ClinicalTrials.gov website (NCT03297905).
From the FULL TEXT Article:
Introduction
Chronic pain is a leading cause of both short- and long-term disability among U.S. Military service members [13]; and causes significant attrition from active duty. Among 34,006 service members evacuated from Operation Iraqi Freedom and Operation Enduring Freedom, diagnoses of spinal pain and musculoskeletal or connective tissue disorders were associated with significantly reduced odds of returning to active duty (odds ratio of 0.41 and 0.46, respectively). [4] The Department of Defense/Veterans Administration Pain Management Taskforce Report emphasizes the need for prevention, early identification, greater emphasis on nonpharmacologic therapies, and proper rehabilitation and reintegration of service members and veterans suffering from acute and chronic pain. [5, 6] To address these goals, each Army medical center has established an interdisciplinary pain management center.
Many Army interdisciplinary pain management centers offer functional restoration (FR) for chronic pain rehabilitation. This interdisciplinary approach combines standard rehabilitative care (SRC), such as physical therapy and occupational therapy, with quantitative progression of exercise and disability management using psychological and case management techniques. [7] Civilian FR programs typically include more than 100 treatment hours. However, active duty service members often find it challenging to commit to the time needed for the most intensive level of FR treatment, and there is a lack of research regarding the ideal duration of treatment for military populations.
Complementary and integrative health (CIH) therapies, in addition to SRC and FR, are also used for injury rehabilitation in Army interdisciplinary pain management centers. These therapies include acupuncture [8, 9], mind-body therapies (e.g., yoga, mindfulness-based therapies, and biofeedback) [1016], and manual therapies (e.g., chiropractic and therapeutic medical massage). [1720] While SRC and CIH treatment approaches have been implemented in Army interdisciplinary pain management centers [21, 22], the optimal sequence of these therapies has yet to be determined. Patients with chronic pain may be reluctant to engage in conventional rehabilitative therapies such as physical and occupational therapies due to kinesiophobia (fear of movement). [23] However, they may be more willing to engage in therapies that require less physical motion such as acupuncture, chiropractic treatment, and some yoga practices. Thus, a sequence of CIH therapies prior to SRC may be more acceptable to patients than the reverse sequence.
Sequential multiple assignment randomized trial (SMART) research design is a good way to evaluate the sequence and duration of therapies that best improves patient outcomes. A SMART design can evaluate sequences of treatment while adapting the treatment to individual responses to achieve optimal long-term outcomes. [24, 25] A SMART design randomly assigns each patient to each stage of treatment, yet allows modifications to treatment intensity, type, or delivery to balance patient benefits with potential risks. [24, 26] A tailoring variable is assessed at baseline and at each stage of treatment to determine if a participant is responding to treatment. [25, 27] This approach resembles the way treatment and treatment decisions naturally evolve over time in clinical practice. [24, 25] The SMART design is thus a powerful tool for pragmatic clinical trials.
In addition to determining optimal treatment sequence, a SMART design can identify predictive variables that may allow tailoring of treatment strategies to the unique needs of patient subgroups. [24] For example, biochemical and genetic biomarkers may be valuable for identifying patients who respond to specific pain management therapies. Biomarkers of psychological and physiological stress constitute intriguing candidates as both (i.e., salivary cortisol and urinary 8-hydroxy- 2'-deoxyguanosine (8-OHdG), respectively) have been implicated in the experience of pain and functional outcomes following rehabilitation programs. [28, 29] Evaluating biomarkers associated with treatment responses makes it possible to develop more precise treatment approaches.
This paper describes a SMART trial protocol designed to[1] determine the optimal treatment combination, sequence, and duration of SRC and CIH therapies among active duty service members with chronic pain; and
[2] identify predictors (e.g., biomarkers) of positive treatment response.
SMART study design and methods
All study procedures were approved by the institutional review board of the U.S. Department of the Army, Regional Health CommandPacific, the Human Research Protection Office of the U.S. Army Medical Research and Materiel Command, and the University of Washington institutional review board. The study is registered on the National Institutes of Health U.S. National Library of Medicine ClinicalTrials.gov website (NCT03297905).
Overview of the SMART study design
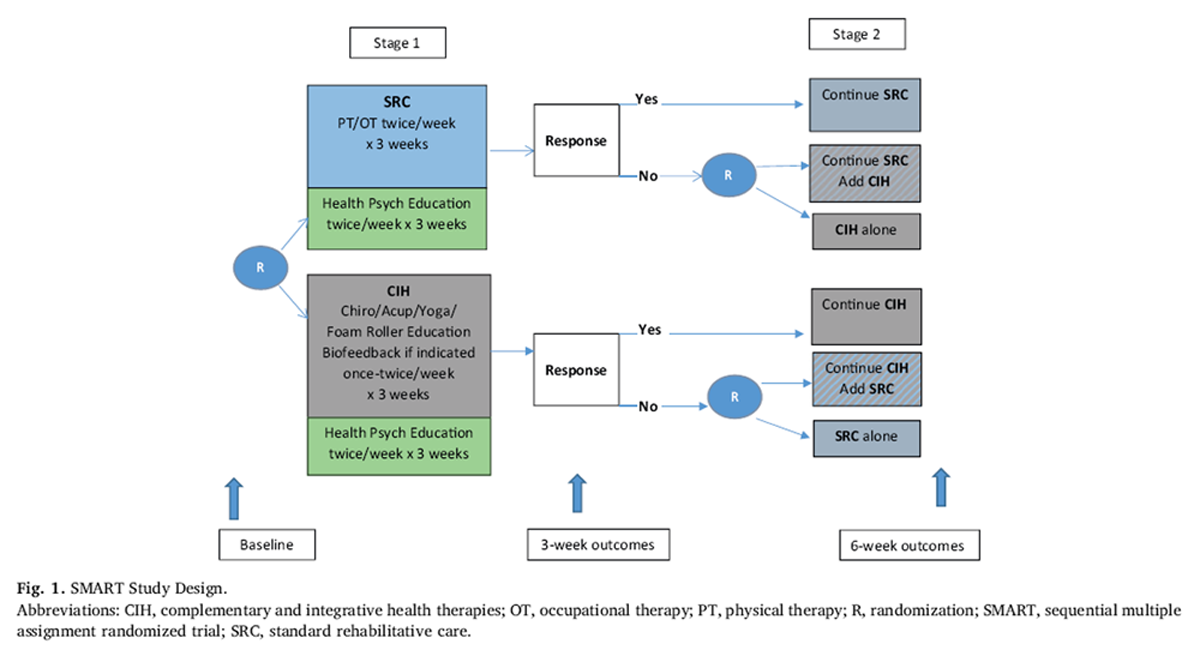
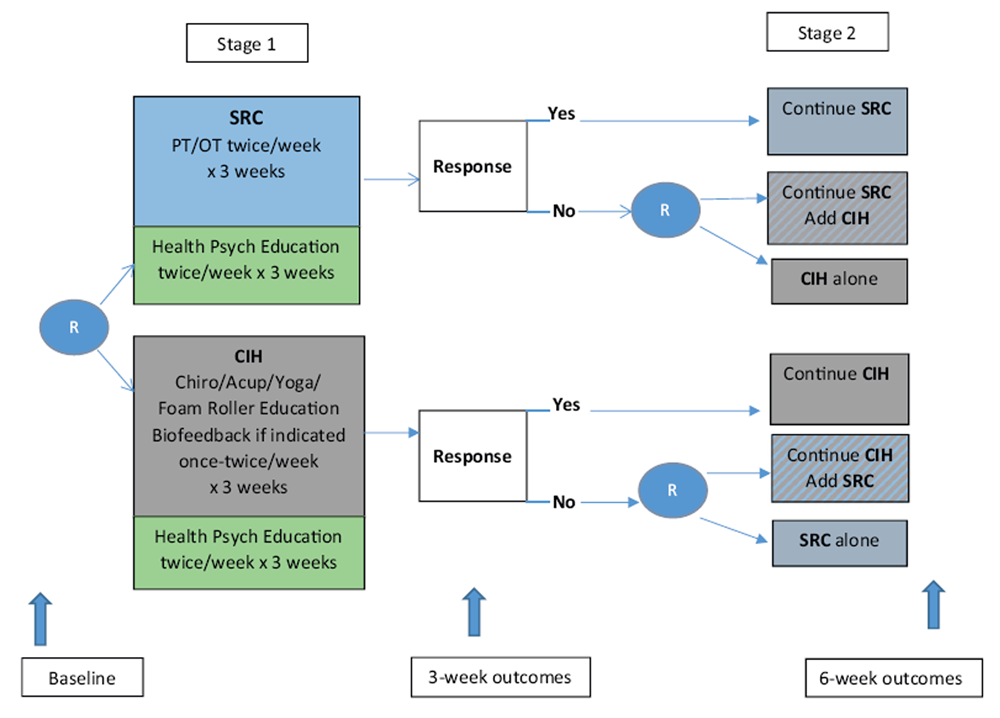
Figure 1 The Determinants of the Optimal Dose and Sequence of Functional Restoration and Integrative Therapies study uses a SMART design, as shown in Figure 1. For the first intervention stage, participants are randomly assigned to 3 weeks of SRC or CIH. The SRC group receives physical and occupational therapy. The CIH group receives chiropractic treatment, acupuncture, yoga, and foam roller instruction; biofeedback may also be included if indicated. Both groups receive psychoeducation about pain management taught by a psychological technician. During the third week of the first stage of intervention, the Pain Impact Score [30] is reassessed and compared with the baseline score. Those participants who show an improvement of 3 points or greater in their Pain Impact Score continue with their assigned treatment for an additional 3 weeks. Although the minimum clinically important difference for pain impact score has not yet been empirically determined, the National Institutes of Health (NIH) Task Force on Research Standards for Chronic Low Back Pain propose that a change in 3 is a reasonable estimate of the minimum clinically important difference for a wide range of musculoskeletal pain conditions based on previous analysis of responsiveness of pain impact score component measures. [31]
Nonresponders are randomly assigned to 3 weeks of[1] the alternative treatment or
[2] a combination of their original treatment plus the alternative treatment.The duration of 3 weeks of therapy for each phase was determined based on the study clinicians' expertise who have found that it requires a minimum of 56 treatments to determine if a patient is responding to therapy.
Specific aimsAim 1 The study's primary aim is to determine the optimal treatment combination, sequence, and duration of a 36 week program of selected CIH therapies alone or in combination with a 36 week SRC program and the corresponding effectiveness in improving pain impact.
Aim 2 The secondary aim is to identify the optimal combination, sequence, and duration of therapies that result in the greatest improvement in[1] patient-reported secondary outcomes (i.e., depressive symptoms, anxiety, anger, sleep disturbance, and fatigue)
[2] functional capacity tests
[3] biological indicators (i.e., cortisol and 8-OHdG levels), and
[4] force readiness.Aim 3 The study's third aim is to identify factors that predict successful primary and secondary outcomes at 6 weeks. These factors might include sex, baseline fitness, pain catastrophizing, kinesiophobia, posttraumatic stress, pain self-efficacy, injury, and biological indicators (i.e., cortisol [levels and genotype], 8-OHdG).
Sample
Study participants are active duty service members referred to the Madigan Army Medical Center Interdisciplinary Pain Management Center for treatment of chronic pain and determined to be candidates for the Determinants of the Optimal Dose and Sequence of Functional Restoration and Integrative Therapies study at initial evaluation by a medical provider.
Eligible participants have[1] chronic pain of 3 or more months' duration
[2] Patient-Reported Outcomes Measurement Information System (PROMIS) scores of at least 1 standard deviation below the mean in the domains of pain interference or physical function and/or average pain intensity of at least 3 on a scale of zero to 10; and
[3] the ability to speak, read, and write in English.Patients are not eligible for the study if they have
[1] prior surgery within the past 6 months, or are scheduled for an upcoming surgery (unless cleared by their surgeon)
[2] unstable psychological disorders; or [3] active substance use disorder.Sample size
To determine the sample size for this study, we used the SMART sample size calculator for continuous outcomes (http://methodologymedia.psu.edu/smart/samplesize). [32] The minimum required sample size to test the primary hypotheses is n = 225, based on a required power of 80% to detect a medium effect size (f = 0.25) in the primary outcome (i.e., pain impact score) at alpha=0.05. From our past experience with clinical trials at Madigan Army Medical Center, we expect to observe approximately 20% attrition in this study, including loss to follow-up. To account for the possibility of a 20% attrition rate (10% following each randomization stage), and to ensure balanced samples in each stage of the treatment arm, the goal is to enroll 280 participants.
ProceduresRecruitment Patients are screened, recruited, and consented after the first visit with a medical provider (physician, nurse practitioner, or physician assistant) at the Madigan Interdisciplinary Pain Management Center. Baseline measures are also completed at this time. A patient who does not consent to the study will receive either SRC or CIH at the discretion of their health care provider.
Randomization After baseline data collection, participants are randomized to either SRC or CIH. A second randomization occurs for participants who do not respond to the first 3 weeks of treatment. Nonresponders are then randomized to either the alternative treatment or to a combination of the same first-stage treatment plus the alternative treatment for 3 weeks. Responders continue with the same first-stage treatment for an additional 3 weeks. In the end, there will be 6 randomization groups to analyze. Two groups will be comprised of those who respond to the initial 3 weeks of therapy and continue the same therapy for another 3 weeks (i.e., SRC-SRC and CIH-CIH). Two groups will be comprised of those who do not show favorable response to the initial 3 weeks of therapy and randomize to the alternate approach (i.e., SRC-CIH and CIH-SRC). The final two groups will be comprised of those who do not respond favorably to the initial 3 weeks of therapy and randomize to a combination of both approaches (i.e., SRC-CIH/SRC and CIH-CIH/SRC).Intervention
The SRC group engages in physical and occupational therapy and psychoeducation. Physical therapy is conducted by a licensed physical therapist alternating with a physical therapy assistant and uses therapeutic exercises, assistive devices if needed, and patient education and training about the preservation, enhancement, or restoration of movement and physical function. Occupational therapy is conducted by a licensed occupational therapist alternating with a certified occupational therapy assistant and focuses on enabling or encouraging participation in meaningful activities of daily life, such as self-care skills, or in work activities. Physical and occupational therapies are scheduled for two 45min sessions of each per week for 3 weeks. The frequency and duration of therapy is standardized for all participants and is based on the clinical input of providers from each discipline.
The CIH group receives chiropractic treatment, acupuncture, and yoga. Chiropractic treatment is conducted by a licensed chiropractor and focuses on manual adjustment or manipulation of the spine and joints and is provided for 15 min twice per week. Acupuncture is conducted by a licensed acupuncture therapist and uses fine needles inserted through the skin at specific points to relieve pain or discomfort. Yoga is conducted by a certified yoga therapist and involves a system of physical postures and breathing techniques. Both acupuncture and yoga are tailored to each patient's presentation and provided 2 times per week for 1 h.
Both SRC and CIH groups participate in health psychology education classes. Classes are taught by a psychological technician, are 1 h in duration, and provided 2 times per week. Class topics include automatic thoughts, resiliency, healthy sleep habits, autogenics/imagery, priorities/ values/goal setting in regards to specific domains (relationships, career, education, personal growth, community), pain behaviors, and stress management tactics to reduce tension. During class, service members are encouraged to disclose appropriately and apply the concepts, strategies, and techniques to manage the impact their chronic pain has had on their functioning, decision-making, and quality of life. In addition, any patients with PASTOR assessments showing severe levels of depression, anxiety or pain catastrophizing or screen positive for problem opioid or alcohol use are referred for assessment by a clinical psychologist.Intervention fidelity Treatment fidelity for the study is assured through established methods outlined in the NIH Behavior Change Consortium Treatment Fidelity Guidelines. [33, 34] Role-playing is used to ensure that team members understand the protocol for interacting with participants. All contact with participants is scripted and randomly and regularly reviewed by the site principal investigator. Treatment delivery is monitored by the interdisciplinary pain management center clinical team leader who ensures that the treatment team discusses each study participant at 3 weeks and 5 weeks. Discussion includes treatment adherence. If participants miss several treatments, they are contacted by the team leader who asks them to sign a letter of commitment in order to proceed with treatment. Receipt of treatment is assessed by the research team by monitoring participants' appointments. Enactment of the treatment skills occurs during follow-up appointments, when participants are asked about their use of the interventions.
Measures
The study uses the Pain Assessment Screening Tool and Outcomes Registry (PASTOR) to collect participant demographic and outcomes data. PASTOR was developed by the Defense and Veterans Center for Integrative Pain Management in collaboration with Northwestern University to collect outcomes data on multiple domains relevant to pain management. [35] PASTOR includes the Defense and Veterans Pain Rating Scale; several PROMIS measures, including sleep, fatigue, anxiety, depression, pain interference, physical function, and satisfaction with social roles; the pain catastrophizing scale; and screens for PTSD, and alcohol and medication misuse. PASTOR is completed online by use of a computer or smart phone and the PROMIS measures apply computer adaptive testing to minimize the survey burden.Primary outcome measures
Pain impact score. The primary outcome assessed in this study is the Pain Impact Score, a validated composite of PROMIS measures including pain intensity, pain interference, and physical function that has been recommended by the NIH Task Force on Research Standards for Chronic Low Back Pain for musculoskeletal pain. [36]
Secondary outcome measures
PROMIS measures. The PROMIS global health item pool [37, 38] assesses health in general with items that include global ratings of the five primary PROMIS domains (physical function, fatigue, pain, emotional distress, social health) as well as of perceptions of general health that span domains. PASTOR also includes the PROMIS item banks for depressive symptoms [38], anxiety [38], emotional distress (anger) [39], sleep disturbance [40], and fatigue. [41] These secondary outcomes are highly relevant to pain management outcomes.
Functional capacity tests. Functional capacity tests on endurance and lifting strength are standard outcomes that FR programs use to complement patient-reported outcomes. These tests include the Modified Naughton treadmill test, which measures the pace and duration of time on a treadmill; the floor-waist lift test, assessing the amount of weight an individual can lift from floor to waist height without experiencing an increase in pain; the waist-shoulder lift test, assessing the amount of weight an individual can lift from waist to shoulder height; and the 40ft carry test, assessing the amount of weight an individual can carry while walking a distance of 40 ft. These functional measures are the standard assessments used by the physical therapists at the study site to determine cardiovascular fitness and motor strength.
Force readiness. Force readiness is assessed based on participants' Military Readiness Category status recorded at baseline and follow-up in the Army Medical Operational Data System. The Medical Readiness Category is determined by whether an Army soldier is physically able to perform military duties versus having a temporary or permanent medical condition that interferes with the ability to perform duties. [42]Potential predictors of treatment response
Data on potential predictors of treatment response are collected with the following instruments included in PASTOR:
A treatment modalities questionnaire asking about 9 types of prior and current treatment modalities and their effectiveness.
PROMIS Prescription Pain Medication Misuse Measure. [43]
PROMIS Alcohol Use [44]
Activity goals, asking the patient to identify 3 important goals and the degree to which pain interferes with those activities.
Defense and Veterans Pain Rating Scale [45], a color-coded 11point numeric pain rating scale (010), with descriptive anchors of pain severity for each number on the scale.
Pain Catastrophizing Scale [46], a 13item, 3dimension survey of trait pain catastrophizing that include items measuring a patient's tendency for [1] rumination [2], magnification, and [3] helplessness.
PROMIS neuropathic pain screen [47]
Primary Care Post-traumatic Stress Disorder Screen for DSM-5 (PC_PTSD-5) [48], a 5item tool that includes an introductory sentence to cue respondents to traumatic events.
In addition, participants complete the following surveys, which are not included in PASTOR:
Month and year pain began
How pain began (injury due to lifting, sports/recreation, training/ job, deployment, motor vehicle accident, following surgery; uncertain cause; or other cause).
The Patient Activation Measure [49], a 22item survey on 4 stages of activation
[1]: believing the patient role is important
[2], having the confidence and knowledge necessary to take action
[3], actually taking action to maintain and improve one's health, and
[4] staying the course even under stress.Tampa Scale for Kinesiophobia [50], an 11item survey assessing pain-related fear in patients with back pain.
Pain Self-Efficacy Questionnaire [51], a 10item survey assessing patients' self-efficacy beliefs with respect to their pain.
Chronic Pain Acceptance Questionnaire [52], an 8item survey of
[1] the degree to which one engages in life activities regardless of pain (activity engagement), and
[2] one's willingness to experience pain, or the inverse of engaging in behaviors to limit pain (pain willingness).All participants are scheduled to complete assessments at baseline, 3 weeks, and 6 weeks, as illustrated in Figure 1.
Biological measures
Salivary cortisol samples are collected at baseline: participants are asked to collect 3 samples using Sarstedt Salivette collection tubes on the day preceding their first day of treatment in their first-stage intervention. Samples are taken upon awakening (0 min), at 30 min after awakening, and in the evening prior to going to bed. This collection schedule allows for analysis of both cortisol awakening response and diurnal cortisol variability (difference between peak cortisol and baseline/evening cortisol levels). [53]Participants are instructed to avoid caffeinated or sugary drinks, food/breakfast, brushing teeth, smoking, and physical exercise thirty minutes prior to taking their morning samples, as these activities have been found to affect the cortisol awakening response. [53] Participants are also instructed to refrigerate the samples immediately following collection and to bring them to the clinic on the first day of their rehabilitation program. [53] Post-treatment salivary samples are collected using the same sampling schedule on the final day of the second-stage intervention. Participants refrigerate these post-treatment samples to the interdisciplinary pain management center.
Oxidative stress (8-OHdG) urine samples are collected at baseline and at the post-treatment period following completion of the secondstage intervention. At baseline, participants are asked to provide a urine sample immediately after their team intake appointment, which takes place between 11 a.m. and 3 p.m. on the day of study enrollment. The post-treatment urine sample is taken in person at the interdisciplinary pain management center upon completion of the second-stage intervention. Urine samples will be stored for the duration of the study at ?80 degrees Celsius for up to one year, until the recruitment period is over and analyses are ready to begin. Of note, urinary 8-OHdg has been shown to be stable when stored at 80 degrees Celsius for more than two years. [54]
For CYP17A1 and CYP11B1 polymorphisms, next generation sequencing is conducted using buccal swabs collected during enrollment. Buccal swab is an efficient, easy, and reliable collection method for targeted genomic DNA (gDNA) analysis. [55] Buccal swabs are collected following saliva collection; samples are stored at 80 degrees Celsius until sufficient numbers are accumulated for processing. gDNA is purified using standard procedures with the Qiagen Gentra Puregene Buccal Cell Kit, with the fidelity of each sample's gDNA confirmed by the Agilent 2100 Bioanalyzer electrophoresis system. Samples that fail quality control are omitted and a second buccal swab collected. Samples that pass quality control are prepared for targeted sequencing of CYP17A1 and CYP11B1 genes using the Illumina TruSeq Custom Amplicon Kits. Sequences are read on the Illumina MiSeq NGS platform; the read alignment is conducted using the Illumina BaseSpace applications. Post-acquisition analysis and comparison of polymorphisms are analyzed with Illumina BaseSpace reference libraries.Statistical analyses
All data are entered into a secure, web-based system. The amount of missing data is quantified first. Where possible, and determined by the amount of missing data, 3 indirect tests of the mechanism of missingness will be explored[1] Baseline demographic characteristics of those who ever and never missed data by group allocation will be compared (an indirect test to rule out missing completely at random)
[2] The difference between mean utility scores of participants with missing and nonmissing data will also be compared (an additional indirect test to rule out missing completely at random)
[3] Controlling for the probability of providing missing data, the mean scores of those that never missed an intervention will be compared with those in the control groups (an indirect test of missing not at random).While analysis using mixed models allows all observed data to be included under the assumption that the data are missing completely at random, where there is substantial missing data, multiple imputation or mean conditional imputation will be used with subsequent sensitivity analyses to explore the impact of imputation on results. To address the issue of artificially reduced estimates of stochastic uncertainty produced by imputation, bootstrapping procedures will be used on the entire imputation and estimation process.
Analyses for aim 1
Hypothesis 1. Among service members who show a favorable response to weeks 13 of either stage 1 treatment (SRC or CIH), they will demonstrate additional reduction in pain impact score following a second 3week course of the same therapeutic approach (SRC-SRC or CIH-CIH).
Hypothesis 2. Among service members who do not respond to CIH during weeks 13, those who have SRC added during weeks 46 (CIHCIH+ SRC), will report greater decrease in pain impact compared to those who randomize to three weeks of SRC alone during weeks 46 (i.e., CIH-SRC).
Hypothesis 3. Among patients who do not respond to SRC during weeks 13, those who have CIH added during weeks 46 (i.e., SRCSRC+ CIH), will report greater decrease in pain impact than those who randomize to three weeks of CIH alone during weeks 46 (i.e., SRCCIH).
Comparisons are carried out using statistical model 1, which relates the outcome Y (pain impact) at weeks 1 to 3 to the group assignment variable x1(SRC or CIH) and to pain impact at baselinex2: Y=X?+Z?+?. In this model, Z is a known design matrix that corresponds to repeated measures, and ? is an unknown vector of random effects. If errors are normally distributed, this model will be fit as a linear mixed effects model. Generalized linear mixed effects modeling will be used with the appropriate link function and error distribution if the outcome is not normally distributed and cannot be normalized using transformations. We are primarily interested in the additive effect of the group variable, and differences in the least square means will be tested according to the levels of variable x1. Next, the definition of pain impact response (improvement of more than 3 points on the Pain Impact Score) will be applied to classify participants in each group as responders or nonresponders to the intervention therapy. The characteristics of responders will be compared to those of nonresponders using t-tests, chi-square, or Fisher's exact tests.
The analytic strategy described above will also be implemented to compare the groups created by the second randomization. The repeated measures of pain impact (one at a time) during weeks 4 to 6 will be related to study group (CIH alone versus CIH+SRC) and to the pain impact measure at week 3. Additionally, this analysis will be conducted among those participants who did not respond to SRC during weeks 1 to 3. Descriptive statistics will be used to analyze demographic data and study variables for all randomization groups to ensure the groups are comparable.Analyses for aim 2
Hypothesis. Service members who complete the six weeks of treatment (CIH-CIH, SRC-SRC, CIH-CIH+SRC OR SRC-SRC+CIH) will show significantly improved outcomes on depressive symptoms, anxiety, anger, sleep disturbance, functional capacity tests, biological indicators, and force readiness compared to those who complete three weeks of treatment. (SRC or CIH).
The analysis for Aim 2 is the same as the analysis for Aim 1, but for the secondary outcome measures. Separate analyses will be performed for the PASTOR patient-reported outcomes, functional capacity tests of strength and endurance, biological measures of salivary cortisol and 8- OHdG, and force readiness status. To derive the quantitative measurement of cortisol, the salivary abundance will be detected by enzymelinked immunosorbent assay (ELISA). A global measure of cortisol response will then be calculated, using area under the curve to determine any changes in cortisol level between baseline and post-treatment intervals. Changes (from baseline to post-treatment) in urinary 8-OHdG levels will also be determined by ELISA and compared across treatment groups. Raw biological measures may be log transformed to ensure that appropriate assumptions are met for statistical analyses. Force readiness among Army participants will be evaluated based on 5 h3 Medical Readiness Category at baseline and follow-up, as recorded in the Army Medical Operational System.Analyses for aim 3
Hypothesis. Service members' sex, baseline fitness, pain catastrophizing, kinesiophobia, post-traumatic stress, pain self-efficacy, and/or biological indicators will predict successful primary and secondary treatment outcomes at 6 weeks.
For the Aim 3 analyses, we will use Q-learning [56], which is a method for using data to construct the decision rules that operationalize the optimal intervention. The Q-learning method will be implemented in SAS PROC QLEARN, developed by Murphy et al. [57] Tailoring for predictive variables that are not embedded in the SMART design (e.g., pain catastrophizing, baseline fitness, cortisol levels, 8-OHdG levels, CYP17A1 and CYP11B1 polymorphisms, sex) will be explored. For example, we will start by assessing the best stage 2 intervention for nonresponders. We will use data only from the nonresponders, to determine (e.g., at a given 8-OHdG level or for a given genotype) if the decision rule recommends increasing the amount of the stage 1 intervention or adding the alternate intervention.
Salivary enzyme immunoassays will be conducted to derive the quantitative measurement of salivary cortisol levels. The cortisol awakening response (area under the curve of 2 morning measures) and diurnal cortisol variability (difference between the 30min post-awakening and the evening measures) will be evaluated as potential biomarkers for successful treatment outcomes. The relationship between baseline urinary 8-OHdG levels and successful treatment outcomes will also be evaluated.
Polymorphisms in CYP17A1 and CYP11B1 examined by NGS will be compared with cortisol levels to determine potential functional effects and with treatment outcomes to investigate potential associations with responses to the intervention(s). Sequence analyses for each participant, as well as cortisol and 8-OHdG measurements, will be conducted blinded to the treatment group, by assigning participants a random code at the time of sample collection. The principal investigator will retain the code key and reveal the treatment group for each sample at the conclusion of the study. At that point, genetic polymorphisms, cortisol, and 8-OHdG values, for each participant sample will be matched with therapy outcomes to provide a comprehensive assessment of the utility of cortisol and 8-OHdG as measures that predict as well as assess treatment response.
Discussion: Benefits of SMART research design
To achieve health care advances that enhance the health of military service members and promote a fit and ready force, it is important that service members participate in well-designed research studies that result in findings directly applicable to their health care and clinical practice. Furthermore, with the growing emphasis on the importance of nonpharmacologic approaches in relieving chronic pain, it is critical that the optimal dose and sequence of these therapies are established. These findings may ultimately shorten the rehabilitation period and improve service member's quality of life; thus, leading to a healthier military force. The potential benefits of nonpharmacologic treatment modalities include pain relief, improved mood, and improved functional status. [58]
The study's SMART design will identify the optimal components and sequence of nonpharmacological interventions for reducing pain impact and improving other patient outcomes.
Specifically, the SMART design allows us to[1] compare the effectiveness of two nonpharmacological interventions
[2] identify subgroups of service members who do and do not respond to the interventions
[3] determine the most effective duration and sequencing of the interventions, and
[4] determine factors associated with treatment response that can support clinical decisionmaking.Identification of service members who respond to treatment and factors that predict successful outcomes will support development of tailored treatment strategies that promote service members' reintegration and fitness. The information gained from this study will be applicable to all integrative rehabilitation programs throughout the Department of Defense and the Department of Veterans Affairs, and the broader rehabilitation community including civilian populations.
Funding sources
National Institute of Nursing Research of the National Institutes of Health under award number K24NR015340. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors (Burney, Flynn, Holmes, Ieronimakis, and McQuinn) are employed by the U.S. Military or by the Federal Government. The views expressed are those of the author(s) and do not reflect the official policy of the U.S. Department of the Army, the U.S. Department of Navy, the U.S. Department of Defense, or the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
References:
H.L. Lew, J.D. Otis, C. Tun, R.D. Kerns, M.E. Clark, D.X. Cifu,
Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in
OIF/OEF veterans: polytrauma clinical triad,
J. Rehabil. Res. Dev. 46 (6) (2009) 697702.R.J. Gironda, M.E. Clark, R.L. Ruff, et al.,
Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies
for the polytrauma rehabilitation,
Rehabil. Psychol. 54 (3) (2009 Aug) 247258.M.E. Clark, R.L. Walker, R.J. Gironda, J.D. Scholten,
Comparison of pain and emotional symptoms in soldiers with polytrauma:
unique aspects of blast exposure,
Pain Med. 10 (3) (Apr 2009) 447455.S.P. Cohen, C. Brown, C. Kurihara, A. Plunkett, C. Nguyen, S.A. Strassels,
Diagnoses and factors associated with medical evacuation and return to duty for service members
participating in Operation Iraqi Freedom or Operation Enduring Freedom:
a prospective cohort study,
Lancet 375 (9711) (Jan 23 2010) 301309.Office of the Army Surgeon General,
Pain Management Task Force Final Report: Providing a Standardized DoD and VHA Vision
and Approach to Pain Management to Optimize the Care for Warriors and their Families, (2010).Office of the Army Surgeon General,
Comprehensive Pain Management Campaign Plan (CPMCP),
http://www.armymedicine.army.mil/reports/U.S._Army_Pain_Management_Campaign.pdf
Accessed date: 18 September 2010.T.G. Mayer, R.J. Gatchel,
Functional Restoration for Spinal Disorders: The Sports Medicine Approach,
Lea & Febiger, Philadelphia, PA, 1998.M. Lam, R. Galvin, P. Curry,
Effectiveness of acupuncture for nonspecific chronic low back pain: a systematic review and meta-analysis,
Spine 38 (24) (Nov 15 2013) 21242138.K.H. Kim, T.H. Kim, B.R. Lee, et al.,
Acupuncture for lumbar spinal stenosis: a systematic review and meta-analysis,
Complement. Ther. Med. 21 (5) (Oct 2013) 535556.S.E. Lakhan, K.L. Schofield,
Mindfulness-based therapies in the treatment of somatization \disorders: a systematic review and meta-analysis,
PLoS One 8 (8) (2013) e71834.S.H. Kim, S.M. Schneider, L. Kravitz, C. Mermier, M.R. Burge,
Mind-body practices for posttraumatic stress disorder,
J. Investig. Med. 61 (5) (Jun 2013) 827834.H. Cramer, R. Lauche, H. Haller, G. Dobos,
A systematic review and meta-analysis of yoga for low back pain,
Clin. J. Pain. 29 (5) (May 2013) 450460.H. Cramer, R. Lauche, C. Hohmann, et al.,
Randomized-controlled trial comparing yoga and home-based exercise for chronic neck pain,
Clin. J. Pain. 29 (3) (Mar 2013) 216223.H. Cramer, R. Lauche, J. Langhorst, G. Dobos,
Yoga for rheumatic diseases: a systematic review,
Rheumatology 52 (11) (Nov 2013) 20252030.G.J. Macfarlane, P. Paudyal, M. Doherty, et al.,
A systematic review of evidence for the effectiveness of practitioner-based complementary and
alternative therapies in the management of rheumatic diseases: osteoarthritis,
Rheumatology 51 (12) (Dec 2012) 22242233.C. Sturmberg, J. Marquez, N. Heneghan, S. Snodgrass, P. van Vliet,
Attentional focus of feedback and instructions in the treatment of musculoskeletal dysfunction:
a systematic review,
Man Ther. 18 (6) (Dec 2013) 458467.J.W. Brantingham, T.K. Cassa, D. Bonnefin, et al.,
Manipulative and multimodal therapy for upper extremity and temporomandibular disorders: a systematic review,
J. Manipulative Physiol. Ther. 36 (3) (Mar-Apr 2013) 143201.A.D. Furlan, M. Imamura, T. Dryden, E. Irvin,
Massage for low back pain: an updated systematic review within the framework of the Cochrane Back Review Group,
Spine 34 (16) (Jul 15 2009) 16691684.L.J. Kong, H.S. Zhan, Y.W. Cheng, W.A. Yuan, B. Chen, M. Fang,
Massage therapy for neck and shoulder pain: a systematic review and meta-analysis,
Evid. Based Complement. Alternat. Med. 2013 (2013) 110.S. Kumar, K. Beaton, T. Hughes,
The effectiveness of massage therapy for the treatment of nonspecific low back pain:
a systematic review of systematic reviews,
Int. J. Gen. Med. 6 (Sep 04 2013) 733741.D.M. Flynn, K.F. Cook, M. Kallen, C. Buckenmaier, et al.,
Use of the Pain Assessment Screening Tool and Outcomes Registry in an Army interdisciplinary
pain management center; lessons learned and future implications of a 10-month beta test,
Mil. Med. 182 (S1) (2017) 167174.D.M. Flynn,
Intensive Functional Rehabilitation for the Soldier and Athlete:
The Madigan Medical Center Experience, Paper presented at:
30th Annual Meeting of the American Academy of Pain Medicine, Phoenix, AZ, 2014.S.H. Kori, R.P. Miller, D.D. Todd,
Kinisophobia: a new view of chronic pain behavior,
Pain Manag. (1990) 3543.D. Almirall, S.N. Compton, M. Gunlicks-Stoessel, N. Duan, S.A. Murphy,
Designing a pilot sequential multiple assignment randomized trial for developing
an adaptive treatment strategy,
Stat. Med. 31 (17) (2012 Jul 30) 18871902.M.K. Song, A.D. Dabbs, S.E. Ward,
A SMART design to optimize treatment strategies for patient and family caregiver outcomes,
Nurs. Outlook 64 (4) (2016 Jul 1) 299305.X. Lu, I. Nahum-Shani, C. Kasari, K.G. Lynch, et al.,
Comparing dynamic treatment regimes using repeated-measures outcomes: modeling considerations in SMART studies,
Stat. Med. 35 (10) (2016) 15951615.D. Almirall, I. Nahum-Shani, N.E. Sherwood, S.A. Murphy,
Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research,
Transl. Behav. Med. 4 (3) (2014) 260274.Y.W. Hsieh, K.C. Lin, M. Korivi, T.H. Lee, C.Y. Wu, K.Y. Wu,
The reliability and predictive ability of a biomarker of oxidative DNA damage on functional outcomes after stroke rehabilitation,
Int. J. Mol. Sci. 15 (4) (Apr 16 2014) 65046516.L.L. Wu, C.C. Chiou, P.Y. Chang, J.T. Wu,
Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics,
Clin. Chim. Acta 339 (12) (2004) 19.R.A. Deyo, S.F. Dworkin, D. Amtmann, G. Andersson, et al.,
Report of the NIH Task Force on Research Standards for Chronic Low Back Pain
Journal of Pain 2014 (Jun); 15 (6): 569585R.A. Deyo, S.F. Dworkin, D. Amtmann, G. Andersson, et al.,
Report of the NIH Task Force on Research Standards for Chronic Low Back Pain
Pain Med. 15 (8) (2014) 12491267.A. Crivello, L. Levy, S. Murphy,
Evaluation of Sample Size Formulae for Developing Adaptive Treatment Strategies Using a SMART Design
(Accessed August 31, 2016).L.H. Eaton, A.Z. Doorenbos, K.L. Schmitz, K.M. Carpenter, B.A. McGregor,
Establishing treatment fidelity in a web-based behavioral intervention study,
Nurs. Res. 60 (6) (2011) 430435.B. Borrelli, D. Sepinwall, D. Ernst, et al.,
A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research,
J. Consult. Clin. Psychol. 73 (5) (Oct 2005) 852860.K.F. Cook, C. Buckenmaier 3rd, R.C. Gershon,
PASTOR/PROMIS (R) pain outcomes system: what does it mean to pain specialists?
Pain Manag. 4 (4) (Jul 2014) 277283.R.A. Deyo, S.F. Dworkin, D. Amtmann, et al.,
Focus article report of the NIH task force on research standards for chronic low back pain,
Clin. J. Pain. 30 (8) (Aug 2014) 701712.D. Cella, W. Riley, A. Stone, et al.,
The patient-reported outcomes measurement information system (PROMIS) developed and tested
its first wave of adult self-reported health outcome item banks: 2005-2008,
J. Clin. Epidemiol. 63 (11) (Nov 2010) 11791194.R.D. Hays, J.B. Bjorner, D.A. Revicki, K.L. Spritzer, D. Cella,
Development of physical and mental health summary scores from the patient-reported outcomes measurement
information system (PROMIS) global items,
Qual. Life Res. 18 (7) (Sep 2009) 873880.P.A. Pilkonis, S.W. Choi, S.P. Reise, et al.,
Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement
Information System (PROMIS(R)): depression, anxiety, and anger,
Assessment 18 (3) (Sep 2011) 263283.L. Yu, D.J. Buysse, A. Germain, et al.,
Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks, Behav.
Sleep Med. 10 (1) (Dec 28 2011) 624.D. Cella, J.S. Lai, S.E. Jensen, C. Christodoulou, D.U. Junghaenel, B.B. Reeve, A.A. Stone,
PROMIS fatigue item bank had clinical validity across diverse chronic conditions,
J. Clin. Epidemiol. 73 (May 2016) 128134.Office of the Surgeon General.
Medical readiness leader guide, version 2.
http://www.kansastag.gov/AdvHTML_Upload/files/Medical%20Readiness%20Leader%20Guide%
20September%2018%202012(1).pdf
[(Accessed July 14, 2018)].P.A. Pilkonis, L. Yu, N.E. Dodds, K.L. Johnston, S.M. Lawrence, T.F. Hilton, D.C. Daley, A.A. Patkar, D. McCarty,
An item bank for abuse of prescription pain medication from the patient-reported outcomes
measurement information system (PROMISฎ),
Pain Med. 18 (8) (2016) 15161527.P.A. Pilkonis, L. Yu, J. Colditz, N. Dodds, K.L. Johnston, C. Maihoefer, A.M. Stover, D.C. Daley, D. McCarty,
Item banks for alcohol use from the Patient-Reported Outcomes Measurement Information System (PROMISฎ):
Use, consequences, and expectancies,
Drug Alcohol Depend. 130 (13) (2013 Jun 1) 167177.C.C. Buckenmaier 3rd, K.T. Galloway, R.C. Polomano, M. McDuffie, N. Kwon, R.M. Gallagher,
Preliminary validation of the defense and veterans pain rating scale (DVPRS) in a military population,
Pain Med. 14 (1) (Jan 2013) 110123.M.J.L. Sullivan, S.R. Bishop, J. Pivik,
The Pain Catastrophizing Scale: Development and validation psychological assessment,
Psychol. Assess. 7 (4) (1995) 137144.R.L. Askew, K.F. Cook, F.J. Keefe, C.J. Nowinski, D. Cella, D.A. Revicki, E.M. Dewitt, K. Michaud,
A PROMIS measure of neuropathic pain quality,
Value Health 19 (5) (Jul 2016) 623630.A. Prins, M.J. Bovin, D.J. Smolenski, et al.,
The Primary Care PTSD Screen for DSM- 5 (PC-PTSD-5): Development and Evaluation Within a Veteran Primary Care Sample,
J. Gen. Intern. Med. 31 (10) (Oct 2016) 12061211.J.H. Hibbard, J. Stockard, E.R. Mahoney, M. Tusler,
Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers,
Health Serv. Res. 39 (4) (Aug 2004) 10051026 Pt 1.S.R. Woby, N.K. Roach, M. Urmston, P.J. Watson,
Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia,
Pain 117 (12) (Sep 2005) 137144.M.K. Nicholas,
The pain self-efficacy questionnaire: Taking pain into account,
Eur J Pain. Feb 11 (2) (2007) 153163.R.A. Fish, B. McGuire, M. Hogan, T.G. Morrison, I. Stewart,
Validation of the chronic pain acceptance questionnaire (CPAQ) in an Internet sample and development
and preliminary validation of the CPAQ-8,
Pain 149 (3) (Jun 2010) 435443.T. Stalder, C. Kirschbaum, B.M. Kudielka, et al.,
Assessment of the cortisol awakening response: Expert consensus guidelines,
Psychoneuroendocrinology 63 (Jan 2016) 414432.Y. Matsumoto, Y. Ogawa, R. Yoshida, A. Shimamori, H. Kasai, H. Ohta,
The stability of the oxidative stress marker, urinary 8-hydroxy-2?-deoxyguanosine (8-OHdG),
when stored at room temperature,
J. Occup. Health 50 (4) (2008) 366372.Y. Yin, J.H. Lan, D. Nguyen, N. Valenzuela, P. Takemura, Y.T. Bolon, B. Springer, K. Saito,
Application of High-Throughput Next-Generation Sequencing for HLA Typing on Buccal Extracted DNA:
Results from over 10,000 Donor Recruitment Samples,
PLoS One 11 (10) (2016) e0165810I. Nahum-Shani, M. Qian, D. Almirall, et al.,
Q-learning: a data analysis method for constructing adaptive interventions,
Psychol Methods. Dec 17 (4) (2012) 478494.A. Ertefaie, D. Almirall, L. Huang, J. Dziak, A. Wagner, S. Murphy,
SAS PROC QLEARN User's Guide.
University Park, The Methodology Center,
Penn State University, PA, 2012.H. Tick, A. Nielsen, K.R. Pelletier, R. Bonakdar, S. Simmons, R. Glick
Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care:
The Consortium Pain Task Force White Paper
Explore (NY). 2018 (May); 14 (3) 177211
Return to OPIOID EPIDEMIC
Return to SPINAL PAIN MANAGEMENT
Return to CHIROPRACTIC CARE FOR VETERANS
Since 12-31-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |