FROM:
European J Pain 2021 (Sep); 25 (8): 1644–1667 ~ FULL TEXT
Hainan Yu, Pierre Côté, Jessica J. Wong, Heather M. Shearer, Silvano Mior, Carol Cancelliere, et. al.
Centre for Disability Prevention and Rehabilitation,
Ontario Tech University and
Canadian Memorial Chiropractic College (CMCC),
Oshawa, Ontario, Canada.
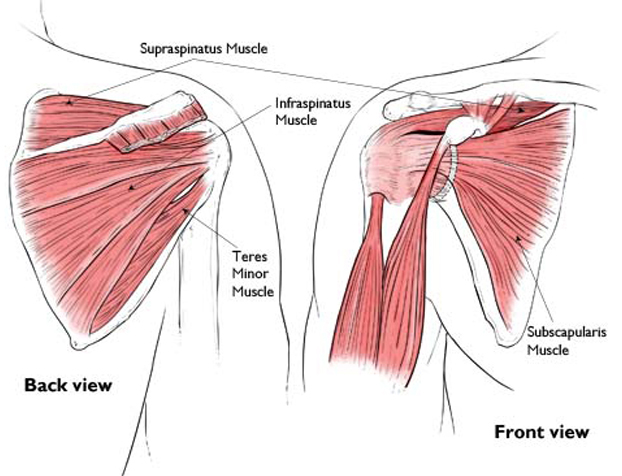
Reproduced with permission from The Body Almanac.
(c) American Academy of Orthopaedic Surgeons, 2003.
Objectives: Objective of this study is to develop an evidence-based guideline for the noninvasive management of soft tissue disorders of the shoulder (shoulder pain), excluding major pathology.
Methods: This guideline is based on high-quality evidence from seven systematic reviews. Multidisciplinary experts considered the evidence of effectiveness, safety, cost-effectiveness, societal and ethical values, and patient experiences when formulating recommendations. Target audience is clinicians; target population is adults with shoulder pain.
Results: When managing patients with shoulder pain, clinicians should (a) rule out major structural or other pathologies as the cause of shoulder pain and reassure patients about the benign and self-limited nature of most soft tissue shoulder pain; (b) develop a care plan in partnership with the patient; (c) for shoulder pain of any duration, consider low-level laser therapy; multimodal care (heat/cold, joint mobilization, and range of motion exercise); cervicothoracic spine manipulation and mobilization for shoulder pain when associated pain or restricted movement of the cervicothoracic spine; or thoracic spine manipulation; (d) for shoulder pain >3-month duration, consider stretching and/or strengthening exercises; laser acupuncture; or general physician care (information, advice, and pharmacological pain management if necessary); (e) for shoulder pain with calcific tendinitis on imaging, consider shock-wave therapy; (f) for shoulder pain of any duration, do not offer ultrasound; taping; interferential current therapy; diacutaneous fibrolysis; soft tissue massage; or cervicothoracic spine manipulation and mobilization as an adjunct to exercise (i.e., range of motion, strengthening and stretching exercise) for pain between the neck and the elbow at rest or during movement of the arm; (g) for shoulder pain >3-month duration, do not offer shock-wave therapy; and (h) should reassess the patient's status at each visit for worsening of symptoms or new physical, mental, or psychological symptoms, or satisfactory recovery.
Conclusions: Our evidence-based guideline provides recommendations for non-invasive management of shoulder pain. The impact of the guideline in clinical practice requires further evaluation.
Significance: Shoulder pain of any duration can be effectively treated with laser therapy, multimodal care (i.e., heat/cold, joint mobilization, range of motion exercise), or cervicothoracic manipulation and mobilization. Shoulder pain (>3 months) can be effectively treated with exercises, laser acupuncture, or general physician care (information, advice, and pharmacological pain management if necessary).
From the FULL TEXT Article:
INTRODUCTION
Background
Shoulder pain is a common musculoskeletal condition in the
general population. In the United States, the annual prevalence of shoulder pain was approximately 9% in adults in the
period of 2002–2009 and shoulder pain ranked fourth behind
low back pain, knee pain, and neck pain as the most prevalent
musculoskeletal condition in 2009 (National Center for
Health Statistics, 2011). Shoulder pain is a major cause of disability worldwide (Vos et al., 2016). Furthermore, shoulder
pain places a significant economic burden on the healthcare
system and society. Employed persons with shoulder pain require a median of 23 days off work in the United States and
an average of 39 days off work in Canada (Saskatchewan
Workers' Compensation Board, 2015; US Department of
Labor, 2015). In Australia, the estimated cost is $56.92 AUD
per day for the management of shoulder pain among employed persons on a waiting list for public hospital orthopaedic care (Marks et al., 2016).
The clinical management of shoulder pain is challenging. For example, three recently developed clinical practice
guidelines for the management of shoulder pain varied in
their recommendations (American College of Occupational
and Environmental Medicine [ACOEM], 2016; Colorado
Division of Workers' Compensation, 2015; Industrial
Insurance Chiropractic Advisory Committee [IICAC],
2014). All three guidelines recommended exercise and manual therapy, but their recommendations varied with regard to
use of other interventions (acupuncture, massage, heat/cold,
transcutaneous electrical nerve stimulation, ultrasound,
shock-wave therapy, taping, and biofeedback; ACOEM,
2016; Colorado Division of Workers' Compensation, 2015;
IICAC, 2014). Furthermore, the quality of these clinical
practice guidelines varies. Specifically, their methodological limitations include searching only one electronic database (i.e., PubMed), using cut-off points to assess risk of
bias, or recommendations based on studies with high risk
of bias, and dated literature searches (search dates up to
2013). An up-to-date, high quality evidence-based clinical
practice guideline is needed to inform the clinical management of soft tissue disorders of the shoulder (shoulder pain)
and promote uniform high-quality care for individuals with
shoulder pain.
Scope and purpose of the guideline
 Table 1
Table 1
|
We developed a clinical practice guideline based on the best
available evidence for the non-invasive management of soft
tissue disorders of the shoulder (Table 1). The target populations were adults (aged 18 years or older) with recent-onset (0- to 3-month duration) or persistent (>3-month duration)
soft tissue disorders of the shoulder. The target audience is
clinicians providing care for patients with shoulder pain in
primary, secondary, and tertiary health care settings. The
recommended interventions in this guideline aim to (a) accelerate recovery; (b) reduce the intensity of symptoms; (c)
promote early restoration of function; (d) prevent chronic
pain and disability; (e) improve health-related quality of life;
(f) reduce recurrences; and (g) promote active participation
of patients in their care.
This guideline was developed by the Ontario Protocol
for Traffic Injury Management (OPTIMa) Collaboration,
which is a multidisciplinary team of clinicians (from medicine, dentistry, physiotherapy, chiropractic, psychology, occupational therapy, and nursing disciplines), academics and
scientists (epidemiologists, clinical epidemiologists, library
sciences, and health economists), a patient liaison, a consumer advocate, a retired judge, and automobile insurance
industry experts. One mandate of the OPTIMa Collaboration
directed by the funding agency was to develop an evidencebased clinical practice guideline for soft tissue disorders of
the shoulder.
METHODS
Systematic reviews and updates
We conducted seven systematic reviews examining the effectiveness and safety of noninvasive interventions for the
management of soft tissue disorders of the shoulder (Abdulla
et al., 2015; Cox et al., 2016; Goldgrub et al., 2016; Piper
et al., 2016; Randhawa et al., 2015; Southerst et al., 2015;
Yu et al., 2015). The literature searches for all systematic reviews were updated on April 26, 2019.
Population, interventions, comparisons and outcomes
The systematic reviews included studies examining the effectiveness and safety of noninvasive interventions for the
management of soft tissue disorders of the shoulder (Table 1;
Appendices I and II). We included grades I to II sprains or
strains, partial thickness tears, nonspecific shoulder pain,
shoulder tendinitis, impingement syndromes, bursitis, shoulder osteoarthritis, and other soft tissue injuries of the shoulder (Table 1; Chan et al., 2012; Noonan & Garrett, 1999;
Woodward & Best, 2000). We excluded studies of shoulder
pain due to major pathology (e.g., fractures, dislocations, infections, neoplasms, systemic disease and others), full thickness tears of the rotator cuff and biceps tendon, and frozen shoulder (Table 1). Noninvasive interventions included acupuncture, exercise, manual therapy, multimodal care, passive physical modalities, soft tissue therapies, and structured
patient education (Appendix I). Pharmacological interventions as a component of multimodal care were included in our guideline. We excluded surgical interventions. Eligible comparators included other interventions, placebo/sham interventions, nonintervention effects associated with wait listing, or no intervention. Eligible outcomes included selfrated recovery, functional recovery, disability, pain intensity, health-related quality of life, psychological outcomes, or adverse events. Eligible study designs included randomized controlled trials (RCTs), cohort studies, case-control studies, and economic studies published in English.
We searched MEDLINE, EMBASE, PsycINFO, and the
Cochrane Central Register of Controlled Trials through Ovid
Technologies, Inc., and CINAHL Plus with Full Text through
EBSCOhost (Appendices IIIA and IIIB).
Original searches
Our initial searches included publication dates from January
1990 to February 2014 (search dates varied between reviews) to identify evidence of effectiveness and safety. We
also searched EconLit through ProQuest, Health Technology
Assessment (Cochrane), and National Health Service
Economic Evaluation Database (Cochrane) for available economic evaluations.
Updated searches
We updated literature searches (first update) of six systematic reviews (Abdulla et al., 2015; Cox et al., 2016; Goldgrub
et al., 2016; Piper et al., 2016; Randhawa et al., 2015;
Southerst et al., 2015) from February 2014 to April 2015
(search dates varied between reviews) before submission for
publication, except for the systematic review of passive physical modalities (published online on November 13, 2014; Yu
et al., 2015). On April 26, 2019, we conducted second updated literature searches using the same search strategies of
all seven systematic reviews to retrieve any new RCTs. We
limited our second updated searches to RCTs because we
did not identify any relevant cohort studies and case-control
studies in our original and first updated searches.
Quality assessment and data synthesis
Random pairs of independent, trained reviewers screened
and critically appraised eligible studies using the Scottish
Intercollegiate Guidelines Network criteria for randomized
trials and economic studies (Harbour & Miller, 2001).
Consensus between the reviewers in each pair was reached
through discussion, with the involvement of an independent third reviewer where necessary. Studies with low risk
of bias were included in our synthesis according to the
best evidence synthesis principle (Slavin, 1995). Studies
with low risk of bias were defined as studies with findings that were likely due to the true treatment effect rather
than selection bias, information bias, or confounding.
Minimal clinically important difference (MCID) thresholds from the literature were considered when determining the clinical importance of the differences in results
between groups (Abdulla et al., 2015; Cox et al., 2016;
Goldgrub et al., 2016; Piper et al., 2016; Randhawa
et al., 2015; Southerst et al., 2015; Yu et al., 2015). These
MCIDs included a between group difference of 1.4/10 cm
on the Visual Analog Scale (VAS; Tashjian et al., 2009),
18/100 on the Shoulder Pain and Disability Index
(SPADI; Breckenridge & McAuley, 2011), 8/100 on the
short form of the Disabilities of the Arm, Shoulder, and
Hand Questionnaire (Quick DASH; Mintken et al., 2009),
10.5/100 on the DASH (Roy et al., 2009), 1.14/7 for
Symptom 1 and 0.91/7 for Symptom 2 on the Measure
Yourself Medical Outcome Profile (Paterson, 1996), and
11% or 4/50 unweighted points on the Shoulder Rating
Questionnaire (Moser et al., 2008).
Development of recommendations
The principle of patient-centered care was fundamental to
developing this guideline. We developed the evidence-based
recommendations according to:
Key decision determinants (overall clinical benefit [effectiveness and safety], value for money [cost-effectiveness data when available], and consistency with expected societal and ethical values) based on the Ontario Health Technology Advisory Committee framework (Johnson
et al., 2009);
Best evidence obtained from systematic reviews of scientific literature; and
Findings from qualitative research exploring patients' lived experiences in receiving care for traffic injuries in Ontario (Lindsay et al., 2016). These findings on patient experiences were considered under the key decision determinant ‘expected societal and ethical values.’
All recommended interventions are supported by evidence of effectiveness, safety and cost-effectiveness (when
cost-effectiveness data were available), and are consistent
with societal and ethical values. We did not recommend
interventions if evidence of the interventions did not meet
the criteria of one or more key decision determinants (i.e.,
evidence of effectiveness, safety, cost-effectiveness, and/
or consistency with societal and ethical values). Our recommendations on assessment, patient education, and reevaluation and discharge are based on universal principles
of health professions' standards of practice (Hopman
et al., 2013; Stiggelbout et al., 2012; Washington State
Department of Labor and Industries, 2014). Initiating care
from assessment to discharging patients reflects current
clinical practice.
 Table 2
Table 2
page 5
|
This guideline adapted the National Institute for Health
and Care Excellence methodology to word guideline recommendations (Table 2; Vargas-Schaffer, 2010). Based on this
methodology, recommendations start with the word “offer”
(for interventions that are of superior effectiveness compared
to other interventions, placebo/sham interventions, or no intervention), “consider” (for interventions providing similar
effectiveness to other interventions), or “do not offer” (for
interventions providing no benefit beyond placebo/sham or
are harmful). An intervention was deemed to have superior
effectiveness if evidence of statistically significant and clinically important benefits was identified in at least one study with low risk of bias.
The frequency and duration of use for recommended
interventions was included in the recommendations. This
was determined according to treatment frequencies and
durations for interventions that were effective in studies
with low risk of bias. Specifically, for recommended interventions that were supported by one low risk of bias
study, we used the frequency and duration of treatment
that was tested in that study. For recommended interventions that were based on more than one low risk of bias
study, we computed the mean frequency and duration of
care across studies with superior outcomes for a specific
intervention.
Development of original recommendations based on evidence from original searches
 Table 3
Table 3
|
All systematic reviews based on the original searches and corresponding recommendations were reviewed and approved
by a multidisciplinary Guideline Expert Panel that included
22 individuals representing emergency medicine, internal
medicine, rehabilitation medicine, orthopedic surgery, dentistry, chiropractic, physical therapy, psychology, nursing,
health economics, epidemiology, clinical epidemiology, law,
patient liaison, consumer representative, and insurers (nonvoting members; Appendices IVA and IVB). We translated
scientific evidence into guideline recommendations following five steps (Table 3).
This evidence-based clinical practice guideline was
developed for the Government of Ontario, Canada. This
guideline (i.e., the original recommendations) was part of
a treatment protocol for common traffic injuries delivered
to the Government Ontario in December 2014 (OPTIMa
Collaboration, 2015). The Government invited stakeholders
(i.e., health care providers, insurers and lawyers) to review
and comment on the guideline. Moreover, the government
held a series of public consultations on this clinical practice
guideline from August 17 to August 21, 2015.
Development of final recommendations based on evidence from original searches and updated searches
Final recommendations were developed by updating the original recommendations using new evidence from updated searches. Final recommendations were reviewed and approved by all coauthors. Coauthors consist of 17 individuals from the multidisciplinary Guideline Expert Panel representing emergency medicine, internal medicine, rehabilitation medicine, dentistry, chiropractic, physical therapy, psychology, health economics, epidemiology, clinical epidemiology, patient liaison, and consumer representative. Five individuals are not listed as co-authors due to various reasons: (a) health issues (2 members); (b) retired (1 member): (c) cannot be reached (1 member); (d) do not meet the criteria for authorship (1 member).
 Figure 1
Figure 1
page 3
|
Each recommendation was integrated into care pathways and algorithms that were approved by all coauthors (Figures 1–4). Interventions for which there was inconclusive evidence of effectiveness were not included in the care pathways (Appendix VA). The development of recommendations and care pathways did not consider interventions that are inconsistent with current practice (Appendix VB).
It is recommended that this guideline be updated in 5 years
to reflect current best evidence (Kung et al., 2012). The update should use methodology similar to the development of
this guideline: (a) form a multidisciplinary guideline expert
panel; (b) conduct systematic reviews of scientific literature
to identify new best available evidence on effectiveness/safety,
cost-effectiveness and qualitative research exploring patients'
and providers' lived experiences; (c) use a comprehensive
framework for evidence-based recommendations (e.g., key decision determinants based on the Ontario Health Technology
Advisory Committee framework).
Reporting
This clinical practice guideline complies with standard reporting elements suggested by the Guidelines International
Network (G-I-N; Qaseem et al., 2012).
Editorial independence
The development of this clinical practice guideline was
funded by the Ministry of Finance and the Financial Services
Commission of Ontario (OSS_00267175). The Ministry of
Finance and Financial Services Commission of Ontario were
not involved in the design, conduct, or interpretation of the
research that informed the development of the care pathways included in this report. The development of the guideline by the Guideline Expert Panel was not influenced by
the Ministry of Finance or Financial Services Commission
of Ontario; the views and interests of the funding body did
not influence the final recommendations. All individuals involved in the project disclosed any conflict of interest (COI)
on a standardized form at the onset and end of the project.
At each Guideline Expert Panel meeting, we asked members
to contact us if their COI forms needed to be updated. Any
individual having a COI at any stage of the guideline development was excused from discussion and voting.
RESULTS OF SYSTEMATIC REVIEWS
Plain text without references provided in Appendix VIA.
Evidence from original searches
The original searches of seven systematic reviews identified 27 RCTs with a low risk of bias (reported in 29 articles; Appendix VIB; Abrisham et al., 2011; Ainsworth
et al., 2007; Albert et al., 2007; Barra Lopez et al., 2013; Bennell et al., 2010; Bergman et al., 2004; Bron et al., 2011; Cacchio et al., 2006; Cook et al., 2014; Engebretsen et al., 2009, 2011; Geraets et al., 2005; Gerdesmeyer et al., 2003;
Ginn & Cohen, 2005; Guerra de Hoyos et al., 2004; Haahr
& Andersen, 2006; Haahr et al., 2005; Hay et al., 2003;
Johansson et al., 2011; Lombardi et al., 2008; Ludewig
& Borstad, 2003; Molsberger et al., 2010; Santamato
et al., 2009; Speed et al., 2002; Szczurko et al., 2009; Van
Der Heijden et al., 1999; Vas et al., 2008). Four articles reported outcomes of different follow-ups based on two RCTs
conducted by Engebretsen et al. (2009, 2011), Haahr and
Andersen (2006), and Haahr et al. (2005). The 29 articles investigated the following interventions: (a) acupuncture (3 articles; Guerra de Hoyos et al., 2004; Molsberger et al., 2010;
Vas et al., 2008); (b) exercise (3 articles; Ginn & Cohen, 2005;
Lombardi et al., 2008; Ludewig & Borstad, 2003); (c) manual
therapy (2 articles; Bergman et al., 2004; Cook et al., 2014);
(d) multimodal care (11 articles; Bennell et al., 2010;
Bron et al., 2011; Engebretsen et al., ,2009, 2011; Geraets
et al., 2005; Ginn & Cohen, 2005; Haahr & Andersen, 2006;
Haahr et al., 2005; Hay et al., 2003; Johansson et al., 2011;
Szczurko et al., 2009); (e) passive physical modalities (12
articles; Abrisham et al., 2011; Ainsworth et al., 2007; Albert
et al., 2007; Cacchio et al., 2006; Engebretsen et al., ,2009,
2011; Gerdesmeyer et al., 2003; Lewis et al., 2005; Rabini
et al., 2012; Santamato et al., 2009; Speed et al., 2002; Van
Der Heijden et al., 1999); and (f) soft tissue therapies (1 articles; Barra Lopez et al., 2013). One article investigated both exercise and multimodal care (Ginn & Cohen, 2005). Two articles investigated both passive physical modalities and multimodal care (Engebretsen et al., ,2009, 2011). We did not
identify any articles related to structured patient education.
We identified one cost-effectiveness study on multimodal
care (Geraets et al., 2006).
Two RCTs evaluating effectiveness of passive physical modalities were not used to inform the development of
recommendations (Lewis et al., 2005; Rabini et al., 2012).
The interventions are not consistent with current practice
(i.e., one application of tape for 20–30 min, three corticosteroid injections over 4 weeks; Lewis et al., 2005; Rabini
et al., 2012). The other 25 RCTs (reported in 27 articles)
were used to inform the original recommendations developed
by the OPTIMa Collaboration (based on original searches
conducted in February 2014; Appendix VII; OPTIMa
Collaboration, 2015).
Evidence from first and second updated searches
Findings from our six systematic reviews were published in
2015 and 2016 (Abdulla et al., 2015; Cox et al., 2016; Goldgrub
et al., 2016; Piper et al., 2016; Randhawa et al., 2015; Southerst et al., 2015). We updated the searches (first updated searches) of these six systematic reviews before submission for publication and identified three recent RCTs with a low risk of bias (reported in four articles): two investigating exercise (Ketola et al., 2009, 2013; Maenhout et al., 2013) and one investigating multimodal care (Rhon et al., 2014; Appendices VIB and VIII). Two of those four articles reported outcomes of different follow-ups based on one RCT (Ketola et al., 2009, 2013). The seventh systematic review was published before the first updated searches (Yu et al., 2015).
The second updated searches of seven systematic reviews (extending from April 15, 2013 to April 26, 2019) for this guideline yielded 842 articles (after duplicates removed), of which 32 were relevant and 19 RCTs (reported in 21 articles) had a low risk of bias (Apeldoorn et al., 2017; Bjornsson Hallgren et al., 2017; CalvoLobo et al., 2018; Chary-Valckenaere et al., 2018; Devereaux et al., 2016; Goksu et al., 2016; Haik et al., 2017; Heron et al., 2017; Holmgren et al., 2012; Kibar et al., 2017; Kolk et al., 2013; Kromer et al., 2014; Kvalvaag et al., 2017, 2018; Li et al., 2017; Littlewood et al., 2016; Mintken et al., 2016; Nazligul et al., 2018; PerezMerino et al., 2016; Perez-Palomares et al., 2017; van der Dolder
et al., 2015) and 11 studies had a high risk of bias (Beaudreuil
et al., 2015; Del Castillo-Gonzalez et al., 2016; Ketola et al., 2017; Lugo et al., 2016; Moezy et al., 2014; Osteras & Torstensen, 2010; Pan et al., 2016; Rueda et al., 2016; Seven et al., 2017; Subasi et al., 2016; Zhang et al., 2016; Appendices VIB and VIII). Four of those 21 articles reported outcomes of different follow-ups based on two RCTs conducted by Kvalvaag et al. (2017, 2018), Bjornsson Hallgren et al. (2017), and Holmgren et al. (2012). The low risk of bias studies from the second updated searches investigated the following interventions: (a) acupuncture (3 articles; Calvo-Lobo et al., 2018; Kibar et al., 2017; Perez-Palomares et al., 2017); (b) exercise (4 articles; Bjornsson Hallgren et al., 2017; Heron et al., 2017;
Holmgren et al., 2012; Littlewood et al., 2016); (c) manual therapy (2 articles; Haik et al., 2017; Mintken et al., 2016); (d) multimodal care (3 articles; Chary-Valckenaere et al., 2018; Kromer et al., 2014; Littlewood et al., 2016); (e) passive physical modalities (9 articles; Apeldoorn et al., 2017; Devereaux et al., 2016; Goksu et al., 2016; Kolk et al., 2013; Kvalvaag et al., 2017, 2018; Li et al., 2017; Nazligul et al., 2018; Perez-Merino et al., 2016); and (f) soft tissue therapies (1 article; van der Dolder et al., 2015).
All 47 RCTs from the original and updated searches were
used to inform the final recommendations in this guideline
(Appendix VII). The identification and selection of articles
in all searches is provided in a summary table and Preferred
Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) flow diagram (Appendices VIB and IX).
FINAL RECOMMENDATIONS BASED ON ORIGINAL AND UPDATED SEARCHES
Our guideline summarized 15 recommended interventions on
managing recent (n = 10; offer/consider 4 interventions, do not
offer 6 interventions) and persistent (n = 14; offer/consider 7
interventions, do not offer 7 interventions) shoulder pain, and
shoulder pain with calcific tendinitis (n = 1; offer 1 intervention) into the following three recommendations: 3, 4, and 5.
Recommendation 1: Evaluation of shoulder pain
Clinicians should rule out major structural or other pathologies as the cause of shoulder pain. Once major pathology has
been ruled out, clinicians should develop an evidence-based
care plan in partnership with the patient. Risks and benefits
of the care plan should be discussed with the patient.
 Table 4
Table 4
 Figure 2-4
Figure 2-4
page 7+
|
Clinicians should conduct a clinical evaluation to rule out
major structural or other pathologies (e.g., grade III sprain/
strain injuries, adhesive capsulitis, fractures, dislocations, infections, neoplasm, inflammatory disorders) as the cause of the patient's presenting symptoms and signs. The presence of risk factors for serious pathologies (also termed ‘red flags’) identified during the history/examination warrants further investigation and referral to the appropriate healthcare professional who is authorized to take appropriate actions and initiate additional examinations (Table 4). Once major pathology has been ruled out, clinicians should develop an evidence-based care plan and involve the patient in care planning and decision-making (Figures 1–4; Stiggelbout et al., 2012). Risks and benefits of the care plan should be discussed with the patient. This recommendation is based on universal principles of health professions' standards of practice when evaluating shoulder pain
(Hopman et al., 2013; Stiggelbout et al., 2012; Washington
State Department of Labor and Industries, 2014).
Recommendation 2: Management of shoulder pain
Clinicians should educate and reassure patients about the
benign and self-limited nature of most soft tissue shoulder
pain. Clinicians need to reassure patients if there are no major
structural or progressive pathologies (e.g., grade III sprain/
strain injuries, dislocations, fractures, or infections) in the
shoulder. Patients with worsening of symptoms or new physical, mental, or psychological symptoms should be referred
to an appropriate health care provider for further evaluation
at any time during their care.
Clinicians should provide information about the nature,
management, and course of shoulder pain as a framework for
initiating a program of care. The patient should be educated
that shoulder pain is most commonly benign and self-limited,
frequently with a natural history of spontaneous recovery. If
an intervention is indicated, it must be effective and timelimited. This recommendation is based on universal principles of health professions' standards of practice wherein
patients are informed and educated about their condition,
and participate in the decision-making process (Stiggelbout
et al., 2012).
Recommendation 3: Management of recent-onset shoulder pain (≤3 months)
 Table 5+6
Table 5+6
page 12
|
For shoulder pain ≤3 months duration, clinicians may consider low-level laser therapy (LLT); multimodal care including heat/cold, joint mobilization, and range of motion
exercise; cervicothoracic spine manipulation and mobilization for shoulder pain when associated pain or restricted
movement of the cervicothoracic spine; or thoracic spine
manipulation (Tables 5 and 6, Figures 1 and 2). In view of
evidence of no effectiveness, clinicians should not offer ultrasound; taping; interferential current therapy; diacutaneous
fibrolysis; soft tissue massage; or cervicothoracic spine manipulation and mobilization as an adjunct to exercise (i.e.,
range of motion, strengthening and stretching exercise) for
shoulder pain (defined as pain between the neck and the
elbow at rest or during movement of the arm).
Passive physical modalities
For subacromial impingement syndrome, clinicians may
offer low-LLT for short-term pain reduction (pulsed laser, 10
sessions over 2 weeks). The parameter of low-LLT is (a) peak
power = 1 kW, average power = 6 W, maximum energy of single impulse = 150 mJ, duration of single impulse < 150 ms,
fluency = 760 mJ/cm2 , wavelength = 1,064 nm; or (b) wavelength = 890 nm, time = 2 min/point, power 2–4 J/cm2 in each point. This recommendation is based on two low risk of bias RCTs suggesting low-LLT was more effective in shortterm pain reduction than placebo (Abrisham et al., 2011) or continuous ultrasound (Santamato et al., 2009).
Clinicians should not offer ultrasound. This recommendation is based on evidence from three low risk of bias RCTs that found similar outcomes: (a) between ultrasound and placebo (Ainsworth et al., 2007; Van Der Heijden et al., 1999); (b) between ultrasound and phonophoresis (Perez-Merino et al., 2016); and (c) between ultrasound and iontophoresis (Perez-Merino et al., 2016).
Clinicians should not offer interferential current therapy.
This recommendation is based on two low risk of bias RCTs
suggesting that interferential current therapy provided similar
outcomes to placebo (Nazligul et al., 2018; Van Der Heijden
et al., 1999).
Clinicians should not offer taping of the shoulder. This
recommendation is based on three low risk of bias RCTs suggesting (a) precut kinesiology tape did not provide added benefits to a shoulder exercise program (Devereaux et al., 2016); (b) kinesiology tape led to similar outcomes as a single
steroid injection (statistically significant differences favoring injection for pain [VAS], and function [SPADI], Goksu
et al., 2016); (c) rigid tape did not provide added benefits to
individualized physiotherapy (Apeldoorn et al., 2017).
Manual therapy
Cervicothoracic spine manipulation and mobilization
For shoulder pain with associated pain or restricted movement
of the cervicothoracic spine, clinicians may consider cervicothoracic spine manipulation and mobilization as an adjunct to usual care (i.e., information and advice, followed by analgesics), provided in 6 sessions over 12 weeks. The recommendation is based on one low risk of bias RCT comparing
manipulation and mobilization in addition to usual care with
usual care alone. The study found that participants receiving
spinal manipulation and mobilization in addition to usual care
were more likely to report “completely recovered” or “much
improved” (Bergman et al., 2004). Spinal manipulation and
mobilization in addition to usual care brings similar benefit
to usual care alone in pain and health-related quality of life.
For shoulder pain (defined as pain between the neck and
the elbow at rest or during movement of the arm), clinicians
should not offer cervicothoracic manipulation and mobilization as adjunct to exercise (i.e., range of motion exercise,
stretching and strengthening exercise). This recommendation
is based on one low risk of bias RCT comparing manipulation and mobilization as an adjunct to exercise with exercise
alone (Mintken et al., 2016). The study suggested that cervicothoracic manipulation and mobilization did not provide
added benefits to exercise.
Thoracic spine manipulation
For shoulder impingement syndrome, clinicians may consider thoracic spine manipulation (two sessions with 3–4 days interval). This recommendation is based on one low risk of bias RCT that thoracic spine manipulation is more effective than sham in reducing shoulder pain (Haik et al., 2017).
Multimodal care
For unilateral pain in shoulder region, clinicians may consider 8–10 sessions over a maximum 5–6 weeks of multimodal care that includes heat, cold, joint mobilization, and range of motion exercise. This recommendation is based on two low risk of bias RCTs examining the effectiveness of multimodal care for recent-onset shoulder pain (Ginn & Cohen, 2005; Hay et al., 2003). This body of evidence suggests that the effective multimodal programs
of care included range of motion, mobilization, heat and
cold.
Soft tissue therapy
Clinicians should not offer diacutaneous fibrolysis. This recommendation is based on one low risk of bias RCT with three
study groups that found diacutaneous fibrolysis did not provide additional benefit to a multimodal program of care and
provided similar outcomes to sham diacutaneous fibrolysis
(Barra Lopez et al., 2013).
Clinicians should not offer soft tissue massage. This recommendation is based on one low risk of bias RCT suggesting that soft tissue massage did not provide added benefits
to a shoulder exercise program (van der Dolder et al., 2015).
Recommendation 4: Management of persistent shoulder pain (>3 months)
For shoulder pain >3-month duration, clinicians may consider
stretching and/or strengthening exercises; laser acupuncture;
low-LLT; multimodal care (heat/cold, joint mobilization,
range of motion exercise); general physician (GP) care (information, advice, and pharmacological pain management if
necessary); cervicothoracic spine manipulation and mobilization for shoulder pain when associated pain or restricted
movement of the cervicothoracic spine; or thoracic spine manipulation (recommendation Tables 5 and 6; Figures 1 and 2).
In view of evidence of no effectiveness, clinicians should not
offer shock-wave therapy; ultrasound; taping; interferential
current therapy; diacutaneous fibrolysis; or soft tissue massage; or cervicothoracic spine manipulation and mobilization
as an adjunct to exercise (i.e., range of motion, strengthening
and stretching exercise) for shoulder pain (defined as pain
between the neck and the elbow at rest or during movement
of the arm).
Exercise
Home-based combined strengthening and stretching
exercise with supervision
For shoulder impingement symptoms, clinicians may
offer home-based combined strengthening and stretching exercises with supervision. This involves home-based
stretching and strengthening exercises for 8 weeks with
supervision in one to two follow-up visits, including five
repetitions per day of stretching of pectoralis minor and
posterior shoulder, and 10–20 repetitions of progressive
strengthening (using hand-held weights and therapeutic
bands) for rotator cuff and serratus anterior of 3 sets per
week (Ludewig & Borstad, 2003). This recommendation is
based on one low risk of bias RCT suggesting that homebased combined strengthening and stretching exercise with
supervision was more effective than no treatment (Ludewig
& Borstad, 2003).
For pain at the shoulder joint and/or the proximal arm that
is exacerbated by active shoulder movements, clinicians may
consider home-based combined strengthening and stretching
exercises with weekly supervision for 5 weeks. This involves
home-based strengthening and stretching of the rotator cuff
and scapulohumeral muscles, with weekly supervision for
5 weeks. This recommendation is specifically for low-grade
shoulder pain (pain intensity <3/10 cm or 30/100 mm on
VAS). This recommendation is based on one low risk of bias
RCT suggesting that home-based combined strengthening
and stretching exercises with supervision provided similar
short-term benefits to a single corticosteroid injection or a
multimodal program of care (Ginn & Cohen, 2005).
For chronic subacromial impingement syndrome, clinicians may consider home-based specific shoulder exercises
with supervision focusing on rotator cuff and scapula stabilizers (Bjornsson Hallgren et al., 2017; Holmgren et al., 2012). This involves home-based exercise with supervision in seven visits: (a) strengthening eccentric exercises for the rotator cuff (supraspinatus, infraspinatus, and teres minor) and concentric/eccentric exercises for the scapula stabilizers (middle and lower trapezius, rhomboideus, and serratus anterior; 15 times/set, 3 sets twice daily for the first 8 weeks and once
daily for week 9–12); and (b) a posterior shoulder stretch
(30–60 s, 3 times/set, 2 sets daily for the first 8 weeks and
once daily for week 9–12). This recommendation is based
on one low risk of bias RCT suggesting specific shoulder
exercise was more effective than non-specific exercise in reducing night pain. Furthermore, participants in the specific
exercise group were more likely to report recovery and less
likely to choose surgery.
Clinic-based strengthening exercise
For shoulder impingement symptoms, clinicians may offer supervised strengthening exercise. This involves 2 sets of 8 repetitions of progressive shoulder flexion/extension/medial rotation/lateral rotation strengthening, supervised twice a week for 8 weeks (Lombardi et al., 2008). These strengthening exercises used multi-pulley musclebuilding equipment (flexion, extension, medial rotation, lateral rotation). This recommendation is based on one low risk of bias RCT suggesting supervised strengthening exercise was more effective than a wait list (Lombardi et al., 2008).
Home-based strengthening exercise with supervision
For shoulder impingement syndrome, clinicians may consider home-based strengthening exercise. This involves
progressive supervised exercises in seven visits and a
home-based exercise program (≥4 times/week using 9
different exercises with 30–40 repetitions 3 times; Ketola
et al., 2009, 2013). These strengthening exercises used
therapeutic bands and light weights; as strength improved,
resistance was increased. This recommendation is based on
one low risk of bias RCT suggesting home-based strengthening exercise with supervision led to similar outcomes as
surgery plus post-surgical rehabilitation at 2 and 5 years
(Ketola et al., 2009, 2013).
Home-based strengthening exercise
For subacromial impingement symptoms, clinicians may
consider home-based rotator cuff strengthening exercise.
This involves home-based internal and external rotation
resisted with a therapeutic band and increased load once
pain decreased (once a day for 3 sets of 10 repetitions at a
speed of 6 s/repetition [2-s concentric phase, 2-s isometric
phase and 2-s eccentric phase] over 12 weeks; Maenhout
et al., 2013). This recommendation is based on one low risk
of bias RCT suggesting home-based heavy load eccentric
loading training provided similar outcomes when added to
home-based rotator cuff strengthening exercise (Maenhout
et al., 2013).
For chronic rotator cuff tendinopathy with shoulder pain
with or without referral into the upper limb, clinicians may
consider home-based strengthening shoulder abduction.
This involves isometric abduction, progressing to isotonic
abduction through increased repetitions and loading using
a resistive therapeutic band or hand weight (3 sets of 10–
15 repetitions, twice per day; Littlewood et al., 2016). This
recommendation is based on one low risk of bias study
suggesting home-based progressive shoulder abduction
provided similar outcomes to individualized physiotherapy
(Littlewood et al., 2016).
Laser acupuncture (laser therapy on acupuncture points)
For subacromial impingement syndrome, clinicians may
offer laser acupuncture (5 sessions per week over 3 weeks).
The parameter of laser acupuncture is: wavelength = 850 nm,
power output = 100 mV, spot area = 0.07 cm2 , 40 s of 4 J/cm2 dosage for each acupuncture point, with a total of 11 acupuncture points (Kibar et al., 2017). This recommendation is based on one low risk of bias RCT suggesting that laser acupuncture was more effective than sham for subacromial impingement syndrome (Kibar et al., 2017).
Passive physical modalities
For subacromial impingement syndrome, clinicians may
offer low-LLT for short-term pain reduction (pulsed laser,
10 sessions over 2 weeks). The parameter of LLT is (a) peak
power = 1 kW, average power = 6 W, maximum energy of single impulse = 150 mJ, duration of single impulse < 150 ms,
fluency = 760 mJ/cm2
, wavelength = 1,064 nm; or (b) wavelength = 890 nm, time = 2 min/point, power 2–4 J/cm2
in
each point. This recommendation is based on two low risk of
bias RCTs suggesting LLT was more effective in short-term
pain reduction than placebo (Abrisham et al., 2011) or continuous ultrasound (Santamato et al., 2009).
Clinicians should not offer shock-wave therapy for shoulder impingement syndrome. This recommendation is based on four low risk of bias RCTs suggesting that (a) shockwave therapy provided similar outcomes to placebo (Kolk et al., 2013; Kvalvaag et al., 2017, 2018; Speed et al., 2002); and (b) shock-wave therapy provided similar outcomes to multimodal care (Engebretsen et al., ,2009, 2011). Furthermore, participants receiving multimodal care were more likely to report improvement in shoulder pain and function (measured by the Shoulder Pain and Disability Index) and returned to work earlier than participants receiving shock-wave therapy (Engebretsen et al., ,2009, 2011). Although one RCT reported shock-wave therapy was more effective than placebo (Li et al., 2017), the preponderance of evidence suggests shock-wave therapy provided similar outcomes to placebo or was less effective than multimodal care.
Clinicians should not offer ultrasound. This recommendation is based on evidence from three low risk of bias RCTs
that found similar outcomes: (a) between ultrasound and placebo for nonspecific shoulder pain (Ainsworth et al., 2007;
Van Der Heijden et al., 1999); (b) between ultrasound and
phonophoresis for subacromial impingement syndrome
(Perez-Merino et al., 2016); and (c) between ultrasound and
iontophoresis (Perez-Merino et al., 2016).
Clinicians should not offer interferential current therapy
for persistent shoulder pain. This recommendation is based
on two low risk of bias RCTs suggesting that interferential
current therapy provided similar outcomes to placebo (Van
Der Heijden et al., 1999; Nazligul et al., 2018).
Clinicians should not offer taping for impingement syndrome. This recommendation is based on two low risk of bias
RCTs suggesting (a) precut kinesiology tape did not provide
added benefits to a shoulder exercise program (Devereaux
et al., 2016) and (b) rigid tape did not provide added benefits
to individualized physiotherapy (Apeldoorn et al., 2017).
Manual therapy
Cervicothoracic spine manipulation and mobilization
For shoulder pain with associated pain or restricted movement of the cervicothoracic spine, clinicians may consider 6 sessions over 12 weeks of cervicothoracic spine manipulation and mobilization as an adjunct to usual care (i.e., information and advice, followed by analgesics). The recommendation is based on one low risk of bias RCT comparing manipulation and mobilization in addition to usual care with usual care alone (Bergman et al., 2004). The study found that participants receiving spinal manipulation and mobilization in addition to usual care were more likely to report “completely recovered” or “much improved” (Bergman et al., 2004). However, spinal manipulation and mobilization in addition to usual care showed similar benefit to usual care alone for measures of pain, and health-related quality of life.
For shoulder pain (defined as pain between the neck and the elbow at rest or during movement of the arm), clinicians should not offer cervicothoracic manipulation and mobilization as an adjunct to exercise (i.e., range of motion exercise, stretching and strengthening exercise). This recommendation is based on one low risk of bias RCT comparing manipulation and mobilization as an adjunct to exercise with exercise alone (Mintken et al., 2016). The study suggested that cervicothoracic manipulation and mobilization did not provide added benefits to exercise in pain and disability.
Thoracic spine manipulation
For shoulder impingement syndrome, clinicians may consider thoracic spine manipulation (two sessions with 3–4 days
interval). This recommendation is based on one low risk of
bias RCT that demonstrated thoracic spine manipulation is
more effective than sham in reducing shoulder pain (Haik
et al., 2017).
Cervical mobilization
For shoulder impingement syndrome, clinicians should not
offer cervical mobilizations as adjunct to multimodal care
including manual therapy, stretching, isotonic strengthening,
and restoration of normative movement. This recommendation is based on one low risk of bias RCT comparing cervical
mobilizations as adjunct to multimodal care with the same
multimodal care without cervical mobilizations. The study
suggested that cervical mobilization did not provide additional benefit to a multimodal program of care in pain and
disability (Cook et al., 2014). Furthermore, similar proportion of participants in the two groups considered their state to
be acceptable (i.e., unlikely to seek further treatment).
Multimodal care
For persistent shoulder pain, clinicians may consider usual
GP care (information, advice, and pharmacological pain
management if necessary). This recommendation is based on
one low risk of bias RCT examining the effectiveness of GP
care (Geraets et al., 2005) and one cost-effectiveness study
of GP care (Geraets et al., 2006). Usual GP care (information
and advice followed by analgesics if necessary, local injection of a corticosteroid if no improvement within 2 weeks)
leads to similar outcomes to behavioral and graded exercise
therapy but cost less than behavioral and graded exercise
therapy from the societal perspective.
For persistent shoulder pain, clinicians may consider 8–10
sessions over a maximum of 5–6 weeks of multimodal care
that includes heat, cold, joint mobilization, and range of motion exercises provided in 8–10 sessions over a maximum
of 5–6 weeks (Ginn & Cohen, 2005; Hay et al., 2003). The
multimodal program of care may only be considered if not
previously provided in the first 3 months of care. However,
a second course may be indicated if the patient has demonstrated ongoing and significant improvement.
Clinicians should not offer multimodal care that combines exercise, mobilization, taping, psychological interventions, and massage for persistent shoulder pain. This
recommendation is based on the body of evidence from five
low risk of bias RCTs examining the effectiveness of multimodal care for persistent shoulder pain (Bennell et al., 2010;
Engebretsen et al., ,2009, 2011; Haahr & Andersen, 2006;
Haahr et al., 2005; Kromer et al., 2014; Rhon et al., 2014).
The body of evidence suggests that a multimodal program
of care including exercise, mobilization, taping, psychological interventions, and massage is not effective for persistent
shoulder pain.
Soft tissue therapy
Clinicians should not offer diacutaneous fibrolysis for subacromial impingement. This recommendation is based on one
low risk of bias RCT with three study groups that found diacutaneous fibrolysis did not provide additional benefit to a
multimodal program of care and provided similar outcomes
to sham diacutaneous fibrolysis (Barra Lopez et al., 2013).
Clinicians should not offer soft tissue massage for nonspecific shoulder pain. This recommendation is based on one
low risk of bias RCT suggesting that soft tissue massage did
not provide added benefits to a shoulder exercise program
(van der Dolder et al., 2015).
Recommendation 5: Management of shoulder pain with calcific tendinitis
For shoulder pain with calcific tendinitis, clinicians may consider shock-wave therapy (Tables 5 and 6; Figures 3 and 4).
Passive physical modalities – Shockwave therapy
Clinicians may offer a maximum of 4 sessions over 4 weeks
of shock-wave therapy with an amplitude ranging from 0.08–
0.60 mJ/mm2 for persistent shoulder calcific tendinitis. This recommendation is based on three low risk of bias RCTs: (a) shock-wave therapy was more effective than sham (Cacchio et al., 2006); (b) high-energy shock-wave therapy was more effective than low-energy shock-wave therapy (Albert
et al., 2007; Gerdesmeyer et al., 2003); and (c) low energy
shock-wave therapy provided similar outcomes to sham
(Gerdesmeyer et al., 2003).
Recommendation 6: Reevaluation and discharge
This recommendation is based on universal principles
of health professions' standards of practice and should
be performed as a component of standard clinical care.
Clinicians should reassess the patient at every visit to determine if
(a) additional care is necessary;
(b) the condition is worsening; or
(c) the patient has recovered.
The decision to
end treatment should be made collaboratively by the patient
and the clinician, on the basis of findings of satisfactory recovery. Health care professionals can use the self-rated recovery question to measure recovery: “How well do you feel
you are recovering from your injuries?” (Carroll et al., 2016;
Fischer et al., 1999).
The response options include
(a) completely better,
(b) much improved,
(c) slightly improved,
(d) no change,
(e) slightly worse,
(f) much worse,
(g) worse than ever.
Patients reporting to be ‘completely better’ or ‘much
improved’ should be considered recovered. The self-rated
recovery question has been demonstrated to be a valid and
reliable global measure of recovery in patients with shoulder
pain (Carroll et al., 2016; Fischer et al., 1999). Patients who
have not recovered should follow the care pathway outlined
in the guideline (Figures 1–4).
DISCUSSION
Our shoulder guideline provides evidence-based recommendations (i.e., recommendations 3, 4, and 5) to help clinicians deliver effective interventions for the management of soft tissue disorders of the shoulder (excluding major pathology). The recommended interventions aim to promote uniform high-quality care based on systematic reviews of the literature and synthesis of best available evidence. Implementing the evidence-based recommendations for shoulder pain will likely improve patient outcomes, reduce regional variations, and improve the efficiency of the healthcare system (Anis et al., 1995; Nichol et al., 1999; Rutten et al., 2010). Our recommendations on assessment, patient education, and reevaluation and discharge (i.e., recommendations 1, 2, and 6) are based on universal principles of health professions' standards of practice (Hopman et al., 2013; Stiggelbout et al., 2012; Washington State Department of Labor and Industries, 2014).
Our guideline focuses on the management of soft tissue disorders of the shoulder. We included grade I to II sprains or strains, nonspecific shoulder pain, shoulder tendinitis, impingement syndromes, bursitis, partial thickness tears, shoulder osteoarthritis, and other soft tissue injuries of the shoulder. These soft tissue shoulder disorders represent most common causes of shoulder pain with similar mechanisms and clinical symptoms and signs in a primary care setting (ACOEM, 2016; Colorado Division of Workers' Compensation, 2015; Ostor et al., 2005). Coexisting etiologies were noted in 77% of patients (House & Mooradian, 2010). Impingement syndromes commonly
coexist with other shoulder pathologies such as rotator cuff partial tears and bursitis (Romeyn & Manske, 2018). Furthermore, clinical tests can elicit symptoms of other coexisting shoulder pathology (Romeyn & Manske, 2018). Therefore, our guideline targets soft tissue disorders of the shoulder as an entity.
Summary of recommendations
Clinicians should rule out major structural or other pathologies as the cause of shoulder pain. In the absence of major
structural or other pathologies, clinicians should develop
a care plan in partnership with the patient. Risks and benefits of the care plan should be discussed with the patient.
Furthermore, clinicians should discuss with the patient the
range of effective interventions available for the management
of shoulder pain.
Clinicians should educate and reassure patients about the
benign and self-limited nature of shoulder pain as a framework for the initiation of the care plan. For recent shoulder
pain (≤3 months), clinicians should consider low-LLT or
multimodal care involving an active component (i.e., heat/
cold, joint mobilization, range of motion exercise) as their first
choice. For persistent shoulder pain (>3 months), clinicians
should consider: (a) home-based combined strengthening and stretching exercise with supervision; (b) clinical-based
strengthening exercise; (c) laser acupuncture; (d) low-LLT;
or (e) multimodal care involving an active component (i.e.,
heat/cold, joint mobilization, range of motion exercise) as
their first choice. For persistent shoulder pain, home-based
exercise and general physical care (information, advice, and
pharmacological pain management if necessary) are alternative options to meet patients' need and treatment goals. With
pain or restricted movement of the cervicothoracic spine,
cervicothoracic spine manipulation and mobilization, or thoracic spine manipulation can be considered for both recent and persistent shoulder pain. For shoulder pain with calcific
tendinitis, clinicians can consider shock-wave therapy.
Our guideline provides parameters on the dosage of interventions that are informed by high quality RCTs. It is important to note that all recommended interventions provide small benefits.
Our guideline identifies clinical interventions that
should not be prescribed because their effectiveness is
not clearly established. We found inconclusive evidence
on needle acupuncture for persistent shoulder pain because the results of three low risk of bias RCTs conflicted
with each other (Molsberger et al., 2010; Perez-Palomares
et al., 2017; Vas et al., 2008) and one low risk of bias RCT
compared acupuncture on different trigger points (CalvoLobo et al., 2018). Furthermore, our guideline does not recommend stand-alone structured patient education because
its effectiveness has not been evaluated in high-quality
studies (Randhawa et al., 2015). Instead, patient education
serves as a framework for initiating the program of care in
this guideline.
Applicability of the guideline
Barriers and facilitators of guideline utilization and adherence have been identified in the literature (Arksey & O'Malley, 2005; Cabana et al., 1999; Levac et al., 2010). The main barriers to guideline utilization in all healthcare practitioners managing musculoskeletal disorders include (a)
disagreement between recommendations and patient expectations; (b) guidelines not specific to individual patients; (c)
unfamiliarity with the term “non-specific” or with the biopsychosocial model of MSDs; (d) time-consuming; and (e) heterogeneity in guideline methods (Sorondo et al., 2021). The
main facilitators include (a) clinician's interest in evidencebased practice; (b) perception from clinicians that the guideline will improve triage, diagnosis and management; (c)
time efficiency; and (d) standardized language (Sorondo
et al., 2021).
To enhance utilization of our guideline, we (a) recommend developing a care plan in partnership with the patient;
(b) clearly defined the target conditions; (c) used a standardized language for the recommendations; (d) created Quick
Reference Guides and Algorithms for the point of care; and
(e) clearly described methodology and steps of developing
the recommendations. Furthermore, we have developed clinicians' and patients' handouts and shoulder exercise videos
(Canadian Chiropractic Guideline Initiative, 2021a, 2021b).
These resources are available in English and French languages for the use of the public.
Comparison to previous guidelines
Overall, our recommendations agree with those of previous
clinical practice guidelines for soft tissue disorders of the
shoulder (ACOEM, 2016; Colorado Division of Workers' Compensation, 2015; IICAC, 2014). Three guidelines recommended exercise and manual therapy (manipulation and mobilization; ACOEM, 2016; Colorado Division of Workers' Compensation, 2015; IICAC, 2014); and two guidelines recommended heat and cold (ACOEM, 2016; Colorado Division of Workers' Compensation, 2015). With new evidence, our guideline provides frequency, duration, dosage of exercise and differentiate first line exercise therapy (i.e., to offer home-based combined strengthening and stretching
exercise with supervision or clinical-based strengthening
exercise) and second line exercise therapy (i.e., to consider
home-based combined strengthening and stretching exercise
with supervision for low grade shoulder pain or home-based
strengthening exercise). Our guideline specifies when to
apply manual therapy (i.e., for restricted movement of the
cervicothoracic spine). Furthermore, we recommend heat
and cold as components of multimodal care (heat/cold, joint
mobilization, range of motion exercise). All these details
should provide clinicians with better direction when planning
treat plans.
There are a few important differences between previous guidelines and ours. Specifically, we recommend offering low-level laser as well as considering GP care and multimodal care (heat/cold, joint mobilization, range of motion exercise). We do not recommend nor refute the use of needle acupuncture. Our systematic reviews identified two low risk of bias studies which found that low-LLT is more effective than placebo or ultrasound (Abrisham et al., 2011; Santamato et al., 2009). We identified three studies suggesting that GP care leads to similar outcomes to multimodal care (behavioral treatment and graded exercise; Geraets et al., 2005); and multimodal care (heat/cold, joint mobilization and range of motion exercise)
leads to similar outcomes to corticosteroid injection (Ginn
& Cohen, 2005; Hay et al., 2003) or exercise (Ginn &
Cohen, 2005). Furthermore, GP care is more cost-effective
than multimodal care (behavioral treatment and graded exercise therapy; Geraets et al., 2006). Moreover, our recent systematic review found inconclusive evidence on the effectiveness of needle acupuncture (Molsberger et al., 2010; Perez-Palomares et al., 2017; Vas et al., 2008).
Comparison between our original and final recommendations
Our updated searches (first and second updated searches from 2014 to 2019) identified 25 articles for the management of soft tissue shoulder pain. The evidence from updated searches added new recommendations to the original recommendations: (a) clinician may consider laser acupuncture (Kibar et al., 2017) and thoracic manipulation (Haik et al., 2017) and (b) do not offer soft tissue massage (van der Dolder et al., 2015) and taping (Apeldoorn et al., 2017; Devereaux et al., 2016; Goksu et al., 2016). The evidence from updated searches strengthened the original recommendations: (a) clinicians may consider stretching and/or strengthening exercise (Bjornsson Hallgren et al., 2017; Heron et al., 2017; Holmgren et al., 2012;
Ketola et al., 2009, 2013; Littlewood et al., 2016;
Maenhout et al., 2013) and (b) do not offer shock-wave
therapy (Kolk et al., 2013; Kvalvaag et al., 2017, 2018),
interferential current therapy (Nazligul et al., 2018), and
ultrasound (Perez-Merino et al., 2016). Furthermore, we
updated original recommendations on manipulation and
mobilization to recommend “do not offer cervicothoracic
spine manipulation and mobilization as an adjunct to exercise (i.e., range of motion, strengthening and stretching exercise) for shoulder pain (defined as pain between the neck and the elbow at rest or during movement of the arm; Mintken et al., 2016).
Despite recent improvement in the quality of the literature on the management of shoulder pain, there are still important gaps. Specifically, the evidence is still inconclusive on the effectiveness of needle acupuncture (Cox et al., 2016;
Perez-Palomares et al., 2017). Therefore, efforts should be
dedicated to determine the effectiveness of this intervention.
In addition, high-quality studies are needed to identify the
most effective dosage to optimize treatment response for all
interventions.
This guideline could be adapted for local use in other
jurisdictions. We recommend that clinicians, insurers
and policy-makers use the ADAPTE framework to adapt
this guideline to their needs and environment (ADAPTE
Collaboration, 2010).
Strengths and limitations
This clinical practice guideline was based on comprehensive literature searches and developed from high-quality
evidence. When developing clinical recommendations, the
Guideline Expert Panel considered effectiveness, safety,
cost-effectiveness, consistency with societal and ethical values, and patient preferences and experiences. Moreover, the
Guideline Expert Panel considered the magnitude of benefit of interventions on patient outcomes using established
MCIDs. Finally, the Guideline Expert Panel exercised editorial independence and disclosed any relevant conflicts of
interest.
Our recommendations were limited by the amount, nature,
and quality of evidence published in the literature (Abdulla
et al., 2015; Cox et al., 2016; Goldgrub et al., 2016; Piper
et al., 2016; Randhawa et al., 2015; Southerst et al., 2015;
Yu et al., 2015). For example, inconclusive evidence on the
effectiveness of needle acupuncture prevents the development of clinical recommendations (Calvo-Lobo et al., 2018;
Cox et al., 2016; Molsberger et al., 2010; Perez-Palomares
et al., 2017; Vas et al., 2008). Very few studies have investigated the effectiveness of stand-alone structured patient
education (Randhawa et al., 2015). We limited our search
to studies published in the English language to increase feasibility, which may have excluded some relevant studies.
However, this is an unlikely source of bias because most
large-scale RCTs are published in English (Jüni et al., 2002).
Also, previous reviews showed that the restriction of systematic reviews to English language studies does not lead to
bias (Moher et al., 1996, 2003; Morrison et al., 2012; Sutton
et al., 2000).
ACKNOWLEDGMENTS
The authors would like to acknowledge the invaluable contributions to this guideline from: Lynn Anderson, Poonam
Cardoso, Gaelan Connell, Brenda Gamble, Willie Handler,
Richard Seto, Viivi Riis, Paula Stern, Thepikaa Varatharajan,
Angela Verven, and Leslie Verville
CONFLICT OF INTEREST
Dr. Pierre Côté has received fees as a medical-legal exert
from the Canadian Chiropractic Protective Association and
NCMIC. Dr. J David Cassidy has received payment for expert
witness testimony from the National Hockey League (NHL)
and for epidemiological advice on sports concussion from both
the NHL and the National Collegiate Athletic Association.
He has also received payment for expert testimony regarding
court actions concerning the risk of stroke from various insurance companies in the United States. Dr. Silvano Mior has
received grants from the Ontario Chiropractic Association,
Canadian Chiropractic Association (paid directly to institution) and consulting/honorarium-Ontario Protocol for Traffic
Injury Management Collaboration. All other individuals involved in the project declared no competing interests
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to all of the
following: (1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be submitted. All authors discussed the results and commented on the manuscript.
References:
Please Refer to Full-Text article

Return to SHOULDER
Since 6-04-2023
|











