

Spinal Manipulative Therapy and Other Conservative Treatments
for Low Back Pain: A Guideline From the Canadian
Chiropractic Guideline InitiativeThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J Manipulative Physiol Ther. 2018 (May); 41 (4): 265–293 ~ FULL TEXT
André E. Bussières, DC, FCCS(C), PhD, Gregory Stewart, DC, Fadi Al-Zoubi, PT, MSc, Philip Decina, DC, Martin Descarreaux, DC, PhD, Danielle Haskett, BSc, Cesar Hincapié, DC, PhD, Isabelle Pagé, DC, MSc, Steven Passmore, DC, PhD, John Srbely, DC, PhD, Maja Stupar, DC, PhD, Joel Weisberg, DC, Joseph Ornelas, DC, PhD
School of Physical and Occupational Therapy,
Faculty of Medicine, McGill University,
Montreal, Québec, Canada.;
Département Chiropratique,
Université du Québec à Trois-Rivières,
Trois-Rivières, Québec, Canada.
Andre.bussieres@mcgill.ca

OBJECTIVE: The objective of this study was to develop a clinical practice guideline on the management of acute and chronic low back pain (LBP) in adults. The aim was to develop a guideline to provide best practice recommendations on the initial assessment and monitoring of people with low back pain and address the use of spinal manipulation therapy (SMT) compared with other commonly used conservative treatments.
METHODS: The topic areas were chosen based on an Agency for Healthcare Research and Quality comparative effectiveness review, specific to spinal manipulation as a nonpharmacological intervention. The panel updated the search strategies in Medline. We assessed admissible systematic reviews and randomized controlled trials for each question using A Measurement Tool to Assess Systematic Reviews and Cochrane Back Review Group criteria. Evidence profiles were used to summarize judgments of the evidence quality and link recommendations to the supporting evidence. Using the Evidence to Decision Framework, the guideline panel determined the certainty of evidence and strength of the recommendations. Consensus was achieved using a modified Delphi technique. The guideline was peer reviewed by an 8-member multidisciplinary external committee.
RESULTS: For patients with acute (0-3 months) back pain, we suggest offering advice (posture, staying active), reassurance, education and self-management strategies in addition to SMT, usual medical care when deemed beneficial, or a combination of SMT and usual medical care to improve pain and disability. For patients with chronic (>3 months) back pain, we suggest offering advice and education, SMT or SMT as part of a multimodal therapy (exercise, myofascial therapy or usual medical care when deemed beneficial). For patients with chronic back-related leg pain, we suggest offering advice and education along with SMT and home exercise (positioning and stabilization exercises).
CONCLUSIONS: A multimodal approach including SMT, other commonly used active interventions, self-management advice, and exercise is an effective treatment strategy for acute and chronic back pain, with or without leg pain.
KEYWORDS: Chiropractic; Conservative Treatment; Disease Management; Low Back Pain; Practice Guideline
From the FULL TEXT Article:
Introduction
In 2015, musculoskeletal (MSK) disorders were the largest contributor to global years lived with disability (YLDs) (18.5% [16.4%-20.9%] of all YLDs). [1] Approximately half (49.6%) of the YLDs stem from low back pain (LBP). [1, 2] The point prevalence of LBP is estimated at nearly 20%, the 1–year prevalence is around 50%, and the lifetime prevalence is about 85% in the general population. [3] Despite the availability of many clinical interventions to manage LBP, [4] a nearly 3–fold increase in the prevalence of chronic LBP was observed between 1992 (3.9%, 95% confidence interval [CI] 3.4%-4.4%) and 2006 (10.2%, 95% CI 9.3%-11.0%). [5]
Affecting more than 630 million people worldwide, [6] LBP results in significant physical, psychological, and social burden and high cost to society. [7] People with LBP tend to experience a higher proportion of functional disability, dysfunctional family relationships, depression, social isolation, work absence, and poor work productivity. [8–14] They have a lower socioeconomic status and a lower quality of life, but tend to be higher users of health care services. [8, 11, 15] Chronic LBP is associated with significant comorbidities, including diabetes, coronary heart disease, [16–18] and depression. [19] The economic burden of LBP is significant. [7, 20, 21] In the United States, the direct and indirect costs of LBP are estimated to exceed 100 billion dollars per year. [5, 22] In Canada, the LBP-related estimate of the medical costs ranges between 6 and 12 billion dollars annually. [23]
Nearly 60% (95% CI 32%-83%) of people with LBP choose to consult a health care provider, including providers of manual therapy such as physiotherapists and chiropractors. [24] However, care-seeking is more common in women and in individuals with previous LBP, poor general health, and more disabling or more painful episodes. [24] Detailed reviews on nonspecific LBP (NSLBP) are available elsewhere. [25]
Approximately 90% of all LBP cases are nonspecific in nature [26] (ie, the pain cannot be attributed to any specific pathology of the spine [27]). In contrast, about 5% of LBP cases present as pain that follows a specific nerve root distribution from a compression, [28] a prolapsed lumbar disk, spinal stenosis, or surgical scarring. [29]
Nonspecific LBP and back-related leg pain (sciatica) with neurological deficit can be further subdivided into the following:(1) acute, defined as pain that restricts daily activities and could last from 1 day to 12 weeks [30]; and
(2) chronic or persistent, defined as pain that restricts daily activities longer than 12 weeks. [5, 31–35]

Table 1 The recent Global Spine Care Initiative (GSCI) [36] classification system covers the spectrum of spine disorders and provides a common language for different types of health providers interested in spine care worldwide. Under this new classification, spine disorders can be classified into 6 classes (class 0 to class V). The classes are distinguished by spine-related symptoms, interference with activities of daily living, presence of neurological deficits, or a severe pathology (Table 1). Patients presenting to primary care clinicians (chiropractors, general physicians, physiotherapists) in Canada would mostly be classified as a class I-III pattern.
Rationale for Developing This Guideline
Clinician adherence to evidence-based clinical practice guidelines (CPGs) can reduce pain and disability in patients with LBP. [37] Numerous national and international CPGs have been produced to address the impact of NSLBP and back related leg pain on people’s health. [38] The Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration recently updated reviews of CPGs on the management of LBP. [39] The review highlighted that the next generation of high-quality guidelines should focus on applicability to specific populations and clear implementation strategies to promote adherence. More recently, guidelines published by the American College of Physicians (ACP) [40] and the Danish National guidelines [41] recommend that clinicians select nonpharmacologic treatment for acute and chronic LBP as first-line treatment, including spinal manipulation therapy (SMT). Other recent CPGs [42, 43] and systematic reviews [44, 45] support recommending SMT for NSLBP. Nonetheless, a paper aimed at updating Canadian family physicians on the effectiveness of SMT for LBP concluded that the research is poor, frequently inconsistent, and almost impossible to interpret. [46] In the light of the important shift toward recommending nonpharmaceutical approaches including SMT as first-line treatment for acute and chronic LBP, and the slow uptake of CPGs by health care providers, [47] it was deemed timely to provide providers of manual therapy and other health care professionals with evidence-informed guidance on the conservative management of NSLBP. This guideline addresses the use of SMT alone or in combination with other commonly used conservative treatments.
Scope and Purpose
The primary aim of this CPG was to synthesize and disseminate the best available evidence on the initial assessment and monitoring of people with LBP and the use of SMT alone or in combination with other conservative treatments for adults (≥18 years of age) and elderly patients with acute (0–3 months) and chronic (>3 months) back pain and back-related leg pain, with the goal of improving clinical decision making and the delivery of care for patients presenting with a class I-III pattern.
The target users of this guideline are providers of manual therapy, other primary care health care professionals, and specialists interested in delivering or referring patients with LBP for manual therapy, as well as policymakers (third-party payers, professional associations, and regulatory boards) making decisions about the organization and delivery of health care. This guideline focuses on the nonsurgical treatment of patients with acute and chronic LBP, with or without radiating leg pain or symptoms (eg, sciatica or radiculopathy). [48] People under the age of 18 years and those presenting with spine-related symptoms with possible spinal stenosis or a class IV or V pattern (ie, stable but severe deformity or serious/systemic pathology, respectively) are excluded from this guideline.
Methods
Guideline recommendations are “Statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.” [49, 50] The framework and methodology used to conduct this study are consistent with the previous guidelines from the Canadian Chiropractic Guideline Initiative (CCGI), which aim to synthesize and disseminate the best available evidence on the management of adults and elderly patients with recent-onset and persistent neck pain and its associated disorders. [51]
Ethics
Because no novel human participant intervention was required, and secondary analyses were considered, the research presented in this guideline is exempt from institutional ethics review board approval.
Selection of Panelists
The CCGI project lead (A.B.) appointed 2 co-chairs (J.O. and G.S.) for the guideline development group and nominated the project executive committee and the remaining guideline panelists. J.O. served as the lead methodologist on the guideline panel. G.S. helped ensure geographic representation of the panel and advised on specific duties of panel members, time commitment, and decision-making process for reaching consensus (development of key questions and of recommendations). The guideline panel included clinicians (P.D., J.W.), clinician researchers (F.A.-Z., M.D., C.H., S.P., I.P., J.S.) methodologists (J.O., A.B., M.S.), a professional leader/decision maker (G.S.), and a patient advocate (D.H.) to ensure that patient values and preferences were considered. One observer (H.C.) monitored the face-to-face meetings of the guideline panel held in Toronto (February 2017). No conflicts of interest were reported through self-declaration among any of the panel members.
Initial Assessment and Monitoring of People With LBP
The project lead (A.B.) and 2 co-chairs (J.O., G.S.) retrieved best practice recommendations on the initial assessment and monitoring of people with LBP issued in prior guidelines, quality standards, and pertinent literature published on the topic in the last decade. The guideline panel then reviewed and approved a short list of recommendations targeting care providers.
Key Question Development on the Conservative Treatment of LBP
The topic areas were chosen based on an Agency for Healthcare Research and Quality (AHRQ) comparative effectiveness review (CER), [43] specific to spinal manipulation as a nonpharmacological intervention. The AHRQ report [43] informed our work because it was the latest, most comprehensive review of the literature on the topic, it considered several highly systematic reviews as an evidence base, and the resources used in developing the AHRQ report were substantial and beyond the capacity of what our group could comparatively develop. Based on this CER, 10 standardized key questions were developed by the panel in a PICO format (ie, population, intervention, comparator, outcome). The comparator is a conservative treatment that may include nonpharmacological approaches such as physical (eg, manual therapy, therapeutic exercise, myofascial therapy) and psychological (eg, cognitive/behavioral) therapies as first-line treatments or usual medical care (Table 2).Search Update and Study Selection

Table 2

Figure 1 The AHRQ CER [43] used systematic methods to search for systematic reviews and randomized controlled trials (RCTs) for each question (Appendix 1 of the AHRQ report, online only) and critically appraise the quality of each study using the AMSTAR tool [52] and its 11 criteria (http://amstar.ca/Amstar_Checklist.php) and Cochrane Back Review Group criteria, respectively. [53] In addition, the panel updated the searches in MEDLINE and the Cochrane Database of Systematic Reviews from April 27, 2015 to February 5, 2017, using the same predefined search strategies [43] (Appendix 2, online only). Our updated search yielded 896 articles (Figure 1). Of the 120 records screened for eligibility based on the AHRQ (CER) [43] inclusion and exclusion criteria (Appendix 3 of the AHRQ report), 3 scientifically admissible RCTs [54–56] and 1 systematic review [57] were included in our synthesis. Updated searches of the systematic review by Ruddock et al [57] using the same databases (March 25, 2015 to February 11, 2017) yielded 260 citations after duplicate removal. Of the 4 records screened for eligibility, 3 studies were not admissible and 1 was a duplicate. The table in Appendix 4 (online only) depicts the studies included for each key question and the reported estimates for each outcome.
This table also highlights which studies were included from the updated search, and the degree to which the estimates from the included studies differed from each other. Each of the 4 additional studies were critically appraised for quality by 2 independent reviewers reaching consensus using the same tools and criteria, [52, 53] with adjudication by a third reviewer if needed (Appendix 4). Furthermore, the risk of bias was incorporated into an evidence profile table of the associated outcome of the associated key question. These summaries suggest in a transparent fashion that the three added studies from the updated review do not substantially change the overall evidence for the two relevant key questions, nor change the certainty or strength of the two relevant recommendations. The articles included and excluded after full text review from the updated search are listed in Appendix 5 (online only).
Recommendation Development
By use of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodological approach, [58] evidence profiles were used to summarize the evidence [59] (Table 2–11). [For Tables 3–11, please refer to the Full Text Article] Using the Evidence-to-Decisions Framework, the panel determined the strength of each recommendation as strong or conditional, using informed judgment on the quality of evidence (confidence in estimates of effect); balance of desirable (eg, reduced pain and disability) and undesirable (eg, adverse reactions) outcomes; confidence in the values and preferences for the target population; and resource implications (costs). [60, 61]
The evidence profiles were used to describe the grading of each recommendation and the outcomes used to address a key question. The outcome estimates and study used for each key question are described in Appendix 6 (online only). Both of these resources provide the supporting evidence gathered for each recommendation.
When deciding to make a recommendation, the panel agreed that there should be evidence of clinically meaningful changes occurring over time in the study population and that a single consensus threshold of clinical effectiveness should be applied consistently. We reached a consensus decision that the thresholds for minimum clinically important change were between-group differences following treatment of 10 points on 0- to 100–point visual analogue scale (VAS), 1 point on a 0- to 10–point numeric rating scale (NRS), 2 points on 0- to 24–point Roland Morris Disability Questionnaire (RMDQ), and 10 points on 0- to 100–point Oswestry Disability Index (ODI), and for any outcome as a standardized mean difference (SMD) of 0.2 to 0.5. These thresholds were informed by the methods in the AHRQ CER. [43] If the desirable and undesirable consequences were judged to be evenly balanced and the evidence was not compelling, the panel’s decision to write or not write a recommendation was taken based on consensus.
An 8–member external committee composed of stakeholders, end users, and researchers from Canada and the United States (Appendix 7, online only) independently reviewed the draft manuscript, recommendations, and supporting evidence. The AGREE II instrument was used to assess the methodological quality of the guideline. [62] Feedback received was collected and considered in a revised draft. For a list of abbreviations and glossary of terms, please see Appendix 8 (online only).
Results
I. Initial Assessment and Monitoring:
What Can Other Guidelines Tell Us About Best Practice?
We first present recommendations on the assessment and monitoring of people with back pain to reflect the algorithm on the management of acute and chronic LBP and back and leg pain (Appendix 9, online only).
Our guideline panel supports the following 10 best practice recommendations on patient’s care issued in prior guidelines, [39–43, 63–65] quality standards, [66–68] and recent literature. [25, 31–34, 44, 69–79]
Care providers are encouraged to:
Give importance to the patient’s individual context, maintain a good relationship and empathy, share information, and use a patient-centered holistic approach by encouraging patients to express their health beliefs, concerns (eg, treatment cost and safety, give a clear explanation of their LBP to help understand the cause[s] of their pain), and personal needs, as well as their preferences for care, treatment management (credibility, effectiveness, individualized), and self-management. [68, 70–73]
Conduct a problem-focused health history and clinical examination at the initial visit to screen for red flags (signs of serious structural or systemic pathologies) with acceptable diagnostic accuracy to rule out malignancies, spinal fractures, and infections. Red flags include a history of malignancy and strong clinical suspicion, older age, prolonged corticosteroid use, major or significant trauma, and presence of a contusion or abrasion for spinal fracture. The likelihood was higher with multiple red flags. [63, 74–76]
Explore the presence of additional MSK complaints and comorbidities.
In the absence of pathology, assess patients for prognostic factors of delayed recovery (ie, risks of poor outcomes or yellow flags). [31–35] The STarT Back screening tool or Örebro Musculoskeletal Pain Screening Questionnaire for screening psychosocial outcomes that might be relevant in patient care are examples of tools clinicians may consider using. [77]
Triage patients with spine pain into 1 of 3 broad categories (specific, nonspecific, and back and leg pain/sciatica)
Consider using the new GSCI classification of spinal disorders, in which back and neck pain can be classified into 6 classes (classes 0 to V), distinguished by the spine-related symptoms, interference with activities of daily living, presence of neurological deficits or a severe pathology. [36]
Avoid the routine use of diagnostic imaging for people with LBP or back-related leg pain regardless of the duration of symptoms unless there are clinical reasons to suspect serious underlying pathology (ie, red flags) [25, 41, 42] (https://choosingwiselycanada.org/spine/).
Consult with or refer the patient to an appropriate provider if co-management is indicated (eg, comorbidities, mental health concerns, significant pain, or functional deficits remain after the maximum therapeutic benefit is reached).
Perform periodic clinical revaluations, monitor patient progression of self-management strategies while discouraging dependence on passive treatment, and evaluate and document side effects.
Consider implementing quality measures aimed at improving the structure, process, and outcomes of care. [66, 67, 78] Electronic data collection systems such as Care Response (https://www.care-response.com/CareResponse/home.aspx) can ease routine collection of patient health measures to monitor and evaluate patients with MSK conditions. Further, electronic health record–linked spine registries can provide feedback on clinicians’ performance and help test and improve spine care pathways. [78]
II. Recommendations on the Conservative Treatment of LBP
We addressed 10 key questions (Table 2). After exploring the evidence for each key question, we decided to combine some with others, yielding a total of 5 recommendations. The panel chose to combine these recommendations because they felt the topic was similar enough to where a single recommendation provided a consistent and not-overlapping message to end-user clinicians. The GRADE evidence profiles supporting each recommendation are presented in Table 3–7. Additional evidence profiles that were developed, yet did not contribute toward developing recommendations from the panel, appear in Table 8–11.
Recommendations. We present five recommendations within three focus areas:(1) acute (0–3 months) classes Ia, IIa, and IIc;
(2) chronic (>3 months) classes Ib, IIb, and IId; and
(3) radicular back-related leg pain.
Recommendations for Acute (0–3 Months) LBP
Key Question 1: Should Spinal Manipulation Versus Another Treatment
Be Used for Acute or Subacute (0–3 months) LBP?
Summary of Evidence. An RCT by Fritz et al [54] randomized patients with LBP of less than 16 days’ duration to receive either early physical therapy (n = 108) consisting of 4 physical therapy sessions over 3 weeks (2 sessions in week 1, followed by 2 weekly sessions) or usual care (n = 112). The early physical therapy group received spinal manipulation using the technique specified in a development of the decision rule. Patients were provided instruction in spinal range-of-motion and trunk-strengthening exercises (10 exercise repetitions 3 to 4 times throughout the day). Usual care consisted of the provision of educational materials and a visit to the primary care physician. All participants were educated about the favorable prognosis of LBP, were advised to remain as active as possible, and were given a copy of The Back Book. The early physical therapy group disability (ODI) improved after 4 weeks (between-group difference, –3.5 (95% CI –6.8 to –0.08) and at 3 months (between-group difference, –3.2 (95% CI –5.9 to –0.47), but not at the 1–year follow-up. There was no improvement in pain intensity (NRS, 0-10) at a 4-week, 3-month, or 1-year follow-up (Table 3).
An RCT by Brennan et al [80] randomized patients with LBP of less than 3 months’ duration to receive manipulation (n = 40), stabilization exercise (n = 46), or specific exercise treatment (n = 37) during a 4-week (twice weekly for a maximum of 8 sessions) period. Disability was assessed in the short term (4 weeks) and long term (1 year). Comparisons were made between patients receiving treatment matched to their subgroup and those receiving unmatched treatment. Manual therapy techniques could include thrust manipulation, or low-amplitude mobilization to the lumbosacral region, along with instruction on lumbar active range-of-motion exercise. Stabilization treatment consisted of a program of trunk strengthening and stabilization exercises. Specific exercise included either flexion or extension exercises as determined by the treating therapist based on patient’s response to movement testing and symptom response to positions of sitting, standing, and walking. All patients who had progressed beyond the acute stage received a general exercise program in keeping with evidence-based recommendations advocating an active, multimodal exercise approach for patients with LBP. Patients receiving matched treatments (n = 50), including manipulation, stabilization, and specific exercise, had less disability (ODI) in the short term (mean difference [MD] = –6.6, 95% CI –0.70 to –12.5) and long term (MD = –8.3, 95% CI –2.5 to –14.1) compared with those receiving unmatched treatments (n = 73).
The panel determined a low certainty in the evidence, with small desirable and undesirable effects and no serious adverse events reported. The resources required for SMT intervention are relatively small (cost of care and equipment needed), with the exception of training to provide the technique. As the intervention of SMT is widely practiced and taught, the panel felt that it is acceptable and feasible to implement.
Recommendation. For patients with acute (0–3 months) LBP, we suggest SMT, other commonly used treatments, or a combination of SMT and commonly used treatments to decrease pain and disability in the short term, based on patient preference and practitioner experience (low quality of evidence, conditional recommendation).
Remarks. Other commonly used treatments may include advice on posture and physical activity, and usual medical care when deemed beneficial.
Recommendations for Chronic (>3 Months) LBP
Key Question 2: Should Spinal Manipulation Versus Inactive Treatment
Be Used For Chronic (>3 Months) LBP?
Summary of Evidence. One RCT by Haas et al [81] evaluated the efficacy of SMT (n = 200) compared with light massage control intervention (n = 200) for patients with nonspecific chronic LBP. Patients were randomized to each of four dose levels of care: 0, 6, 12, or 18 sessions (3 sessions per week) of SMT over 6 weeks to affected segments of the lumbar region. On non-SMT visits, they received a brief (5–minute) light massage treatment to symptomatic areas of the lower back. Participants also received hot pack and low-dose pulsed ultrasound at each visit. Modest improvements in pain were observed in the SMT groups receiving both 12 and 18 sessions of SMT compared with the group receiving light massage only. A greater reduction in pain (modified Von Korff pain intensity, 0–100 scale) was observed at 12 weeks in the group receiving 12 SMT sessions (MD = 8.6, 95% CI 3.2-14.0) and at 39 weeks in the group receiving 18 SMT sessions (MD 7.6, 95% CI 2.0-13.2). Greater reduction in disability (0–100 scale) was also observed at 6 weeks in the 12-session SMT group (–7.5, 95% CI –1.7 to 13.3) and at 39 weeks in the 18-session group (MD = –8.8, 95% CI –3.3 to –14.4). Changes in the SF-12 mental health component and EuroQol Health States scales did not significantly differ between SMT and light massage (Table 4).
An RCT by Gibson et al [82] compared SMT with short-wave diathermy (SWD) to placebo (detuned SWD) for patients with chronic LBP (n = 109) of 2 to 12 months’ duration. Patients in the SWD and detuned SWD groups each received 3 treatments per week for 4 weeks by a physiotherapist, whereas the SMT group received 1 treatment per week for 4 weeks by an osteopath. The SMT intervention consisted of soft tissue manipulation, passive articulation of stiff spinal segments, and manipulation of the lumbosacral region using minimal rotation. Statistically nonsignificant reduction in pain (VAS) was observed at 1 and 3 months following SMT treatment compared with detuned SWD.
Another RCT by Balthazard et al [83] evaluated the effects of SMT plus active exercise (n = 21) with the effects of sham therapy (detuned ultrasound) plus active exercise (n = 21), with each group receiving 8 treatments over 4 to 8 weeks. The SMT intervention was performed by a physiotherapist and consisted of 1 or more of the following techniques: passive intervertebral movements on a painful/stiff vertebral segment, muscle energy technique, and high-velocity low-amplitude (HVLA) manipulation to a stiff vertebral segment(s). Sham therapy was performed by 2 physiotherapists and consisted of application of detuned ultrasound to the painful region of the spine. Both groups received active exercise consisting of spinal mobility, passive stretching, motor control, and strengthening exercises. The SMT plus specific active exercise group had lower disability (ODI; MD = –7.14, 95% CI –12.8 to –1.52) at 6 months.
The panel determined very low certainty in the evidence, with small desirable and undesirable effects and no serious adverse events. Overall, the panel decided the balance between the desirable and undesirable effects probably favors SMT, and based on the available evidence, a conditional recommendation could be made in favor of SMT over minimal intervention therapy. When reported, adverse events in patients undergoing SMT for LBP were limited to muscle soreness, stiffness, and/or a transient increase in pain.
Recommendation. For patients with chronic (>3 months) LBP, we suggest SMT over minimal intervention to decrease pain and disability in the short term (very low evidence, conditional recommendation).
Remarks. SMT may consist of any 1 or more of the following: passive intervertebral movements and/or HVLA thrust applied to a dysfunctional spinal segment(s), muscle energy, and/or soft tissue technique applied to the affected area. Minimal intervention includes manually applied forces with diminished magnitude or 5 minutes of light massage. Inactive treatment (inert or sham therapy) includes detuned SWD or detuned ultrasound.
Key Question 3: Should Spinal Manipulation Versus Another Treatment
Be Used for Chronic (>3 Months) LBP?
Summary of Evidence. One RCT by Xia et al [56] compared a brief course of SMT (n = 72) with nonthrust flexion-distraction spinal manipulation (n = 72) in patients with chronic LBP over 2 weeks. Both SMT and nonthrust spinal manipulation reduced pain (VAS, 0–100 mm scale; MD = –17.1; 95% CI –27.5 to –6.7, and MD = –12.8, 95% CI –23.1 to –2.6) and disability (RMDQ) (MD = –3.0, 95% CI –4.7 to –1.4, and MD = –3.1, 95% CI –4.8 to –1.4) compared with the control group, respectively. No difference in outcomes was observed between SMT and nonthrust spinal manipulation (Table 5).
A single-blind parallel group pragmatic RCT by Castro-Sanchez et al, [55] conducted in Spain, compared the effectiveness of SMT (n = 31) with that of a low-force “functional technique” (n = 31) in patients with chronic LBP. Patients received 3 treatment sessions over 3 weeks. The SMT group demonstrated lower disability (ODI) immediately posttreatment (MD = 2.9, 95% CI 1.4-4.4) and at 1 month (MD = 1.4, 95% CI 0.2-2.6).
Another pragmatic RCT by Ferreira et al [84] compared spinal manipulation (n = 77) including SMT and joint mobilization directed to the lumbopelvic region up to a maximum of 12 visits with motor control exercise (n = 73) and general exercise (n = 74) in patients with LBP of more than 3 months’ duration. Motor control exercise included retraining specific trunk muscles using ultrasound feedback. General exercise included strengthening, stretching, and aerobic exercise while considering physical activity level. Motor control exercise and SMT produce slightly better short-term function and perceptions of effect than general exercise. In general, therapies included extension exercises, advice plus exercise, myofascial therapy, and usual medical care. Pain relief was most effective within the first 6 months, and functional improvement was most effective at 1 month.
The decision for referral for SMT should be based on factors including benefits, costs, patient preferences of providers, and the relative safety of treatment options. The panel determined the conditional recommendation based on the high level of evidence, with small undesirable effects and no serious adverse events. A high level of evidence was defined by Rubinstein et al, as further research is very unlikely to change the confidence in the estimate of effect. [85] The data themselves are considered sufficient with narrow CIs present. Adverse events in the SMT group included muscle soreness and stiffness, with or without transient increase in pain. Low costs are required for the SMT intervention and no specific equipment is needed, with the exception of training to provide the technique. As the intervention of SMT is widely practiced and taught, the panel felt that it was acceptable and feasible to implement and sustain.
Recommendation. For patients with chronic (>3 months) LBP, we recommend SMT or other treatments for short-term reduction in pain and disability (high quality of evidence, conditional recommendation).
Remarks. “Other treatments" includes extension exercises, advice plus exercise, myofascial therapy, or usual medical care when deemed beneficial. Pain relief is most effective within the first 6 months and functional improvement was most effective at 1 month.
Key Question 4: Should Spinal Manipulation Plus Other Treatments
Versus Other Treatments Alone Be Used for Chronic (>3 Months) LBP?
Summary of Evidence. Five RCTs evaluated the effectiveness of SMT plus another treatment compared with treatment without manipulation to reduce pain and disability for patients with chronic LBP. In 4 trials, multimodal therapy consisting of SMT plus another treatment was as effective as treatment without manipulation on pain (VAS) at 1 month (pooled estimates of 3 trials, [86–88] n = 228, MD = –5.88, 95% CI –10.85 to –0.90); 3 months (pooled estimates of 2 trials, [87, 89] n = 1,016, MD = –7.23, 95% CI –11.72 to –2.74); 6 months (pooled estimates of 2 trials, [87, 89] n = 143, MD = –6.77, 95% CI –14.07 to 0.53); and 12 months (pooled estimates of 2 trials, [88, 89] n = 1,000, MD = –3.31, 95% CI –6.60 to –0.02) (Table 6).
Multimodal therapy (SMT plus another treatment) was also as effective as treatment without manipulation in reducing disability (RMDQ 0-24) at 1 month (pooled estimates of 2 trials, [87, 89] n = 158, SMD = –0.40, 95% CI –0.73 to –0.07, I2 = 0%); 3 months (pooled estimates of 2 trials, [87, 89] n = 1078, SMD = –0.22, 95% CI –0.38 to –0.06); 6 months (pooled estimates of 2 trials, [87, 89] n = 142, SMD = –0.30, 95% CI –0.64 to –0.03); and 12 months (pooled estimates of 1 trial, [89] n = 994, SMD = –0.21, 95% CI –0.34 to –0.09, I2 = 0%).
A fifth RCT by Goertz et al [90] evaluated the effectiveness of SMT plus standard medical care (n = 45) compared with standard medical care alone (n = 46) in improving pain and function. Standard medical care included any or all of the following: a focused history and physical examination, diagnostic imaging as indicated, education about self-management including maintaining activity levels as tolerated, pharmacological management with the use of analgesics and anti-inflammatory agents, and physical therapy and modalities such as heat/ice and referral to a pain clinic. [90] Spinal manipulation therapy plus standard medical care was more effective than standard medical care alone for reducing pain (NRS) at 2 weeks (MD = 2.2, 95% CI 1.2-3.1) and 1 month (MD = 1.2, 95% CI 0.2-2.3) and reducing disability (RMDQ) at 2 weeks (MD = –3.9, 95% CI –1.8 to –6.1), and 1 month (MD = –4.0, 95% CI –1.3 to –6.7).
The panel determined the overall certainty of the evidence was moderate, with small desirable effects for short-term and trivial undesirable effects of the intervention. There is no important uncertainty or variability for both pain and function outcomes. The balance between desirable and undesirable effects probably favors the intervention in the short term. This option is acceptable to stakeholders and probably feasible to implement. Nonetheless, barriers to implement this intervention for chronic cases may include the need for more complex and costly multidimensional management, the presence of psychosocial overlays, and a perceived link with the opioid crisis. Management of chronic LBP patients may require a team approach, which is more challenging to establish clinically.
Recommendation. For patients with chronic (>3 months) LBP, we suggest multimodal therapy with or without SMT to decrease pain and disability (moderate quality of evidence, conditional recommendation).
Remarks. Multimodal therapy with SMT treatment may also include exercise, myofascial therapy, advice, educational material, and usual medical care. Spinal manipulation therapy (2 sessions per week for 4 weeks) plus standard medical therapy has resulted in better pain and functional outcomes than standard medical care alone. Pain and functional improvement were also observed at 3 and 12 months.
Radicular Back-related Leg Pain
Key Question 5: Should Spinal Manipulation Plus Other Treatments
Versus Another Treatment Alone Be Used for Back-Related Leg Pain
(Sciatica or Radicular LBP)?
Summary of Evidence. One controlled pragmatic trial by Brønfort et al [91] evaluated the effectiveness SMT plus home exercise and advice (n = 96) compared with home exercise and advice alone (n = 96) in reducing leg pain in the short and long term in adults with subacute to chronic back-related leg pain of at least 4 weeks’ duration. Patients in the SMT group received up to 20 visits of SMT, each lasting 10 to 20 minutes, and attended 4 home exercise and advice visits. Patients in the home exercise and advice group received four 1–hour, 1-on-1 visits during the 12–week intervention. Trial participants were followed up at 3, 12, 26, and 52 weeks. SMT plus home exercise and advice was associated with reduced back and leg pain (NRS) at 12 weeks compared with home exercise and advice alone (MD = 10, 95% CI 2-19) and disability (RMDQ) (MD = –2.5; 95% CI –4 to –1.1) (Table 7). Improvement of the secondary outcomes was generally greater in the SMT plus home exercise and advice group at 12 weeks. However, only global improvement, satisfaction, and medication use had sustained improvements at 52 weeks. No serious treatment-related adverse events or deaths occurred.
The primary focus of the SMT treatment was on manual techniques, including HVLA thrust procedures or low-velocity, variable-amplitude mobilization maneuvers to the lumbar vertebral or sacroiliac joints. The main goals of the home exercise and advice group program were to provide patients with the tools to manage existing pain, prevent pain recurrences, and facilitate engagement in daily activities. Instruction and practice were provided for positioning and stabilization exercises to enhance mobility and increase trunk endurance. Adherence to exercise was encouraged through reminders in both intervention groups.
Given that back-related leg pain is associated with greater disability, health care use, and intervention compared with nonspecific LBP, [92, 93] the panel reached consensus that this is a priority problem in the area of LBP management. The panel deemed that the quality and quantity of evidence informing SMT for back-related leg pain was low and sparse, thus limiting the panel’s decision to a conditional recommendation. Nonetheless, there was consensus among the panel that there is probably no important uncertainty or variability in how much patients experiencing back-related leg pain value pain relief and functional improvement for this problem. The panel, therefore, deemed that the balance of desirable and undesirable effects likely favors SMT for back-related leg pain. On the basis of patient preference and positive safety profile, [94, 95] those who seek a conservative treatment for their back-related leg pain and are appropriate candidates may be offered spinal manipulative care as a desirable, feasible and viable therapeutic option.
Recommendation. For patients with chronic (>3 months) back-related leg pain, we suggest SMT plus home exercise and advice to reduce back pain and disability (low quality of evidence, conditional recommendation).
Remarks. Treatments includes home exercise (positioning and stabilization exercises) and advice. Reduced chronic back-related leg pain and disability were observed at 3 months follow-up.
Discussion
This evidence-based guideline establishes best practices for the use of SMT in the management of LBP. The guideline covers acute (0–3 months) and chronic (>3 months) LBP with or without leg pain. It does not cover the management of MSK thoracic spine or chest wall pain. The primary outcomes reported in the selected studies were LBP intensity and related disability. All recommendations included in this guideline are based on low or moderate risk of bias RCTs. Further, the overall quality of evidence is generally low to moderate considering other factors suggested by GRADE, such as imprecision and risks of bias, and thus the strength of recommendations is weak at this time. Weak recommendations mean that clinicians need to devote more time to the process of shared decision making with patients and ensure that the informed choice reflects patient values and preferences.
Recent guidelines and literature on the assessment and monitoring of patients with LBP encourage care providers to use a patient-centered holistic approach, conduct a problem-focused health history and clinical exam, explore the presence of additional MSK complaints and comorbidities, assess patients for prognostic factors, avoid the routine use of diagnostic imaging, triage patients, consult with or refer the patient to an appropriate provider if co-management is indicated, perform periodic clinical revaluations, monitor patient progression while discouraging dependence on passive treatment, evaluate and document side effects, and consider implementing quality measures. [25, 31–34, 39–44, 63–79]
Similarities and Differences With Recommendations From Other CPGs
on the Conservative Treatment of LBP
Findings from systematic reviews on SMT [45] and of CPGs by the OPTIMa collaboration [39] and CPGs published since the review [40, 41, 42, 43, 63] on LBP treatment and assessment were compared with the current guideline.
For patients with acute and chronic LBP, the current guideline recommends SMT, other commonly used treatments, or a combination of SMT and commonly used treatments in addition to advice (posture, staying active), reassurance, education, and self-management strategies for patients for reduction of back pain and disability. Most guidelines suggest using multimodal strategies including patient education and advice on self-care, different types of exercise, manual therapy (myofascial therapy, joint mobilization, SMT) or soft tissue techniques such as massage, and usual medical care (OPTIMa, [39] National Institute for Care Excellence [NICE], [42] Danish National Guidelines [DNGs], [41] and the Minor Injury Treatment Protocol Project [63]). The ACP clinical guideline [40] recommends that clinicians select nonpharmacologic treatment for acute and chronic LBP (superficial heat, massage, acupuncture, SMT) before considering pharmacologic treatment options.
Generally, recent guidelines recommend that patients remain physically active and that clinicians offer supervised group exercise over home-based exercise for acute and chronic LBP. National Institute for Care Excellence [42] recommends motor control exercise, aerobic exercise, mind-body exercise, or a combination of approaches; OPTIMa39 suggests supervised exercise or yoga; and the Minor Injury Treatment Protocol Project [63] recommends considering aerobic activity, movement instruction, muscle strengthening, postural control, and stretching.
For acute LBP, the DNGs41 recommend supervised exercise, broadly defined as exercise or physical activity aimed directly at the back or general health and fitness (eg, back-specific strengthening, stretching, motor control exercise or mobilizing exercises, and cardiovascular training).
Although the present guideline included usual medical care as a treatment comparator, pharmacological treatments were often poorly described or standardized across studies. Considering this limitation, no inference should be made from a recommendation in favor or against usual medical care. Clinicians may consider the following recommendations from recent guidelines on pharmacological treatment for patients with acute LBP. If pharmacologic treatment is desired, the ACP suggests offering nonsteroidal anti-inflammatory drugs (NSAIDs) or skeletal muscle relaxants. [40] The AHRQ CER also recommends NSAIDs, skeletal muscle relaxants, and opioids, but recommends against paracetamol and systemic corticosteroids. [43] Similarly, NICE recommends against paracetamol and opioid use, but suggests NSAIDs may be offered at the lowest effective dose only after careful consideration of comorbidities and other risk factors for adverse effects. [42] In contrast, the DNGs recommend against NSAIDs, paracetamol, opioids, extraforaminal glucocorticoid injection, acupuncture, and targeted treatment for acute LBP. [41]
For patients with chronic LBP, this guideline suggests providing SMT over minimal intervention or SMT as part of a multimodal therapy (other commonly used treatments include exercise, advice and education, and myofascial therapy). Both AHRQ CER [43] and NICE [42] specify manual therapy only as part of a multimodal approach including exercise, with or without psychological therapy. Of interest, recent evidence suggests that manual therapy may be more effective in people with higher baseline symptom severity. [96] The ACP recommends exercise, multidisciplinary rehabilitation, acupuncture, exercises (mindfulness-based stress reduction, tai chi, yoga, motor control exercise), progressive relaxation, electromyography biofeedback, low-level laser therapy, operant conditioning (behavioral therapy involving reinforcement), cognitive-behavioral therapy, and SMT. [40] For patients with high levels of disability or significant distress, OPTIMa [39] recommends combining exercise with psychological interventions such as a behavioral approach.
A recent overview of Cochrane reviews reported that physical activity and exercise can reduce the severity of pain, improve physical function, and have a variable effect on both psychological function and quality of life and few adverse events. [97] Type of exercise should be determined based on patient needs and preferences. The current guideline recommendations did not specifically address psychological therapy as this approach was not used as a comparator. Nonetheless, because these other recent guidelines recommend psychological therapy for chronic LBP, clinicians may wish to consider including this approach as part of a multimodal therapy. In patients with an inadequate response to nonpharmacologic therapy, the ACP suggests considering NSAIDs as first-line therapy or tramadol or duloxetine as second-line therapy for chronic LBP. Opioids should only be considered as an option in patients who have failed to respond to the aforementioned treatments and only if the potential benefits outweigh the risks for individual patients and after a discussion of known risks and realistic benefits with patients. [40, 98, 99]
For patients with chronic back-related leg pain (sciatica or radicular LBP), this guideline suggests providing SMT along with home exercise and advice to improve pain and disability. The NICE guidelines recommend considering manual therapy (spinal manipulation, mobilization, or soft tissue techniques such as massage) as part of a treatment package including exercise and potentially psychological therapy. [42] For recent-onset lumbar radiculopathy, the DNGs [41] recommend advising patients to stay active within pain tolerance (eg, walking, working, participating in leisure-time activities, exercises), offering supervised exercise therapy, directional exercise or motor control exercise, and spinal manual therapy (any mobilization or spinal manipulation technique) as an add-on to the usual treatment. The course of care should be chosen based on a collaborative process including clinician expertise and patient preference, and it should be modified based on changes in clinical presentation over time.
Stakeholder Considerations
When choosing the right therapy for the right patient, it is necessary to compare the effectiveness, as well as the risk of adverse events and related costs, of a given treatment with that of other commonly used approaches and to consider patient experience and satisfaction with care. [69, 100] Current evidence on(1) treatment effectiveness presented above;
(2) lower risk of adverse events following SMT [45, 101] compared with pharmacological agents including commonly prescribed NSAIDs [102–105] and opioids [99, 106–111];
(3) equivalent costs of guideline-endorsed treatments for acute and chronic LBP [112] offered by chiropractors, physical therapists, and general practitioners [113–117];
(4) recent evidence favoring combined physical and psychological treatments, yoga, educational programs, acupuncture, and SMT as likely cost-effective options for LBP [118]; and
(5) high satisfaction with care from providers of manual therapy including chiropractors [102, 119] suggests that nonpharmacologic therapies, including manual therapy should be the first line of treatment for acute and chronic LBP.This, however, represents a major shift. Third-party payers should discourage the prescription of expensive, marginally effective, and potentially harmful drugs such as opioids and NSAIDs, [120] but rather encourage patients to be referred for equally effective lower-risk alternative therapies, including spinal manipulation, mobilization, massage, and supervised exercise. [39–43, 45, 63] Recent evidence suggests behavioral and CAM therapies (education about nonpharmacological methods for pain management and taught mindfulness techniques, movement, guided imagery, relaxation training, yoga, qigong, tai chi, physical therapy, exercise classes, chiropractic therapy, osteopathic treatment) can help reduce pain and the use of opioids. [121]
The projection of societal burden related to MSK conditions and recent research evidence on the effectiveness and risks of adverse events of common approaches for managing these patients raises the question of whether the current general practitioner–led primary-care model for patients with MSK disorders and back pain is the best approach. [122] Alternative options include transferring first-contact care to other professional groups (chiropractors, physiotherapists, and osteopaths) whose clinical interests and expertise more clearly focus on MSK problems. Furthermore, the use of multidisciplinary care models in which a variety of professionals work together to share the responsibility for the early assessment and management of patients with MSK disorders should be considered. [122]
Spinal manipulative care is not inherently resource intensive. A single regulated professional may be able to deliver treatment, dependent on training, practice patterns, and legal scope of practice. This could help limit health care access inequalities. In addition to chiropractors, some physical therapists, general physicians, and osteopathic physicians provide SMT. Considering the skills required to deliver manual therapy and other forms of therapies (eg, exercise prescription) and based on individual patient preference, lumbar SMT as part of multimodal care should be delivered by properly trained licensed professionals. [123]
The level of knowledge about the extent of evidence supporting chiropractic care is ubiquitously low among health care professionals (physicians, physiotherapists) [124–126] and students (medical, nurse practitioners, physician assistants). [127, 128] Many develop their opinions on chiropractic care during or after medical school. Further, knowledge often derives from nonauthoritative, often anecdotal, sources such as patients, family, and friends, and relationships with chiropractors. [126] Nonetheless, most care providers and students report wanting to learn more about the evidence supporting chiropractic care. Education about chiropractic may optimally be implemented during training. [126–128]
Dissemination and Implementation Plan
Numerous professional (eg, lack of knowledge, skills, self-capacity, misperceptions about evidence-based CPGs, lack of time) and organizational/contextual barriers (eg, leadership, organizational culture, years involved in quality improvement, data infrastructure/information systems, and resources) impede the uptake of guideline recommendations in clinical practice. [129–131] The field of knowledge translation has produced a plethora of tools and methods to address these barriers and enhance the uptake of guidelines by clinicians. Knowledge translation is focused on closing the gap between what is known to work best and what is routinely done in practice. [132] The closure of this gap can be achieved by developing and implementing knowledge translation strategies targeting care providers, patients, and wider health care organizations. [133] Such initiatives include Choosing Wisely Canada (http://choosingwiselycanada.org/about/), Inter-professional Spine Assessment and Education Clinics (http://www.isaec.org/), Bone and Joint Canada (http://boneandjointcanada.com/), and the Center for Effective Practice (https://thewellhealth.ca/low-back-pain/).
To prepare for guideline implementation, we considered the Guideline Implementation Planning Checklist [134] and available strategies and supporting evidence to increase guideline uptake. [135, 136] To raise awareness, chiropractic professional organizations are encouraged to inform their members of new CCGI guidelines, resources, and tool kits easily accessible on our website (http://www.chiroguidelines.org/) to help with “front line” dissemination. The potential resource implications (specialized staff, cost) of applying the guideline recommendations are considered small.
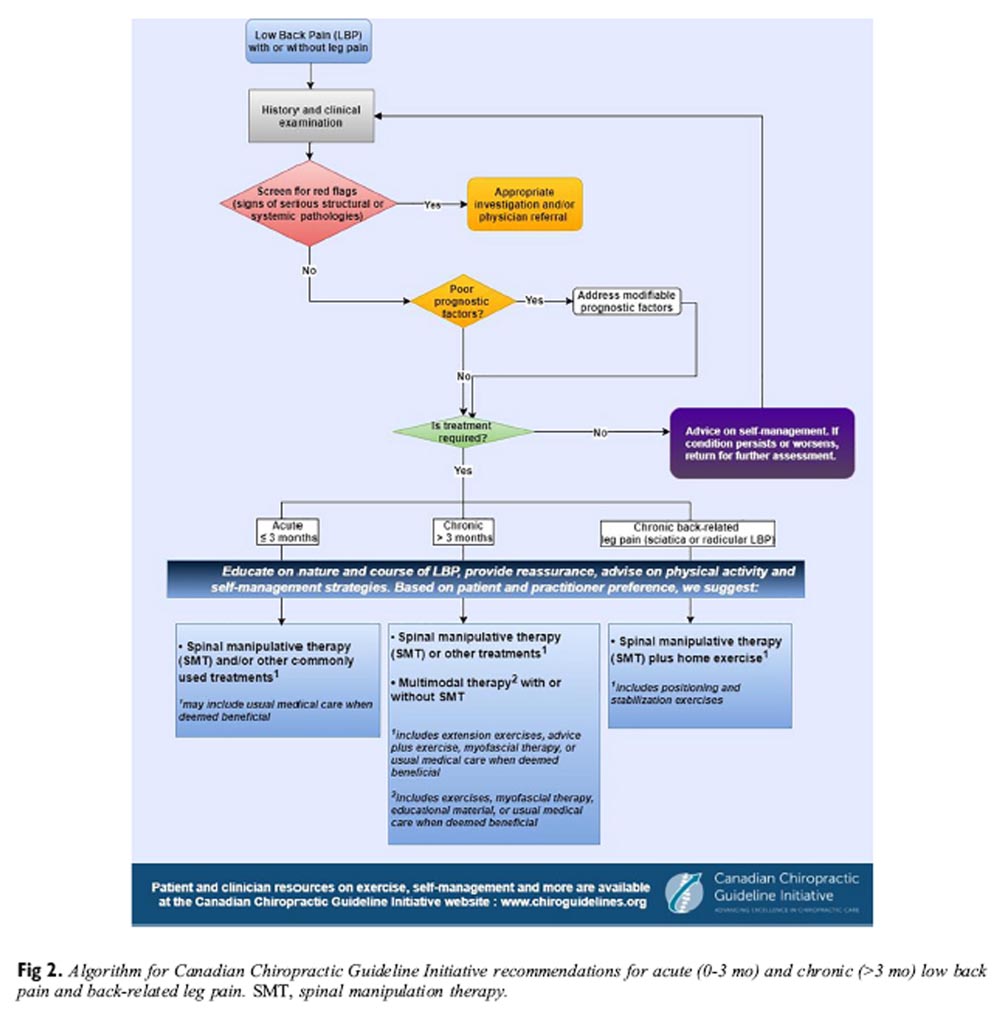
Figure 2 The guideline implementation tools framework was used to clarify the objectives of the tools; identify end users, as well as context and setting where tools will be used; provide instructions for use; and describe methods for developing the tools, related evidence, and methods to evaluate the tools. [137] Implementation tools designed to increase guideline uptake include practitioner and patients’ handouts (Appendix 9, Appendix 10, online only), algorithms (Figure 2), webinars, videos and learning modules produced in collaboration with the Canadian Chiropractic Protective Association (http://www.ccpaonline.ca), point-of-care checklists, and health status reminders. [138–140] The CCGI has also established a network of opinion leaders across Canada to enhance the uptake of research among chiropractors. [141]
Patient versions of guidelines are increasingly valued. For the design of a patient guideline on LBP, we will consider the following recommendations: the purpose of the guideline for patients, the health care system, and clinicians, as well as the applicability and the properties of guidelines. [142] Additional themes emerging from a qualitative study among patients and the public included better access to and awareness of available guidelines and suggestions on how best to present the evidence and the format of the guideline. [143]
People with chronic LBP are more likely to prefer and participate in exercise or training programs and activities that are designed with consideration of their preferences, circumstances, fitness levels, and previous exercise experience. [144] Importantly, exercises alone (strengthening the spinal muscles, stretching or aerobic exercise) or in combination with education may reduce the subsequent occurrence of LBP by approximately 30%. [145, 146] An online CCGI evidence-based exercise video series for people with spinal pain is available at www.chiroguidelines.org.
To select exercises for the video series, we reviewed the clinical trials included in recent CPGs [41–43] for supervised and home exercises (stretching, strengthening, motor control, directional, physical activity) found to be effective in improving back pain. Descriptions of specific exercises were extracted from the literature and organized within 5 themes: stretching, mobility, proprioception, motor control, and strengthening. In parallel, 4 expert clinicians each provided a list of 20 exercises they commonly prescribe to patients with LBP. We excluded duplicates, exercises with no supporting evidence, and programs requiring certification such as the McKenzie method. The expert clinicians reached consensus over exercises to retain for chronic LBP (n = 15) and the progression to recommend (from easy to more difficult) to clinicians and patients. A 7–member international external review committee (Canada, United States, and England) reviewed those choices prior to producing the exercise videos.
Research
Research on LBP is at times difficult to interpret, often because of poor reporting and high heterogeneity of randomized controlled trials (patients, settings, treatments, outcomes). [116, 147] New standards from the National Institutes of Health (NIH) for conducting research on chronic LBP are expected to improve the comparability of studies, facilitate pooling of data from multiple sources, and improve the ability to define phenotypes (ie, prognostic stratification) among patients with LBP. [148]
Guideline Update
The methods for updating the CCGI guidelines have been reported elsewhere. [51] These include(1) monitoring changes in evidence, available interventions, importance and value of outcomes, resources available, and relevance of the recommendations to clinicians (limited systematic literature searches each year for 3–5 years and survey to experts in the field annually);
(2) assessing the need to update (relevance of the new evidence or other changes, type and scope of the update); and
(3) communicating the process, resources, and timeline to the Guideline Advisory Committee of the CCGI, who will submit a recommendation to the Guideline Steering Committee to make a decision to update and schedule the process.Strengths and Limitations
Strengths of this guideline include the rigorous adherence to current scientific standards. Further, the guidelines were peer-reviewed by international experts who provided detailed comments that resulted in revisions and clarifications prior to release of the final report. Shortcomings of this guideline include the low to moderate quality of supporting evidence found during the searches. Most of the downgrading of evidence supporting the outcomes occurred because of imprecision and risks of bias. In addition, our updated search of the published reports included 2 databases (Medline and Cochrane Central Register of Controlled Trials), but was limited to reports published in English, which possibly excluded some relevant studies. This, however, is an unlikely source of bias. [149, 150] Further, poor descriptions of the SMT interventions evaluated in included trials were common. The new Consensus on Interventions Reporting Criteria List for Spinal Manipulative Therapy is expected to improve the reporting of SMT intervention in future studies. [147] Although the composition of the guideline panel was diverse, with experienced methodologists, expert clinicians, and stakeholder and patient representatives, only one member was from another health discipline (physiotherapist). The scope of this guideline focused on selected outcomes such as pain and disability, although included studies assessed several additional outcomes.
Conclusion
Current evidence on the effectiveness, lower risks of adverse events, and equivalent costs suggests that nonpharmacological therapies including SMT should be the first line of treatment for acute and chronic LBP. Based on patient preference and resources available, a mixed multimodal approach including manual therapy, advice on self-management, and exercise (supervised/unsupervised or at home) may be an effective treatment strategy for acute and chronic LBP and back and leg pain. Progress, particularly with respect to pain alleviation and reduction of disability, should be regularly monitored for evidence of benefit.
Practical Application
A multimodal approach including manual therapy, self-management advice,
and physical activity is an effective treatment strategy for acute and chronic LBP.Acknowledgements
We thank the following people for their contributions to this article: Dr. Henry Candelaria, DC, observer; Heather Owens, Research Manager, and Siobhan Milner, research assistant, proofreading; Mona Shah and the Ontario Chiropractic Association, for assistance in producing the companion document intended for patients with neck and back pain; members of the guideline panel who served on the Delphi consensus panel and members of the external review committee (refer to Appendix 7), who made this project possible by donating their expertise and clinical judgment.
Funding Sources and Conflicts of Interest
Funds were provided by the Canadian Chiropractic Research Foundation. The views of the funding body have not influenced the content of the guideline. A conflict of interest disclosure or declaration form was completed by all participants involved in this guideline. In the past 3 years, no conflicts of interest were reported for this study.
Guideline Disclaimer
The evidence-based practice guidelines published by the Canadian Chiropractic Guideline Initiative (“CCGI”) include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options. Guidelines are intended to inform clinical decision making, are not prescriptive in nature, and do not replace professional chiropractic care or advice, which always should be sought for any specific condition. Furthermore, guidelines may not be complete or accurate because new studies that have been published too late in the process of guideline development or after publication are not incorporated into any particular guideline before it is disseminated. CCGI and its working group members, executive committee, and stakeholders (the “CCGI Parties”) disclaim all liability for the accuracy or completeness of a guideline and disclaim all warranties, expressed or implied. Guideline users are urged to seek out newer information that might impact the diagnostic and/or treatment recommendations contained within a guideline. The CCGI Parties further disclaim all liability for any damages whatsoever (including, without limitation, direct, indirect, incidental, punitive, or consequential damages) arising out of the use, inability to use, or the results of use of a guideline, any references used in a guideline, or the materials, information, or procedures contained in a guideline, based on any legal theory whatsoever and whether or not there was advice of the possibility of such damages.
Through a comprehensive and systematic literature review, CCGI evidence-based clinical practice guidelines incorporate data from the existing peer-reviewed literature. This literature meets the prespecified inclusion criteria for the clinical research question, which CCGI considers, at the time of publication, to be the best evidence available for general clinical information purposes. This evidence is of varying quality from original studies of varying methodological rigor. CCGI recommends that performance measures for quality improvement, performance-based reimbursement, and public reporting purposes should be based on rigorously developed guideline recommendations.
References:
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators.
Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases
and Injuries, 1990-2015: a Systematic Analysis for the Global Burden of Disease Study 2015
Lancet. 2016 (Oct 8); 388 (10053): 1545–1602Hoy, D., L. March, P. Brooks, F. Blyth, A. Woolf, et al.
The Global Burden of Low Back Pain: Estimates from the Global Burden of Disease 2010 study
Ann Rheum Dis. 2014 (Jun); 73 (6): 968–974Hoy, D, Bain, C, Williams, G et al.
A systematic review of the global prevalence of low back pain.
Arthritis Rheum. 2012; 64: 2028–2037Haldeman S, Dagenais S.
A Supermarket Approach to the Evidence-informed Management of Chronic Low Back Pain
Spine Journal 2008 (Jan); 8 (1): 1–7Freburger, JK, Holmes, GM, Agans, RP et al.
The rising prevalence of chronic low back pain.
Arch Intern Med. 2009; 169: 251–258Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, et al.:
Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990-2010:
A Systematic Analysis for the Global Burden of Disease Study 2010
Lancet. 2012 (Dec 15); 380 (9859): 2163–2196Dagenais S, Caro J, Haldeman S.
A Systematic Review of Low Back Pain Cost of Illness Studies
in the United States and Internationally
Spine J 2008 (Jan); 8 (1): 8–20Andersson, G.
Epidemiological features of chronic low-back pain.
Lancet. 1999; 354: 581–585Frank, AO and De Souza, LH.
Conservative management of low back pain.
Int J Clin Pract. 2001; 55: 21–31Scheermesser, M, Bachmann, S, Schämann, A, Oesch, P, and Kool, J.
A qualitative study on the role of cultural background in patients’ perspectives on rehabilitation.
BMC Musculoskelet Disord. 2012; 13: 5Horng, YS, Hwang, YH, Wu, HC et al.
Predicting health-related quality of life in patients with low back pain.
Spine (Phila Pa 1976). 2005; 30: 551–555Di Iorio, A, Abate, M, Guralnik, JM et al.
From chronic low back pain to disability, a multifactorial mediated pathway: the InCHIANTI study.
Spine (Phila Pa 1976). 2007; 32: E809–E815Widanarko, B, Legg, S, Stevenson, M, Devereux, J, and Jones, G.
Prevalence of low back symptoms and its consequences in relation to occupational group.
Am J Ind Med. 2013; 56: 576–589Schofield, DJ, Callander, EJ, Shrestha, RN, Passey, ME, Kelly, SJ, and Percival, R.
Back problems, comorbidities, and their association with wealth.
Spine J. 2015; 15: 34–41Balagué, F, Mannion, AF, Pellisé, F, and Cedraschi, C.
Non-specific low back pain.
Lancet. 2012; 379: 482–491Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D.
The Burden of Chronic Low Back Pain: Clinical Comorbidities, Treatment Patterns,
and Health Care Costs in Usual Care Settings
Spine (Phila Pa 1976). 2012 (May 15); 37 (11): E668–677Dario, A, Ferreira, M, Refshauge, K et al.
Mapping the association between back pain and type 2 diabetes:
a cross-sectional and longitudinal study of adult Spanish twins.
PLoS One. 2017; 12: e0174757Fernandez, M, Ordoñana, JR, Hartvigsen, J et al.
Is chronic low back pain associated with the prevalence of coronary heart disease when genetic
susceptibility is considered? A co-twin control study of Spanish twins.
PLoS One. 2016; 11: e0155194Bletzer, J, Gantz, S, Voigt, T, Neubauer, E, and Schiltenwolf, M.
Chronische untere Rückenschmerzen und psychische Komorbidität.
Schmerz. 2017; 31: 93–101Wenig, CM, Schmidt, CO, Kohlmann, T, and Schweikert, B.
Costs of back pain in Germany.
Eur J Pain. 2009; 13: 280–286Australian Institute of Health and Welfare.
Health-Care Expenditure on Arthritis and Other Musculoskeletal Conditions 2008–09.
(Arthritis series no. 20, Cat. no. PHE 177)AIHW, Canberra; 2014Katz, J.
Lumbar disc disorders and low-back pain: socioeconomic factors and consequences.
J Bone Joint Surg Am. 2006; 88: 21–24Church, J, Saunders, D, Wanke, M, Pong, R, Spooner, C, and Dorgan, M.
Citizen participation in health decision-making: past experience and future prospects.
J Public Health Policy. 2002; 23: 12–32Ferreira, ML, Machado, G, Latimer, J, Maher, C, Ferreira, PH, and Smeets, RJ.
Factors defining care-seeking in low back pain–a meta-analysis of population based surveys.
Eur J Pain. 2010; 14: 747.e1-e7.Maher, C, Underwood, M, and Buchbinder, R.
Non-specific low back pain.
Lancet. 2017; 389: 736–747van Tulder, M, Koes, B, and Bombardier, C.
Low back pain.
Best Pract Res Clin Rheumatol. 2002; 16: 761–775Manek, NJ and MacGregor, AJ.
Epidemiology of back disorders: prevalence, risk factors, and prognosis.
Curr Opin Rheumatol. 2005; 17: 134–140Frymoyer, JW.
Back pain and sciatica.
N Engl J Med. 1988; 318: 291–300Waddell, G.
The Back Pain Revolution. 2nd ed.
Churchill Livingstone, Edinburgh, UK; 2004Karayannis, NV, Jull, GA, and Hodges, PW.
Physiotherapy movement based classification approaches to low back pain:
comparison of subgroups through review and developer/expert survey.
BMC Musculoskelet Disord. 2012; 13: 24Steenstra, IA, Munhall, C, Irvin, E et al.
Systematic review of prognostic factors for return to work in workers with sub acute
and chronic low back pain.
J Occup Rehabil. 2017; 27: 369–381Chou R, Huffman LH; American Pain Society.
Nonpharmacologic Therapies for Acute and Chronic Low Back Pain:
A Review of the Evidence for an American Pain Society/
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 492–504Staal, JB, Hlobil, H, van Tulder, MW et al.
Occupational health guidelines for the management of low back pain: an international comparison.
Occup Environ Med. 2003; 60: 618–626Burton, AK, Balagué, F, Cardon, G et al.
Chapter 2. European Guidelines for Prevention in Low Back Pain: November 2004
Eur Spine J. 2006; 15: S136–S168Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr., Shekelle P, Owens DK:
Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline
from the American College of Physicians and the American Pain Society
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 478–491Haldeman S., Johnson C.D., Chou R. et al.
The Global Spine Care Initiative: Classification System for Spine-related Concerns
European Spine Journal 2018 (Sep); 27 (Suppl 6): 889–900Golec, SJ and Valier, AR.
The effect of following clinical practice guidelines on the pain and disability outcomes of patients
with low back pain – a critically appraised topic [e-pub ahead of print].
(Available at: http://dx.doi.org/10.1123/jsr.2015-0185
Accessed February 14, 2018)J Sport Rehabil. 2017; : 1–11Côté, P, Wong, J, Sutton, D et al.
Management of Neck Pain and Associated Disorders: A Clinical Practice Guideline
from the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration
Eur Spine J. 2016 (Jul); 25 (7): 2000–2022Wong JJ, Cote P, Sutton DA, et al.
Clinical Practice Guidelines for the Noninvasive Management of Low Back Pain: A Systematic Review
by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration
European J Pain 2017 (Feb); 21 (2): 201–216Qaseem A, Wilt TJ, McLean RM, Forciea MA;
Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain:
A Clinical Practice Guideline From the American College of Physicians
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 514–530Stochkendahl MJ, Kjaer P, Hartvigsen J et al.
National Clinical Guidelines for Non-surgical Treatment of Patients with
Recent Onset Low Back Pain or Lumbar Radiculopathy
European Spine Journal 2018 (Jan); 27 (1): 60–75National Institute for Health and Care Excellence (NICE):
Low Back Pain and Sciatica in Over 16s: Assessment and Management (PDF)
NICE Guideline, No. 59 2016 (Nov): 1–1067Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al.
Noninvasive Treatments for Low Back Pain
Comparative Effectiveness Review no. 169
Agency for Healthcare Research and Quality; (February 2016)Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al.
Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 493–505Paige NM, Myiake-Lye IM, Booth MS, et al.
Association of Spinal Manipulative Therapy with Clinical Benefit and Harm
for Acute Low Back Pain: Systematic Review and Meta-analysis
JAMA. 2017 (Apr 11); 317 (14): 1451–1460Manning, MA and Allan, GM.
Spinal manipulative therapy for low back pain.
Can Fam Phys. 2017; 63: 294Amorin-Woods LG, Beck RW, Parkin-Smith GF, Lougheed J, Bremner AP.
Adherence to Clinical Practice Guidelines Among Three Primary
Contact Professions: A Best Evidence Synthesis of the Literature
for the Management of Acute and Subacute Low Back Pain
J Can Chiropr Assoc 2014 (Sept); 58(3): 220–237Lin, CW, Verwoerd, AJ, Maher, CG et al.
How is radiating leg pain defined in randomized controlled trials of conservative treatments in primary care?
A systematic review.
Eur J Pain. 2014; 18: 455–464in: G Graham, M Mancher, D Miller Wolman, S Greenfield, E Steinberg (Eds.)
Clinical Practice Guidelines We Can Trust.
Institute of Medicine, Shaping the Future for Health.
National Academies Press, Washington, DC; 2011Rosenfeld, RM, Shiffman, RN, and Robertson, P.
Clinical practice guideline development manual, third edition:
a quality-driven approach for translating evidence into action.
Otolaryngol Head Neck Surg. 2013; 148: S1–S55Bussières, AE, Stewart, G, Al Zoubi, F et al.
The Treatment of Neck Pain-Associated Disorders and
Whiplash-Associated Disorders: Clinical Practice Guideline
J Manipulative Physiol Ther. 2016 (Oct); 39 (8): 523–564Shea, BJ, Hamel, C, Wells, GA et al.
AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews.
J Clin Epidemiol. 2009; 62: 1013–1020Robinson, KA, Chou, R, Berkman, ND et al.
Integrating Bodies of Evidence: Existing Systematic Reviews and Primary Studies.
(Rockville, MD: Agency for Healthcare Research and Quality; 2008)
in: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. ; 2008Fritz, JM, Magel, JS, McFadden, M et al.
Early physical therapy vs usual care in patients with recent-onset low back pain: a randomized clinical trial.
JAMA. 2015; 314: 1459–1467Castro-Sánchez, AM, Lara-Palomo, IC, Matarán-Peñarrocha, GA et al.
Short-term effectiveness of spinal manipulative therapy versus functional technique in patients with chronic
nonspecific low back pain: a pragmatic randomized controlled trial.
Spine J. 2016; 16: 302–312Xia, T, Long, CR, Gudavalli, MR et al.
Similar effects of thrust and nonthrust spinal manipulation found in adults with subacute and chronic
low back pain: a controlled trial with adaptive allocation.
Spine. 2016; 41: E702–E709Ruddock, JK, Sallis, H, Ness, A, and Perry, RE.
Spinal manipulation vs sham manipulation for nonspecific low back pain:
a systematic review and meta-analysis.
J Chiropr Med. 2016; 15: 165–183Guyatt, GH, Oxman, AD, Schünemann, HJ, Tugwell, P, and Knottnerus, A.
GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology.
J Clin Epidemiol. 2011; 64: 380–382Guyatt, G, Oxman, AD, Akl, EA et al.
GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables.
J Clin Epidemiol. 2011; 64: 383–394Andrews, J, Guyatt, G, Oxman, AD et al.
GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations.
J Clin Epidemiol. 2013; 66: 719–725Andrews, JC, Schünemann, HJ, Oxman, AD et al.
GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength.
J Clin Epidemiol. 2013; 66: 726–735Brouwers, MC, Kho, ME, Browman, GP et al.
AGREE II: advancing guideline development, reporting and evaluation in health care.
J Clin Epidemiol. 2010; 63: 1308–1311Côté, P, Shearer, H, Ameis, A et al.
Enabling recovery from common traffic injuries: A focus on the injured person.
(Available at: https://www.fsco.gov.on.ca/en/auto/documents/2015-cti.pdf
Accessed February 14, 2018)UOIT-CMCC Centre for the Study of Disability Prevention and Rehabilitation, ; January 31, 2015Globe, G, Farabaugh, RJ, Hawk, C et al.
Clinical Practice Guideline: Chiropractic Care for Low Back Pain
J Manipulative Physiol Ther. 2016 (Jan); 39 (1): 1–22Bussières, AE, Taylor, JA, and Peterson, C.
Diagnostic Imaging Practice Guidelines for Musculoskeletal Complaints
n Adults — An Evidence-Based Approach: Part 3: Spinal Disorders
J Manipulative Physiol Ther 2008 (Jan); 31 (1): 33-88Royal College of Chiropractors. (2012)
Acute Low Back Pain: Chiropractic Quality StandardRoyal College of Chiropractors. (2014)
Chronic Low Back Pain: Chiropractic Quality StandardNational Clinical Guideline Centre.
Patient Experience in Adult NHS Services: Improving the Experience of Care for People Using Adult NHS Services.
Patient Experience in Generic Terms. NICE Clinical Guidelines, No. 138.
National Clinical Guideline Centre (UK). London, England:
Royal College of Physicians (UK); 2012. (Available at:) (Updated November 2016)
http://pathways.nice.org.uk/pathways/patient-experience-in-adult-nhs-servicesHopayian, K and Notley, C.
A systematic review of low back pain and sciatica patients’ expectations and experiences of health care.
Spine J. 2014; 14: 1769–1780Dima, A, Lewith, GT, Little, P, Moss-Morris, R, Foster, NE, and Bishop, FL.
Identifying patients’ beliefs about treatments for chronic low back pain in primary care: a focus group study.
Br J Gen Pract. 2013; 63: e490–e498Ellis, S.
The patient-centred care model: holistic/multiprofessional/reflective.
Br J Nurs. 1999; 8: 296–301Meeker, WC, Watkins, RW, Kranz, KC, Munsterman, SD, and Johnson, C.
Improving Our Nation's Health Care System: Inclusion of Chiropractic in Patient-Centered
Medical Homes and Accountable Care Organizations
Journal of Chiropractic Humanities 2014 (Dec); 21 (1); 49–64Kitson, A, Marshall, A, Bassett, K, and Zeitz, K.
What are the core elements of patient-centred care? A narrative review and synthesis of the literature
from health policy, medicine and nursing.
J Adv Nurs. 2013; 69: 4–15Verhagen, AP, Downie, A, Maher, CG, and Koes, BW.
Most red flags for malignancy in low back pain guidelines lack empirical support: a systematic review.
Pain. 2017; 158: 1860–1868Downie, A, Williams, CM, Henschke, N et al.
Red flags to screen for malignancy and fracture in patients with low back pain: systematic review.
Br J Sports Med. 2014; 48: 1518Verhagen, AP, Downie, A, Popal, N, Maher, C, and Koes, BW.
Red flags presented in current low back pain guidelines: a review.
Eur Spine J. 2016; 25: 2788–2802Fuhro, FF, Fagundes, FR, Manzoni, AC, Costa, LO, and Cabral, CM. Örebro
musculoskeletal pain screening questionnaire short-form and STarT back screening tool:
correlation and agreement analysis.
Spine (Phila Pa 1976). 2016; 41: E931–E936Goertz, CM, Weeks, WB, Justice, B, and Haldeman, S.
A Proposal to Improve Health-care Value in Spine Care Delivery: The Primary Spine Practitioner
Spine J. 2017 (Oct); 17 (10): 1570–1574Chou, R, Fu, R, Carrino, JA, and Deyo, RA.
Imaging strategies for low-back pain: systematic review and meta-analysis.
Lancet. 2009; 373: 463–472Brennan, GP, Fritz, JM, Hunter, SJ, Thackeray, A, Delitto, A, and Erhard, RE.
Identifying subgroups of patients with acute/subacute “nonspecific” low back pain:
results of a randomized clinical trial.
Spine (Phila Pa 1976). 2006; 31: 623–631Haas M, Vavrek D, Peterson D, Polissar N, Neradilek MB.
Dose-response and Efficacy of Spinal Manipulation for Care of Chronic Low Back Pain:
A Randomized Controlled Trial
Spine J. 2014 (Jul 1); 14 (7): 1106–1116Gibson, T, Grahame, R, Harkness, J, Woo, P, Blagrave, P, and Hills, R.
Controlled comparison of short-wave diathermy treatment with osteopathic treatment in non-specific low back pain.
Lancet. 1985; 1: 1258–1261Balthazard, P., de Goumoens, P., Rivier, G., Demeulenaere, P., Ballabeni, P.
Manual Therapy Followed by Specific Active Exercises Versus a Placebo Followed by
Specific Active Exercises on the Improvement of Functional Disability in Patients
with Chronic Non Specific Low Back Pain: A Randomized Controlled Trial
BMC Musculoskelet Disord. 2012 (Aug 28); 13: 162Ferreira ML, Ferreira PH, Latimer J, et al.
Comparison of General Exercise, Motor Control Exercise and Spinal Manipulative Therapy
for Chronic Low Back Pain: A Randomized Trial
Pain. 2007 (Sep); 131 (1-2): 31–37Rubinstein, S, van Middelkoop, M, Assendelft, WJ, de Boer, MR, and van Tulder, MW.
Spinal manipulative therapy for chronic low-back pain: an update of a Cochrane review.
Spine (Phila Pa 1976). 2011; 36: E825–E846Hsieh, CY, Adams, AH, Tobis, J et al.
Effectiveness of four conservative treatments for subacute low back pain: a randomized clinical trial.
Spine (Phila Pa 1976). 2002; 27: 1142–1148Licciardone, JC, Stoll, ST, Fulda, KG et al.
Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial.
Spine (Phila Pa 1976). 2003; 28: 1355–1362Rasmussen, J, Laetgaard, J, Lindecrona, AL, Qvistgaard, E, and Bliddal, H.
Manipulation does not add to the effect of extension exercises in chronic low-back pain (LBP).
A randomized, controlled, double blind study.
Joint Bone Spine. 2008; 75: 708–713Underwood M, UK BEAM Trial Team.
United Kingdom Back Pain Exercise and Manipulation (UK BEAM) Randomized Tial:
Effectiveness of Physical Treatments for Back Pain in Primary Care
British Medical Journal 2004 (Dec 11); 329 (7479): 1377–1384Goertz CM, Long CR, Hondras MA, Petri R, Delgado R, Lawrence DJ, et al.
Adding Chiropractic Manipulative Therapy to Standard Medical Care
for Patients with Acute Low Back Pain: Results of a Pragmatic
Randomized Comparative Effectiveness Study
Spine (Phila Pa 1976). 2013 (Apr 15); 38 (8): 627–634Bronfort G, Hondras MA, Schulz CA, Evans RL, Long CR et al (2014)
Spinal Manipulation and Home Exercise With Advice for Subacute and Chronic Back-related Leg Pain:
A Trial With Adaptive Allocation
Annals of Internal Medicine 2014 (Sep 16); 161 (6): 381—391Konstantinou, K, Hider, SL, Jordan, JL, Lewis, M, Dunn, KM, and Hay, EM.
The impact of low back-related leg pain on outcomes as compared with low back pain alone:
a systematic review of the literature.
Clin J Pain. 2013; 29: 644–654Hider, SL, Whitehurst, DG, Thomas, E, and Foster, NE.
Pain Location Matters: The Impact of Leg Pain on Health Care Use, Work Disability
and Quality of Life in Patients with Low Back Pain
European Spine Journal 2015 (Mar); 24 (3): 444–451Hincapié, CA, Tomlinson, GA, Côté, P, Rampersaud, Y, Jadad, AR, and Cassidy, JD.
Chiropractic Care and Risk for Acute Lumbar Disc Herniation:
A Population-based Self-controlled Case Series
European Spine Journal 2018 (Jul); 27 (7): 1526–1537Hincapié CA, Cassidy JD, Côté P, Rampersaud YR, Jadad AR et al (2017)
Chiropractic Spinal Manipulation and the Risk for Acute Lumbar Disc Herniation:
A Belief Elicitation Study
European Spine Journal 2018 (Jul); 27 (7): 1517–1525Licciardone, JC, Kearns, CM, and Minotti, DE.
Outcomes of osteopathic manual treatment for chronic low back pain according to baseline pain severity:
results from the OSTEOPATHIC Trial.
Man Ther. 2013; 18: 533–540Geneen, LJ, Moore, RA, Clarke, C, Martin, D, Colvin, LA, and Smith, BH.
Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews.
Cochrane Database Syst Rev. 2017; 4: CD011279Dowell D, Haegerich TM, Chou R.
CDC Guideline for Prescribing Opioids for Chronic Pain: United States, 2016
Morbidity and Mortality Weekly Report
Recommendations and Reports Vol. 65 No. 1 March 18, 2016Manchikanti, L, Kaye, AM, Knezevic, NN et al.
Responsible, safe, and effective prescription of opioids for chronic non-cancer pain:
American Society of Interventional Pain Physicians (ASIPP) Guidelines.
Pain Phys. 2017; 20: S3–S92Bussières, A, Gauthier, C, Fournier, G, and Descarreaux, M.
Spinal manipulative therapy for low back pain – time for an update. Tools for Practice. e-Letter.
Can Fam Physician. 2017; 63: 669–672Hebert JJ, Stomski NJ, French SD, et al.
Serious Adverse Events and Spinal Manipulative Therapy
of the Low Back Region: A Systematic Review of Cases
J Manipulative Physiol Ther 2015 (Nov); 38 (9): 677–691Deyo, RA.
The Role of Spinal Manipulation in the Treatment of Low Back Pain
JAMA. 2017 (Apr 11); 317 (14): 1418–1419Whelton, A and Hamilton, CW.
Nonsteroidal anti-inflammatory drugs: effects on kidney function.
J Clin Pharmacol. 1991; 31: 588–598Hörl, WH.
Nonsteroidal anti-inflammatory drugs and the kidney.
Pharmaceuticals (Basel). 2010; 3: 2291–2321Vonkeman, HE and van de Laar, MA.
Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention.
Semin Arthritis Rheum. 2010; 39: 294–312Deyo, RA, Hallvik, SE, Hildebran, C et al.
Association between initial opioid prescribing patterns and subsequent long-term use among
opioid-naïve patients: a statewide retrospective cohort study.
J Gen Intern Med. 2017; 32: 21–27Volkow, ND and McLellan, AT.
Opioid abuse in chronic pain–misconceptions and mitigation strategies.
N Engl J Med. 2016; 374: 1253–1263Busse, JW, Craigie, S, Juurlink, DN et al.
Guideline for Opioid Therapy and Chronic Noncancer Pain
CMAJ. 2017 (May 8); 189 (18): E659–E666National Institute of Drug Abuse:
Advancing Addiction Science. Opioid Overdose Crisis.
(Available at: https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis
Accessed February 14, 2018.)
Publisher Name: National Institute of Health (NIH). Bethesda, MD Date: 2017
Date accessed: May 22, 2017Canadian Centre on Substance Use and Addiction.
Joint Statement of Action to Address the Opioid Crisis.
(Available at: http://www.ccsa.ca/Resource%20Library/CCSA-Joint-Statement-of-Action
-Opioid-Crisis-Annual-Report-2017-en.pdf
Accessed February 14, 2018.) Publisher Name: A Collective Response.
Annual Report 2016-2017. Health Canada Date: 2016
Date accessed: May 22, 2017Nunn, ML, Hayden, JA, and Magee, K.
Current management practices for patients presenting with low back pain to a large emergency department in Canada.
BMC Musculoskelet Disord. 2017; 18: 92Lin C, Haas M, Maher C, et al.
Cost-Effectiveness of General Practice Care for Low Back Pain: A Systematic Review
European Spine Journal 2011 (Jul); 20 (7): 1012–1023Blanchette M, Stochkendahl M., Da Silva RB, et al.
Effectiveness and Economic Evaluation of Chiropractic Care for the Treatment of
Low Back Pain: A Systematic Review of Pragmatic Studies
PLoS One. 2016 (Aug 3); 11 (8): e0160037Baldwin ML, Cote P, Frank JW, et al.
Cost-effectiveness Studies of Medical and Chiropractic Care for
Occupational Low Back Pain. A Critical Review of the Literature
Spine J. 2001 (Mar); 1 (2): 138–147Brown, A, Angus, D, Chen, S et al.
Costs and Outcomes of Chiropractic Treatment for Low Back Pain
Technology report no 56. (July 2005)
Canadian Coordinating Office for Health Technology Assessment, Ottawa, Canada (CCOHTA)Furlan AD, Yazdi F, Tsertsvadze A, Gross A, Van Tulder M, Santaguida L, et. al.
A Systematic Review and Meta-analysis of Efficacy, Cost-effectiveness, and Safety
of Selected Complementary and Alternative Medicine for Neck and Low-back Pain
Evidence-Based Complementary and Alternative Medicine. 2012 (Nov 24); 2012: 953139Dagenais, S, Brady, O, Haldeman, S, and Manga, P.
A Systematic Review Comparing the Costs of Chiropractic Care to other Interventions
for Spine Pain in the United States
BMC Health Serv Res. 2015 (Oct 19); 15: 474Andronis L, Kinghorn P, Qiao S, Whitehurst DG, Durrell S, McLeod H.
Cost-Effectiveness of Non-Invasive and Non-Pharmacological Interventions for Low Back Pain:
A Systematic Literature Review
Applied Health Econ and Health Policy 2017 (Apr); 15 (2): 173–201Consumer, R.
Relief for Your Aching Back: What Worked for Our Readers.
(Available at:) (Accessed February 14, 2018)
http://www.consumerreports.org/cro/2013/01/relief-for-your-aching-back/index.htm
Date: 2013 Date accessed: April 26, 2017Gore, M, Tai, KS, Sadosky, A, Leslie, D, and Stacey, BR.
Use and costs of prescription medications and alternative treatments in patients with
osteoarthritis and chronic low back pain in community-based settings.
Pain Pract. 2012; 12: 550–560Mehl-Madrona, L, Mainguy, B, and Plummer, J.
Integration of complementary and alternative medicine therapies into primary-care pain management
for opiate reduction in a rural setting.
J Altern Complement Med. 2016; 22: 621–626Foster, NE, Hartvigsen, J, and Croft, PR.
Taking responsibility for the early assessment and treatment of patients with musculoskeletal pain:
a review and critical analysis.
Arthritis Res Ther. 2012; 14: 205World Health Organization (WHO)
WHO Guidelines on Basic Training and Safety in Chiropractic
Geneva, Switzerland: (November 2005)Hughes, CM, Quinn, F, and Baxter, GD.
Complementary and alternative medicine: perception and use by physiotherapists in the management of low back pain.
Complement Ther Med. 2015; 19: 149–154Busse, JW, Jim, J, Jacobs, C et al.
Attitudes towards chiropractic: an analysis of written comments from a survey of North American orthopaedic surgeons.
Chiropr Man Therap. 2011; 19: 25Weis, CA, Stuber, K, Barrett, J et al.
Attitudes toward chiropractic: a survey of Canadian obstetricians.
J Evid Based Compl Altern Med. 2015; 21: 92–104Wong, JJ, Di Loreto, L, Kara, A et al.
Assessing the change in attitudes, knowledge, and perspectives of medical students towards
chiropractic after an educational intervention.
J Chiropr Educ. 2014; 28: 112–122Bowden, BS and Ball, L.
Nurse practitioner and physician assistant students’ knowledge, attitudes, and perspectives of chiropractic.
J Chiropr Educ. 2016; 30: 114–120Slade, S, Kent, P, Patel, T, Bucknall, T, and Buchbinder, R.
Health care professional clinical practice guidelines adherence for low back pain:
a systematic review and meta-synthesis of qualitative studies.
in: Physiother. 101(Suppl. 1).
WCPT Congress, ; 2015: eS1238–eS1642Cochrane, LJ, Olson, CA, Murray, S, Dupuis, M, Tooman, T, and Hayes, S.
Gaps between knowing and doing: understanding and assessing the barriers to optimal health care.
J Contin Educ Heal Prof. 2007; 27: 94–102Bussieres AE, Al Zoubi F, Stuber K, French SD, Boruff J, Corrigan J, et al.
Evidence-based Practice, Research Utilization, and Knowledge Translation
in Chiropractic: A Scoping Review
BMC Complement Altern Med. 2016 (Jul 13); 16 (1): 216Straus, S, Tetroe, J, and Graham, I.
Knowledge Translation in Health Care: Moving from Evidence to Practice.
Wiley-Blackwell/BMJ Books, Oxford, UK; 2009: 318Scott, S, Albrecht, L, O’Leary, K et al.
Systematic review of knowledge translation strategies in the allied health professions.
Implement Sci. 2012; 7: 70Gagliardi, A, Marshall, C, Huckson, S, James, R, and Moore, V.
Developing a checklist for guideline implementation planning: review and synthesis of guideline
development and implementation advice.
Implement Sci. 2015; 10: 19Canadian Agency for Drugs and Technologies in Health (CADTH).
Rx for Change Database. (Available at:) (Accessed February 14, 2018.)
https://www.cadth.ca/rx-change
Date: 2011 Date accessed: July 27, 2017Flodgren, G, Hall, AM, Goulding, L et al.
Tools developed and disseminated by guideline producers to promote the uptake of their guidelines.
Cochrane Database Syst Rev. 2016; 8: CD010669Gagliardi, AR, Brouwers, MC, and Bhattacharyya, OK.
A framework of the desirable features of guideline implementation tools (GItools):
Delphi survey and assessment of GItools.
Implement Sci. 2014; 9: 98Okelo, SO, Butz, AM, Sharma, R et al.
Interventions to modify health care provider adherence to asthma guidelines: a systematic review.
Pediatrics. 2013; 132: 517–534Murthy, L, Shepperd, S, Clarke, MJ et al.
Interventions to improve the use of systematic reviews in decision-making by health system managers,
policy makers and clinicians.
Cochrane Database Syst Rev. 2012; 9: CD009401Garg, AX, Adhikari, NK, McDonald, H et al.
Effects of computerized clinical decision support systems on practitioner performance and patient outcomes:
A systematic review.
JAMA. 2005; 293: 1223–1238Bussières, A, Grondin, D, Maiers, M, and Brockhusen, S.
Identifying and training opinion leaders in chiropractic.
J Can Chiropr Assoc. 2017; 61: 1Loudon, K, Santesso, N, Callaghan, M et al.
Patient and public attitudes to and awareness of clinical practice guidelines:
a systematic review with thematic and narrative syntheses.
BMC Health Serv Res. 2014; 14: 321Fearns, N, Kelly, J, Callaghan, M et al.
What do patients and the public know about clinical practice guidelines and what do they want from them?
A qualitative study.
BMC Health Serv Res. 2016; 16: 1–13Slade SC, Patel S, Underwood M, et al.
What Are Patient Beliefs and Perceptions About Exercise for Nonspecific Chronic Low Back Pain?
A Systematic Review of Qualitative Studies
Clin J Pain. 2014 (Nov); 30 (11): 995–1005Steffens D, Maher CG, Pereira LS, et al.
Prevention of Low Back Pain: A Systematic Review and Meta-analysis
JAMA Intern Med. 2016 (Feb); 176 (2): 199–208Shiri, R, Coggon, D, and Falah-Hassani, K.
Exercise for the prevention of low back pain: systematic review and meta-analysis of controlled trials
[e-pub ahead of print].
(Available at: https://doi.org/10.1093/aje/kwx337
Accessed February 14, 2018.)
Am J Epidemiol. 2017;Groeneweg, R, Rubinstein, SM, Oostendorp, RA, Ostelo, RW, and van Tulder, MW.
Guideline for reporting interventions on spinal manipulative therapy: Consensus on interventions
reporting criteria list for spinal manipulative therapy (CIRCLeSMT).
J Manip Physiol Ther. 2017; 40: 61–70R.A. Deyo, S.F. Dworkin, D. Amtmann, G. Andersson, et al.,
Report of the NIH Task Force on Research Standards for Chronic Low Back Pain
Journal of Pain 2014 (Jun); 15 (6): 569–585Moher, D, Pham, B, Lawson, ML, and Klassen, TP.
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
Health Technol Assess. 2003; 7: 1–90Morrison, A, Polisena, J, Husereau, D et al.
The effect of English-language restriction on systematic review-based meta-analyses:
a systematic review of empirical studies.
Int J Technol Assess Health Care. 2012; 28: 138–144Cherkin, DC, Deyo, RA, Battie, M, Street, J, and Barlow, W.
A Comparison of Physical Therapy, Chiropractic Manipulation, and Provision of an Educational Booklet
for the Treatment of Patients with Low Back Pain
New England Journal of Medicine 1998 (Oct 8); 339 (15): 1021-1029Bergquist-Ullman, M and Larsson, U.
Acute low back pain in industry. A controlled prospective study with special reference to therapy
and confounding factors.
Acta Orthop Scand. 1977; 170: 1–117Farrell, JP and Twomey, LT.
Acute low back pain. Comparison of two conservative treatment approaches.
Med J Aust. 1982; 1: 160–164Skargren, EI, Oberg, BE, Carlsson, PG, and Gade, M.
Cost and effectiveness analysis of chiropractic and physiotherapy treatment for low back and neck pain
Six-month follow-up.
Spine (Phila Pa 1976). 1997; 22: 2167–2177Bronfort G, Goldsmith CH, Nelson CF, Boline PD, Anderson AV.
Trunk Exercise Combined with Spinal Manipulative or NSAID Therapy
for Chronic Low Back Pain: A Randomized, Observer-blinded Clinical Trial
J Manipulative Physiol Ther. 1996 (Nov); 19 (9): 570–582Hemmilä, HM, Keinänen-Kiukaanniemi, SM, Levoska, S, and Puska, P.
Long-term effectiveness of bone-setting, light exercise therapy, and physiotherapy for prolonged back pain:
a randomized controlled trial.
J Manipulative Physiol Ther. 1996 (Nov); 19 (9): 570–582Hondras MA, Long CR, Cao Y, et al.
A Randomized Controlled Trial Comparing 2 Types of Spinal Manipulation
and Minimal Conservative Medical Care for Adults 55 Years and Older
With Subacute or Chronic Low Back Pain
J Manipulative Physiol Ther. 2009 (Jun); 32 (5): 330–343Hurwitz EL, Morgenstern H, Harber P, Kominski GF, Belin TR, Yu F, Adams AH
A Randomized Trial of Medical Care with and without Physical Therapy
and Chiropractic Care with and without Physical Modalities for
Patients with Low Back Pain: 6-month Follow-up Outcomes
From the UCLA Low Back Pain Study
Spine (Phila Pa 1976) 2002 (Oct 15); 27 (20): 2193–2204Skillgate, E, Vingård, E, and Alfredsson, L.
Naprapathic manual therapy or evidence-based care for back and neck pain: a randomized, controlled trial.
Clin J Pain. 2007; 23: 431–439Petersen T, Larsen K, Nordsteen J, Olsen S, Fournier G, Jacobsen S.
The McKenzie Method Compared with Manipulation When Used
Adjunctive to Information and Advice in Low Back Pain Patients
Presenting with Centralization or Peripheralization:
A Randomized Controlled Trial
Spine (Phila Pa 1976) 2011 (Nov 15); 36 (24): 1999-2010Xia, T, Long, CR, Gudavalli, MR et al.
Similar effects of thrust and nonthrust spinal manipulation found in adults with subacute and chronic
low back pain: a controlled trial with adaptive allocation.
Spine (Phila Pa 1976). 2016; 41: E702–E709Hoiriis KT, Pfleger B, McDuffie FC, Cotsonis G, Elsangak O, Hinson R, et al.
A Randomized Clinical Trial Comparing Chiropractic Adjustments to Muscle Relaxants
for Subacute Low Back Pain
J Manipulative Physiol Ther 2004 (Jul); 27 (6): 388-398Cramer, GD, Humphreys, CR, Hondras, MA, McGregor, M, and Triano, JJ.
The Hmax/Mmax ratio as an outcome measure for acute low back pain.
J Manip Physiol Ther. 1993; 16: 7–13Jüni, P, Battaglia, M, Nüesch, E et al.
A randomised controlled trial of spinal manipulative therapy in acute low back pain.
Ann Rheum Dis. 2009; 68: 1420–1427Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al.
A Clinical Prediction Rule To Identify Patients With Low Back Pain Most Likely To Benefit
from Spinal Manipulation: A Validation Study
Annals of Internal Medicine 2004 (Dec 21); 141 (12): 920–928MacDonald, RS and Bell, CM.
An open controlled assessment of osteopathic manipulation in nonspecific low-back pain.
Spine (Phila Pa 1976). 1990; 15: 364–370Ghroubi, S, Elleuch, H, Baklouti, S, and Elleuch, MH.
Chronic low back pain and vertebral manipulation.
Ann Readapt Med Phys. 2007; 50: 570–576Waagen, G, Haldeman, S, Cook, G, Lopez, D, and DeBoe, K.
Short term trial of chiropractic adjustments for the relief of chronic low back pain.
Manual Med. 1986; 2: 63–67Triano JJ, McGregor M, Hondras MA, Brennan PC.
Manipulative Therapy Versus Education Programs in Chronic Low Back Pain
Spine (Phila Pa 1976). 1995 (Apr 15); 20 (8): 948–955
Return to LOW BACK PAIN
Return to LOW BACK GUIDELINES
Return NON-PHARMACOLOGIC THERAPY
Since 4-11-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |