Food Groups and Risk of All-cause Mortality:
A Systematic Review and Meta-Analysis
of Prospective StudiesThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Am J Clin Nutr. 2017 (Jun); 105 (6): 1462-1473 ~ FULL TEXT
Lukas Schwingshackl, Carolina Schwedhelm, Georg Hoffmann, Anna-Maria Lampousi,
Sven Knüppel, Khalid Iqbal, Angela Bechthold, Sabrina Schlesinger,
and Heiner Boeing
German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE),
Nuthetal, Germany;
lukas.schwingshackl@dife.deBackground: Suboptimal diet is one of the most important factors in preventing early death and disability worldwide.
Objective: The aim of this meta-analysis was to synthesize the knowledge about the relation between intake of 12 major food groups, including whole grains, refined grains, vegetables, fruits, nuts, legumes, eggs, dairy, fish, red meat, processed meat, and sugar-sweetened beverages, with risk of all-cause mortality.
Design: We conducted a systematic search in PubMed, Embase, and Google Scholar for prospective studies investigating the association between these 12 food groups and risk of all-cause mortality. Summary relative risks (RRs) and 95% CIs were estimated with the use of a random effects model for high-intake compared with low-intake categories, as well as for linear and nonlinear relations. Moreover, the risk reduction potential of foods was calculated by multiplying the RR by optimal intake values (serving category with the strongest association) for risk-reducing foods or risk-increasing foods, respectively.
Results: With increasing intake (for each daily serving) ofwhole grains (RR: 0.92; 95% CI: 0.89, 0.95),
vegetables (RR: 0.96; 95% CI: 0.95, 0.98),
fruits (RR: 0.94; 95% CI: 0.92, 0.97),
nuts (RR: 0.76; 95% CI: 0.69, 0.84), and
fish (RR: 0.93; 95% CI: 0.88, 0.98),the risk of all-cause mortality decreased; higher intake of
red meat (RR: 1.10; 95% CI: 1.04, 1.18) and
processed meat (RR: 1.23; 95% CI: 1.12, 1.36)was associated with an increased risk of all-cause mortality in a linear dose-response meta-analysis. A clear indication of nonlinearity was seen for the relations between vegetables, fruits, nuts, and dairy and all-cause mortality. Optimal consumption of risk-decreasing foods results in a 56% reduction of all-cause mortality, whereas consumption of risk-increasing foods is associated with a 2-fold increased risk of all-cause mortality.
Conclusion: Selecting specific optimal intakes of the investigated food groups can lead to a considerable change in the risk of premature death.
KEYWORDS: diet; dose response; food groups; meta-analysis; mortality
From the FULL TEXT Article:
INTRODUCTION
In 2013, the number of deaths worldwide and among all age groups amounted to nearly 55 million; 70% of these were caused by noncommunicable diseases. [1] One-third of these fatalities were caused by cardiovascular disease (CVD), followed by cancer at 15%. [1] A high-quality diet comprising abundant amounts of whole grains, fruits, vegetables, nuts, and fish is one of the most important factors in preventing early death and disability worldwide. [2]
During the past 50 y, lifestyle factors have been identified as modifiable factors associated with death. Thus, despite often unclear direct biological mechanisms due to the many potential underlying disease mechanisms, epidemiologic risk factors that can change the probability of death are important public health indicators. Studies that were able to translate risk reduction into measures of life expectancy calculated that populations with a low-risk profile (no smoking, physically active, healthy dietary pattern) differ in 10–15 y from those with a high-risk profile. [3]
Previous meta-analyses showed that whole grains, fruits and vegetables, nuts, and fish were associated with a lower risk of all-cause mortality [4-7], whereas red and processed meats were associated with an increased risk. [8] In addition, a recent meta-analysis showed that high adherence to diet quality indexes such as the Healthy Eating Index and Dietary Approaches to Stop Hypertension were associated with a 22% decrease in the risk of all-cause mortality. [9]
When investigating diet-disease relations in terms of their meaning for public health, the best approach is the study of foods or food groups instead of nutrients. Moreover, foods are directly linked to food-based dietary guidelines, which are based on the preventive actions of foods and should be released for each country depending on dietary practices and intake amounts. [10] Evidence for food consumption–death risk relations from systematic reviews is a key component of this process.
Up-to-date evidence about the association of all-cause mortality risk with consumption of legumes, eggs, and sugar-sweetened beverages (SSBs) has not been synthesized. Moreover, meta-analyses rarely assess the quality of evidence. Because meta-analyses are a crucial building block in generating guidelines and recommendations, it is of the utmost importance to implement a method to evaluate the quality of evidence.
Thus, we investigated the associations of 12 food groups defined a priori, including whole grains, refined grains, vegetables, fruits, nuts, legumes, eggs, dairy, fish, red meat, processed meat, and SSBs, with risk of all-cause mortality by evaluating all available data from prospective studies. Special attention was given to the strength and shape of the dose-response relationship for finding an optimal intake for lowest all-cause mortality risk. Finally, using the NutriGrade scoring system, we aimed to determine the quality of meta-evidence of the protective or detrimental effects of the food groups on all-cause mortality risk.
METHODS
The review was registered in PROSPERO International Prospective Register of Systematic Reviews
(www.crd.york.ac.uk/PROSPERO/; identifier CRD42016037069).
This systematic review was planned and conducted according to the standards of the Meta-analysis of Observational Studies in Epidemiology. [11]
Search strategy
The literature published through December 2016 was searched using the electronic databases PubMed, Embase, and Google Scholar, with no restrictions on language or calendar date. The search terms used are listed in Supplemental Material 1.
Moreover, the reference lists from the retrieved articles, systematic reviews, and meta-analyses were checked for further relevant studies. The literature search was conducted by 2 authors (LS and A-ML); disagreements were resolved by consensus after discussion with another reviewer (HB).
Study selection
Studies were included in the meta-analysis if they 1) were cohort studies, case-cohort studies, follow-ups of randomized controlled trials, and case-control studies nested in a prospective study that were peer-reviewed and for which the full text was available; 2) provided information about the association for ≥1 of the following 12 food groups: whole grains and cereals, refined grains and cereals, vegetables, fruits, nuts, legumes, eggs, dairy products, fish, red meat, processed meat, and SSBs [these 12 food groups are the focus because most diet quality indexes or scores are based on them [9, 12, 13], as previously reported [14]]; 3) included participants aged ≥18 y; and 4) considered all-cause mortality as an outcome. We excluded studies including populations suffering from chronic disease and studies reporting only cause-specific mortality.
Data extraction
After selecting studies, 2 reviewers (LS and A-ML) extracted the following information: name of first author, year of publication, study origin, cohort name, sample size, number of subjects, age at entry, sex, study duration (follow-up in years), outcome, outcome assessment, assessment of food group, quantity of food, risk estimate [most adjusted measures; HRs, RRs, or ORs with their corresponding 95% CIs], and adjustment factors.
When a study provided several risk estimates, the multivariable adjusted model was chosen. When only separate risk estimates for male and female participants were available in a study, we combined the RRs using a fixed effects model before inclusion in the meta-analysis.
Risk of bias assessment
To determine the risk of bias of the prospective studies, we assessed how the studies ascertained exposure, how they assessed outcomes, whether the follow-up was adequate (≥10 y), and whether they adjusted the basic model and made any outcome-relevant adjustments (age, sex, education, BMI, smoking, physical activity, or energy intake). [15] Studies were classified as being at low risk of bias in general only if none of the domains established a high or unclear risk of bias.
Statistical analysis
A random effects model was used to calculate summary RRs and 95% CIs for the associations between all-cause mortality and the highest compared with the lowest intake category for each of the 12 food groups (whole grains and cereals, refined grains and cereals, vegetables, fruits, nuts, legumes, eggs, dairy products, fish, red meat, processed meat, and SSBs) and for the dose-response analysis [16], which incorporated both within-study and between-study variabilities. To evaluate the weighting of each study, the SE for the logarithm RR for each study was calculated and regarded as the estimated variance of the logarithm RR, through the use of an inverse variance method. [16]
A method described by Greenland and Longnecker [17] and Orsini et al. [18] was applied for the dose-response analysis; this computed study-specific slopes (linear trends) and 95% CIs from the natural logs of the RRs and CIs across intake categories for the 12 food groups. The method requires knowing the distribution of cases and person-years or noncases and the RRs with the 95% CIs for ≥3 quantitative exposure categories.
When studies reported only the total number of cases or total person-years and the exposure was defined in quantiles, the distribution of cases or person-years was calculated by dividing the total number by the number of quantiles. Whenever reported, the mean or median intake by category was assigned to the corresponding RR. The midpoint was calculated for studies that reported only a range of intake by category. When the intake values were open-ended, we assumed that their range was the same as that of the adjacent category. For studies presenting the exposure per given unit of energy intake, we rescaled the exposure using the mean energy intake provided.
The dose response was expressed in the following servings: 30 g whole grains or cereals/d, 30 g refined grains or cereals/d, 100 g vegetables/d, 100 g fruits/d, 28 g nuts/d, 50 g legumes/d, 50 g eggs/d, 200 g dairy products/d, 100 g fish/d, 100 g red meat/d, 50 g processed meat/d, and 250 mL SSBs/d. For studies that reported intake only as serving size (and did not specify the quantitative amount), we used recommended conversions (Supplemental Table 1).
To examine possible nonlinear associations, we calculated restricted cubic splines for each study with ≥3 categories of exposure, using 3 fixed knots at 10%, 50%, and 90% through the total distribution of the reported intake, and combined them using multivariate meta-analysis. [19]
Moreover, the risk reduction potential of foods was calculated by multiplying the RR by optimal intake values (serving category with the strongest association) of risk-decreasing foods, and risk-increasing foods
, respectively. The optimal intake value was defined as the number of servings of a single food group with the strongest association for all-cause mortality.
To explore heterogeneity between studies, we used the Cochran Q test and the I2 statistic [with I2 >50% considered to represent potentially important statistical heterogeneity [20]]. In addition, to identify potential sources of heterogeneity, we stratified the meta-analysis by subgroups: sex, duration of follow-up (mean or median ≥10 compared with <10 y), geographic location (Europe, America, Asia, or Australia), number of cases (≥1000 compared with <1000), and dietary assessment (validated compared with nonvalidated). Furthermore, we analyzed sensitivity for studies with a low risk of bias.
Potential small-study effects such as publication bias were explored using the Egger test and funnel plots [21] if ≥10 studies were available, as recommended by the Cochrane Handbook. [22]
Review Manager 5.3 (Nordic Cochrane Center), and Stata software version 14 (StataCorp) were used for the statistical analyses.
Quality of meta-evidence
To evaluate the meta-evidence for the association between 12 predefined food groups and all-cause mortality (quality of evidence of meta-analyses was defined as the confidence in the estimate), we applied the NutriGrade scoring system (a maximum of 10 points), which comprises the following items: 1) risk of bias, study quality, and study limitations; 2) precision; 3) heterogeneity; 4) directness; 5) publication bias; 6) funding bias; 7) study design (only for meta-analyses of randomized controlled trials); and 8) effect size. [15] Based on this scoring system, we recommend 4 categories to judge the meta-evidence: high (≥8 points), moderate (6 to <8 points), low (4 to <6 points), and very low (0 to <4 points).
RESULTS
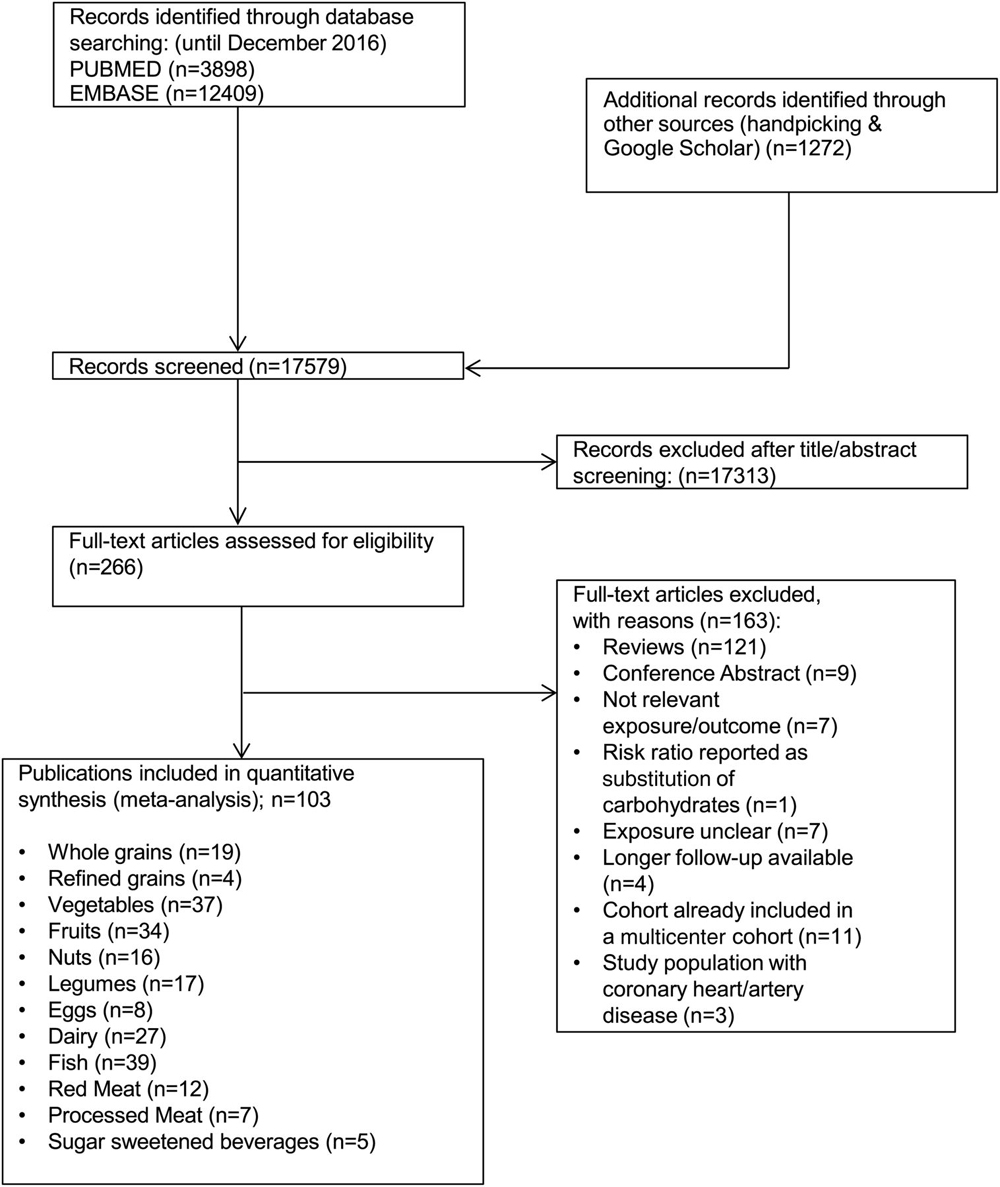
Of the 17,579 records that were identified by the literature search, 266 full-text articles were assessed in detail because they reported ≥1 of the 12 food groups and all-cause mortality in the title or abstract (Figure 1).
Included in the meta-analysis were19 prospective studies (18 reports) of consumption of whole grains [23-40]
(Supplemental Table 2),
4 studies of refined grains [27, 30, 33, 36]
(Supplemental Table 3),
37 studies of vegetables (36 reports) [23–25, 29, 31, 34–36, 38, 39, 41–66]
(Supplemental Table 4),
34 studies of fruits (33 reports) [23–25, 29, 31, 34–36, 38, 39, 41, 42, 45, 46, 48, 49, 51–67]
(Supplemental Table 5),
16 for nuts (14 reports) [36, 42, 68–79]
(Supplemental Table 6),
17 studies of legumes (16 reports) [34, 36, 42, 47, 50, 51, 56, 57, 63, 65, 78, 80–84]
(Supplemental Table 7),
8 studies of egg consumption [36, 56, 77, 78, 80, 85–87]
(Supplemental Table 8),
27 studies of dairy products (25 reports) [23, 24, 35, 44, 46–48, 50, 65, 77, 78, 80, 82, 84, 88–98]
(Supplemental Table 9),
39 studies of fish (37 reports) [31, 34, 35, 38, 39, 42, 46, 50, 53, 56, 59, 61, 63–65, 77, 80, 81, 99–117]
(Supplemental Table 10),
12 studies of red meat (10 reports) [42, 61, 105, 106, 112, 118–122]
(Supplemental Table 11),
7 studies of processed meat (6 reports) [61, 105, 119–122]
(Supplemental Table 12), and
5 studies of SSBs [78, 95, 123–125]
(Supplemental Table 13).
Whole grains
Nineteen studies with 121,141 mortality cases were included in the high- compared with low-intake meta-analysis (overall intake range: 0–110 g/d). When we compared extreme categories, an inverse association between all-cause mortality and whole-grain intake was observed, with an indication for high heterogeneity between studies (RR: 0.88; 95% CI: 0.84, 0.92; I2 = 91%; P-heterogeneity < 0.001) (Supplemental Figure 1). Each additional daily 30 g of whole grains was inversely associated with mortality risk (RR: 0.92; 95% CI: 0.89, 0.95; I2 = 80%; P-heterogeneity < 0.001; n = 11) (Supplemental Figure 2). The heterogeneity persisted in additional analyses stratified by sex, follow-up duration, geographic location, number of cases, and dietary assessment (Supplemental Table 14). The inverse association was not observed in European, Asian, Australian, or short-term studies. These subgroup differences were not statistically significant. Some evidence of heterogeneity between studies applying validated compared with nonvalidated dietary assessment methods was observed. An inverse association was observed only for studies applying validated dietary assessment methods.
There was significant evidence for small study effects in the high-intake compared with the low-intake meta-analysis (P = 0.02) but not in the dose-response meta-analysis (P = 0.64). Visual inspection of the funnel plot (dose-response analysis) indicated moderate symmetry (Supplemental Figure 3). Although the test for nonlinearity was significant (P-nonlinearity < 0.01; n = 10 studies), a clear dose-response relation was observed. The risk of mortality decreased by 25% with increasing intake of whole grains up to ~ 100 g/d (Figure 2).
Refined grains
Four studies with 11,034 mortality cases were included in the high-intake compared with low-intake meta-analysis (overall intake range: 0–183 g/d). No association was observed for the highest compared with the lowest refined grain intake category (RR: 0.99; 95% CI: 0.94, 1.05; I2 = 26%; P-heterogeneity = 0.26) (Supplemental Figure 4), or for each additional daily 30 g (RR: 0.99; 95% CI: 0.97, 1.01; I2 = 7%; P-heterogeneity = 0.36; n = 4) (Supplemental Figure 5). No significant association was observed in any of the stratified analyses (Supplemental Table 15). Furthermore, no evidence of a nonlinear dose-response association was found (P-nonlinearity = 0.11; n = 3 studies) (Figure 2).
Vegetables
Thirty-seven studies with 121,067 mortality cases were included in the high-intake compared with the low-intake meta-analysis (overall intake range: 5–663 g/d). An inverse association was observed for the high intake compared with the low intake (RR: 0.93; 95% CI: 0.90, 0.95; I2 = 75%; P-heterogeneity < 0.001) (Supplemental Figure 6) and dose-response analyses (RR/100 g: 0.96; 95% CI: 0.95, 0.98; I2 = 67%; P-heterogeneity < 0.001; n = 17) (Supplemental Figure 7).
The observed heterogeneity persisted largely in additional analyses stratified by sex, follow-up duration, geographic location, and number of cases. The inverse association was not observed in studies conducted in the United States or in studies including only women (Supplemental Table 16). No evidence of heterogeneity was detected between subgroups in stratified analyses.
Significant evidence for small study effects was found in both the high- compared with the low-intake (P < 0.01) and the dose-response analyses (P = 0.096). Visual inspection of the funnel plots (dose-response analysis) suggests little symmetry (Supplemental Figure 8). Evidence exists of a nonlinear dose-response association (P-nonlinearity < 0.001; n = 14 studies). The risk of mortality decreased by 11% with increasing intake of vegetables up to ~ 300 g/d. No benefit was apparent when increasing intake above this value (Figure 2).
Fruit
Thirty-four studies with 120,033 mortality cases were included in the high- compared with the low-intake meta-analysis (overall intake range: 0–626 g/d). An inverse association was observed (RR: 0.91; 95% CI: 0.89, 0.94; I2 = 77%; P-heterogeneity < 0.001) (Supplemental Figure 9). Each additional daily 100 g of fruits was inversely associated with mortality risk (RR: 0.94; 95% CI: 0.92, 0.97; I2 = 82%; P-heterogeneity < 0.001; n = 17) (Supplemental Figure 10).
The heterogeneity persisted largely in additional analyses stratified by sex, follow-up duration, geographic location, number of cases, and dietary assessment (Supplemental Table 17). The inverse association was not observed in studies from the United States or studies including only women. However, no evidence of heterogeneity was detected between subgroups in stratified analyses.
Significant evidence was found for small study effects in the high- compared with the low-intake (P < 0.001) and dose-response analyses (P < 0.001). Visual inspection of the funnel plots (dose-response analysis) suggests little symmetry (Supplemental Figure 11). Evidence exists of a nonlinear dose-response association (P-nonlinearity < 0.001; n = 13 studies). The risk of all-cause mortality decreased by ~ 10%, with increasing intake of fruit up to ~ 250–300 g/d. No benefit was apparent when increasing intake above this value (Figure 2).
Nuts
Sixteen studies with 80,204 mortality cases were included in the high- compared with the low-intake meta-analysis (overall intake range: 0–52 g/d). A strong inverse association was observed for the highest compared with the lowest nut intake category (RR: 0.80; 95% CI: 0.74, 0.86; I2 = 84%; P-heterogeneity < 0.001) (Supplemental Figure 12), and for each additional daily 28 g (RR: 0.76; 95% CI: 0.69, 0.84; I2 = 82%; P-heterogeneity < 0.001; n = 16) (Supplemental Figure 13).
The strong inverse association and heterogeneity observed persisted in additional stratified analyses (Supplemental Table 18). Evidence was seen of heterogeneity between subgroups stratified by follow-up duration and geographic location, showing stronger inverse associations in European and Asian studies, and in studies with a shorter-term follow-up. Significant evidence for small study effects was detected in the high- compared with the low-intake (P < 0.001) and dose-response analyses (P < 0.01). Visual inspection of the funnel plots suggests little symmetry (Supplemental Figure 14). Evidence exists of a nonlinear dose-response association (P-nonlinearity < 0.001; n = 13 studies). The risk of all-cause mortality decreased by ~ 17% with increasing intake of nuts up to ~ 15–20 g/d. No benefit was apparent when increasing intake above this value (Figure 2).
Legumes
Seventeen studies with 53,085 mortality cases were included in the high- compared with low-intake meta-analysis (overall intake range: 6–166 g/d). An inverse association was observed for the highest compared with lowest legume intake categories (RR: 0.96; 95% CI: 0.93, 1.00; I2 = 48%; P-heterogeneity = 0.01) (Supplemental Figure 15), but not for each additional daily 50 g (RR: 0.96; 95% CI: 0.90, 1.01; I2 = 48%; P-heterogeneity = 0.09; n = 6) (Supplemental Figure 16).
We observed an inverse association for studies conducted in Asia and Australia, and studies with long-term follow-up (Supplemental Table 19). We found evidence of heterogeneity between subgroups stratified by geographic location. No evidence for small study effects was detected in the high- compared with low-intake analysis (P = 0.32). No evidence of a nonlinear dose-response association was observed (P-nonlinearity = 0.39; n = 4 studies). The risk of all-cause mortality decreased by ~ 16% with increasing intake of legumes up to ~ 150 g/d (Figure 2).
Eggs
Eight studies with 30,352 mortality cases were included in the analysis of the highest compared with the lowest intake category (overall intake range: 4–68 g/d). A positive association was observed for the highest compared with the lowest egg intake category (RR: 1.06; 95% CI: 1.00, 1.12; I2 = 71%; P-heterogeneity < 0.001) (Supplemental Figure 17), and for each additional daily 50 g (RR: 1.15; 95% CI: 0.99, 1.34; I2 = 87%; P-heterogeneity < 0.001; n = 5) (Supplemental Figure 18).
The observed heterogeneity persisted in additional analyses stratified by sex, follow-up duration, geographic location, and number of cases. We observed a positive association for studies of men, short-term studies, and studies applying a validated dietary assessment (Supplemental Table 20). Evidence of heterogeneity between subgroups was detected for follow-up duration and dietary assessment. Moreover, significant positive associations were observed for studies with ≥1000 mortality cases. We found evidence of a nonlinear dose-response association (P-nonlinearity < 0.001; n = 3 studies). The risk of all-cause mortality increased by ~ 10% with increasing intake of eggs up to ~ 60 g/d (Figure 2).
Dairy
Twenty-seven studies with 126,759 mortality cases were included in the meta-analysis of the highest compared with the lowest intake category (overall intake range: 0–1041 g/d). No association was observed for the high compared with the low intake (RR: 1.03; 95% CI: 0.98, 1.07; I2 = 94%; P-heterogeneity < 0.001) (Supplemental Figure 19), or for each additional daily 200 g of dairy products (RR: 0.98; 95% CI: 0.93, 1.03; I2 = 96%; P-heterogeneity < 0.001; n = 16) (Supplemental Figure 20).
The observed heterogeneity persisted in additional analyses stratified by sex, duration of follow-up, geographic location, and number of cases. In subgroup analyses no significant difference comparing low-fat and high-fat dairy products was observed (Supplemental Table 21). Some evidence of heterogeneity between subgroups in stratified analyses was observed (number of cases).
We found evidence for small study effects in the dose-response meta-analysis (P = 0.09), but not in the high- compared with the low-intake analysis (P = 0.59). Visual inspection of the funnel plots (dose-response analysis) suggests moderate symmetry (Supplemental Figure 21). Evidence of a nonlinear dose-response association was seen between dairy products and all-cause mortality (P-nonlinearity = 0.01; n = 12 studies). No detrimental effects were observed up to an intake of ~ 750 g/d, whereas intakes of ≤1000 g/d were associated with a 15% increased risk of mortality (Figure 2).
Fish
Thirty-nine studies with 157,688 mortality cases were included in the meta-analysis of the highest compared with the lowest intake category (overall intake range: 0–225 g/d). An inverse association was observed for the highest compared with the lowest fish intake category (RR: 0.95; 95% CI: 0.92, 0.98; I2 = 51%; P-heterogeneity < 0.001) (Supplemental Figure 22), and for each additional daily 100 g (RR: 0.93; 95% CI: 0.88, 0.98; I2 = 53%; P-heterogeneity < 0.01; n = 19) (Supplemental Figure 23).
We observed statistically significant heterogeneity in subgroups stratified for geographic location but no significant associations for studies conducted in Europe (Supplemental Table 22). No evidence for small study effects was observed (P = 0.30), and visual inspection of the funnel plots suggests symmetry (Supplemental Figure 24). We found no evidence of a nonlinear dose-response association (P-nonlinearity = 0.09; n = 19 studies). The risk decreased by 10% with increasing intake of ≤200 g fish/d (Figure 2).
Red meat
Twelve studies with 177,655 mortality cases were included in the high- compared with low-intake meta-analysis (overall intake range: 0–200 g/d). A positive association was observed (RR: 1.10; 95% CI: 1.00, 1.22; I2 = 93%; P-heterogeneity < 0.001) (Supplemental Figure 25). Each additional daily 100 g of red meat was positively associated with risk of all-cause mortality (RR: 1.10; 95% CI: 1.04, 1.18; I2 = 92%; P-heterogeneity < 0.001; n = 10) (Supplemental Figure 26).
The observed positive associations and heterogeneity persisted in additional analyses stratified by sex, follow-up duration, geographic location, and number of cases. We observed a positive association for studies of men, those conducted in the United States, long-term studies, and studies including ≥1000 mortality cases (Supplemental Table 23). Evidence of heterogeneity between subgroups in stratified analyses was detected for geographic location and number of cases.
We found evidence of small study effects in the dose-response analysis (P = 0.051) and the analysis of high compared with low intake (P = 0.08). Visual inspections of the funnel plots suggest low symmetry (Supplemental Figure 27). No evidence of a nonlinear dose-response association was found (P-nonlinearity = 0.30; n = 10 studies) (Figure 2).
Processed meat
Seven studies with 143,572 mortality cases were included in the high- compared with the low-intake meta-analysis (overall intake range: 0–200 g/d). A positive association was observed (RR: 1.21; 95% CI: 1.16, 1.26; I2 = 56%; P-heterogeneity = 0.03) (Supplemental Figure 28). Each additional daily 50 g of processed meat was associated with a risk of all-cause mortality (RR: 1.23; 95% CI: 1.12, 1.36; I2 = 94%; P-heterogeneity < 0.001; n = 7) (Supplemental Figure 29). The observed positive associations and heterogeneity persisted in additional analyses stratified by sex, follow-up duration, geographic location, and number of cases (Supplemental Table 24). We detected evidence of heterogeneity in stratified analyses for geographic location, showing stronger associations in studies from the United States compared with Europe.
Although the test for nonlinearity was significant (P-nonlinearity = 0.02; n = 7 studies), a clear dose-response relation was observed. The risk of all-cause mortality increased by ~ 60% with increasing intake of processed meat up to ~ 200 g/d (Figure 2).
Sugar-sweetened beverages
Five studies with 81,407 mortality cases were included in the high- compared with the low-intake meta-analysis (overall intake range: 0–930 mL/d). No association between all-cause mortality and SSBs was observed in the analysis of high compared with low intake (RR: 1.02; 95% CI: 0.97, 1.06; I2 = 78%; P-heterogeneity < 0.01) (Supplemental Figure 30) or the linear dose-response meta-analysis (RR: 1.03; 95% CI: 0.91, 1.18; I2 = 71%; P-heterogeneity = 0.02; n = 4) (Supplemental Figure 31). We observed a positive association in studies conducted in America (Supplemental Table 25). Some evidence of heterogeneity between subgroups in stratified analyses (follow-up duration and geographic location) was observed, indicating positive associations only for studies conducted in the United States and those with a shorter follow-up. We found no evidence of a nonlinear dose-response association (P-nonlinearity = 0.66; n = 3 studies). The risk of all-cause mortality increased by ~ 7% with increasing intake of SSBs up to ~ 250 mL/d (Figure 2).
Summary across food groups

Table 1 Table 1 shows the RR for all-cause mortality from nonlinear dose-response analysis of the 12 food groups according to servings per day. Optimal consumption (the smallest serving with significant results and no further substantial change in risk or no further data for larger amounts) of risk-decreasing foods [3 servings whole grains/d (RR = 0.79), 3 servings vegetables/d (RR = 0.89), 3 servings fruit/d (RR = 0.90), 1 serving nuts/d (RR = 0.85), 1 serving legumes/d (RR = 0.90), and 2 servings fish/d (RR = 0.90)] results in a 56% reduction (calculated by Graphic) compared with no consumption of these foods. The highest reduction in risk for all-cause mortality in terms of servings could be observed for whole grains: 90 g/d (3 servings/d) was associated with a 21% reduction in risk compared with no consumption of this food group. An ~ 15% risk reduction was observed for 1 serving nuts/d. Furthermore, Table 1 shows that increasing the daily consumption of food with an inverse relation to risk of all-cause mortality beyond 3 servings/d of vegetables and of fruits (~ 250 g/d for both), and one-half serving nuts/d (~ 15 g/d) will not further reduce risk (Figure 2). We could also calculate that a consumption of risk-increasing food—2 servings red meat/d (170 g; RR = 1.35), 4 servings processed meat/d (120 g; RR = 1.35), 1 serving eggs/d (55 g; RR = 1.07), and 1 serving SSBs/d (250 mL; RR = 1.07)—is associated with a 2-fold increased risk (Graphic) compared with no consumption. Not consuming these foods would reduce the risk of all-cause mortality by about 52% [calculated by Graphic]. Because lower intakes of dairy consumption showed a reduced risk of mortality and higher intake values showed increased risk (largely driven by 2 cohort studies), we did not consider this food group in the optimal consumption calculations.
Risk of bias
The results varied little by methodologic assumption, including only studies with a low risk of bias (Supplemental Tables 14–25). Findings including studies with a low risk of bias suggest a smaller inverse association between fruits (n = 4) and nuts (n = 5) and all-cause mortality in the linear dose-response meta-analysis. Moreover, all of the studies included that investigated the association between eggs and all-cause mortality were rated with an unclear or high risk of bias.
Quality of meta-evidence
We rated the quality of meta-evidence for the 12 food groups. The NutriGrade meta-evidence rating was “very low” for eggs; “low” for refined grains, vegetables, fruits, and SSBs; “moderate” for nuts, legumes, dairy, fish, red meat, and processed meat; and “high” for whole grains (Supplemental Table 26).
DISCUSSION
The associations between 12 food groups defined a priori and risk for all-cause mortality were systematically assessed in this meta-analysis through comparison of extreme categories and dose-response analyses both for linear and nonlinear relations. Nine of the 12 food groups showed an association with all-cause mortality in the categorical or continuous dose-response analyses; an inverse association was present for whole grain, vegetable, fruit, nut, legume, and fish consumption, whereas a positive association was present for red meat, processed meat, egg, and SSB consumption. We found a clear indication for nonlinear relations between vegetables, fruits, nuts, and dairy with all-cause mortality. The NutriGrade tool for evaluating the meta-evidence suggested high confidence in the effect estimate for whole grains. In agreement with this statement, this food group has been deemed important in the prevention of early death and disability. [2]
Previous meta-analyses have shown findings similar to ours. These publications reported an inverse association of all-cause mortality with consumption of whole grains, fruits and vegetables, nuts, and fish [4-7]; a positive association with consumption of red and processed meats [8]; and no significant linear associations with consumption of refined grains and dairy products. [5, 126] Mostly concentrating on a single food group, none of these meta-analyses is as comprehensive as ours, and most did not investigate nonlinear dose-response relations or the quality of meta-evidence. Finally, this is, to our knowledge, the first meta-analytic synthesis of any associations between legumes, eggs, and SSBs and all-cause mortality.
The observed association between whole-grain intake and all-cause mortality is consistent with results from meta-analyses that associated whole-grain intake with reduced risk of CVDs, overall cancer, and especially colorectal cancer. [5, 127]
The inverse association we observed between fruit and vegetable consumption and all-cause mortality risk was similar to results from a previous meta-analysis. [128]
The inverse linear association between nut consumption and risk of all-cause mortality seen in this study was the strongest of all associations in the 12 food groups. This finding is consistent with the results of a recent meta-analysis [129] and umbrella review [130] considering multiple outcomes.
Despite the absence of a linear association between legumes and all-cause mortality risk, the nonlinear analysis showed a 16% reduced risk of all-cause mortality when consuming up to ~ 150 g/d.
Consistent with the conviction that fish consumption is good for health, our study showed an inverse association with risk of all-cause mortality when comparing the highest with the lowest intake category.
According to a meta-analysis of 15 randomized controlled trials, consumption of marine n-3 PUFAs can lead to a 17% reduction in premature deaths [131], whereas a more recent meta-analysis showed no association between n-3 fatty acid supplementation and risk of all-cause mortality and CVD. [132] Although the risk-decreasing potential of fish is well established and confirmed by the results of our meta-analysis, the unavoidable presence of environmental contaminants should be also taken into account when larger amounts are consumed. [133]
Many large US cohort studies have consistently found evidence of an association between red and processed meats and an increased risk of all-cause mortality. [119, 121] Analyses of the EPIC (European Prospective Investigation into Cancer and Nutrition) cohort showed a slight J-shaped positive association between red meat consumption and all-cause mortality; the lowest risk was identified among participants with low to moderate meat consumption. [120]
Despite not finding a linear association between SSBs and all-cause mortality risk in our study, the nonlinear analyses showed a 7% higher risk with increasing intake of SSBs up to ~ 250 mL/d. Furthermore, a recent meta-analysis showed a 22% increase in the risk of CVD for each additional serving of SSBs per day. [134]
We are not arguing that the consumption of the investigated food groups are per se causally related to mortality, but rather that the study results and their synthesis via meta-analyses reflect underlying specific biological relations related to the etiology of chronic diseases and/or preclinical disorders and risk factors. Therefore, the end point “mortality” and even “cause-specific mortality” could be considered as important public health markers for the impact of dietary factors on the disease spectrum, and as general measures of potential disease reduction. The investigation of single food groups (or other dietary factors) with respect to mortality is based on the paradigm that compounds of the food groups can be linked to specific disease mechanisms because of their biological activity and other physiologic properties.
For example, protective effects of whole grains, fruits, vegetables, nuts, and fish might be explained by anti-inflammatory, antioxidative, antiproliferative, or chemopreventive mechanisms, which have been described for a number of bioactive compounds (e.g., fiber, minerals, trace elements, vitamins, carotenoids, polyphenols, alkylresorcinols, omega-3 fatty acids). [135, 136]
On the other hand, unfavorable effects of food groups such as red and processed meats might be based on opposite effects (proinflammatory, pro-oxidative, or carcinogenic compounds) triggered by nitrosamines, iron, or SFAs. [137] The detrimental effects observed for SSBs may be partly attributed to impairments of the otherwise working regulation of hunger and satiety. [138]
The investigated food groups are often part of dietary patterns or diet quality indexes, such as the Mediterranean diet, vegetarian diet, or Healthy Eating indexes. A meta-analysis of nearly 5 million subjects reported an 8% reduction of overall mortality for each 2-point increase in adherence to the Mediterranean diet. [139] Moreover, in a recent meta-analysis high adherence to the Healthy Eating Index was associated with a 22% lower risk of mortality. [9]
Strengths and limitations
Dietary information of most of the included studies derives from food frequency questionnaires, which represent a subjective approximation of past dietary behaviors rather than an assessment of absolute intakes. Hence, our results may reveal higher accuracy than is actually available. [140] Substantial heterogeneity was found with respect to the analyzed population size, follow-up duration, baseline age, and food consumption. We conducted subgroup analyses for sex, follow-up duration, geographic location, number of cases, and dietary assessment methods in order to explore high degrees of statistical heterogeneity. Overall, for most food groups, high levels of statistical heterogeneity persisted in subgroup analyses. People with a high intake of whole grains, fruits, vegetables, fish, nuts, or legumes might have different lifestyles or a different socioeconomic status from those with lower intakes, representing important confounders. [141] However, our main results were confirmed by sensitivity analyses including only studies with a low risk of bias [adjusted for important lifestyle factors (smoking, physical activity, and BMI)]. Another important limitation was the indication of small study effects such as publication bias in the analyses of vegetables, fruits, nuts, dairy products, and red meat. The results of the nonlinear association between dairy and all-cause mortality should be interpreted with caution because these observations were largely influenced by 2 cohort studies showing a strong positive association. [93] Among the strengths of the present meta-analysis are the a priori published systematic review protocol [14], the comprehensive literature search, and the large numbers of prospective studies, death cases, and food groups included. Furthermore, we performed different types of analyses (high compared with low intake, dose-response meta-analysis, nonlinear dose-response analysis, and subgroup and sensitivity analyses), which allowed us to detect associations where the relation was nonlinear and find an optimal consumption with the lowest risk of all-cause mortality. Finally, we assessed the quality of the studies using meta-evidence for each food group through use of the NutriGrade scoring system.
In conclusion, an optimal intake of whole grains, vegetables, fruits, nuts, legumes, and fish, as well as reduced consumption of red and processed meats and SSBs, can lead to an important decrease—by ~ 80%—in the relative risk of premature death when compared with intakes always from the highest risk category. To obtain a complete picture, it seems useful to extend the type of food groups and the clinical end points to be considered. We will in the future develop methods that are able to rank foods and diseases according to their contribution to the prevention of chronic diseases.
Acknowledgments
We are grateful to Peter Elwood and Janet Pickering for providing additional data for the current meta-analysis.
The authors’ responsibilities were as follows—LS, SS, KI, GH, and HB: conceived and designed the systematic review and meta-analysis; LS and A-ML: acquired and analyzed the data; LS, AB, SK, KI, GH, and HB: interpreted the results; LS, CS, GH, SK, AB, SS, KI, and HB: wrote the article; and all authors: critically revised the meta-analysis and approved submission of the final manuscript. None of the authors reported a conflict of interest related to the study.
References:
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60. Erratum in: Lancet 2013;381:628
O’Doherty MG, Cairns K, O’Neill V, Lamrock F, Jorgensen T, Brenner H, Schottker B, Wilsgaard T, Siganos G, Kuulasmaa K, et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur J Epidemiol 2016;31:455–68
Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716
Mayhew AJ, de Souza RJ, Meyre D, Anand SS, Mente A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr 2016;115:212–25
Zhao LG, Sun JW, Yang Y, Ma X, Wang YY, Xiang YB. Fish consumption and all-cause mortality: a meta-analysis of cohort studies. Eur J Clin Nutr 2016;70:155–61
Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr 2016;19:893–905
Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115:780–800.e5
EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on establishing food-based dietary guidelines. EFSA J 2010;8:1460
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12
Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med 2015;4:1933–47
Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer 2014;135:1884–97
Schwingshackl L, Chaimani A, Bechthold A, Iqbal K, Stelmach-Mardas M, Hoffmann G, Schwedhelm C, Schlesinger S, Boeing H. Food groups and risk of chronic disease: a protocol for a systematic review and network meta-analysis of cohort studies. Syst Rev 2016;5:125
Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopanteils E, Iqbal K, et al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 2016;7:994–1004
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9
Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–57
Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. London: The Cochrane Collaboration; 2011 [updated 2011 Mar]. Available from: www.handbook.cochrane.org
Yu D, Sonderman J, Buchowski MS, McLaughlin JK, Shu XO, Steinwandel M, Signorello LB, Zhang X, Hargreaves MK, Blot WJ, et al. Healthy eating and risks of total and cause-specific death among low-income populations of African-Americans and other adults in the southeastern United States: a prospective cohort study. PLoS Med 2015;12:e1001830
Boggs DA, Ban Y, Palmer JR, Rosenberg L. Higher diet quality is inversely associated with mortality in African-American women. J Nutr 2015;145:547–54
Buil-Cosiales P, Zazpe I, Toledo E, Corella D, Salas-Salvado J, Diez-Espino J, Ros E, Fernandez-Creuet Navajas J, Santos-Lozano JM, Aros F, et al. Fiber intake and all-cause mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr 2014;100:1498–507
Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med 2015;13:59
Jacobs DR Jr.., Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am J Clin Nutr 2007;85:1606–14
Johnsen NF, Frederiksen K, Christensen J, Skeie G, Lund E, Landberg R, Johansson I, Nilsson LM, Halkjaer J, Olsen A, et al. Whole-grain products and whole-grain types are associated with lower all-cause and cause-specific mortality in the Scandinavian HELGA cohort. Br J Nutr 2015;114:608–23
Key TJ, Thorogood M, Appleby PN, Burr ML. Dietary habits and mortality in 11,000 vegetarians and health conscious people: results of a 17 year follow up. BMJ 1996;313:775–9
Liu S, Sesso HD, Manson JE, Willett WC, Buring JE. Is intake of breakfast cereals related to total and cause-specific mortality in men? Am J Clin Nutr 2003;77:594–9
Roswall N, Sandin S, Lof M, Skeie G, Olsen A, Adami HO, Weiderpass E. Adherence to the healthy Nordic food index and total and cause-specific mortality among Swedish women. Eur J Epidemiol 2015;30:509–17
Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr 2006;83:124–31
Steffen LM, Jacobs DR Jr.., Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:383–90
van den Brandt PA. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am J Clin Nutr 2011;94:913–20
Vormund K, Braun J, Rohrmann S, Bopp M, Ballmer P, Faeh D. Mediterranean diet and mortality in Switzerland: an alpine paradox? Eur J Nutr 2015;54:139–48
Wang JB, Fan JH, Dawsey SM, Sinha R, Freedman ND, Taylor PR, Qiao YL, Abnet CC. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep 2016;6:22619
Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med 2015;175:373–84
Tognon G, Nilsson LM, Lissner L, Johansson I, Hallmans G, Lindahl B, Winkvist A. The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J Nutr 2012;142:1547–53
Tognon G, Rothenberg E, Eiben G, Sundh V, Winkvist A, Lissner L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. Age (Dordr) 2011;33:439–50
Jacobs DR Jr.., Meyer HE, Solvoll K. Reduced mortality among whole grain bread eaters in men and women in the Norwegian County Study. Eur J Clin Nutr 2001;55:137–43
Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, Singh-Manoux A, Ritchie K, Shipley MJ, Kivimaki M. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53
Fraser GE, Sumbureru D, Pribis P, Neil RL, Frankson MAC. Association among health habits, risk factors, and all-cause mortality in a black California population. Epidemiology 1997;8:168–74
Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 2004;160:1223–33
Hays JC, Keller HH, Ostbye T. The effects of nutrition-related factors on four-year mortality among a biracial sample of community-dwelling elders in the North Carolina piedmont. J Nutr Elder 2005;25:41–67
Hjartåker A, Knudsen MD, Tretli S, Weiderpass E. Consumption of berries, fruits and vegetables and mortality among 10,000 Norwegian men followed for four decades. Eur J Nutr 2015;54:599–608
Knoops KT, Groot de LC, Fidanza F, Alberti-Fidanza A, Kromhout D, van Staveren WA. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: the HALE project. Eur J Clin Nutr 2006;60:746–55
Kouris-Blazos A, Gnardellis C, Wahlqvist ML, Trichopoulos D, Lukito W, Trichopoulou A. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br J Nutr 1999;82:57–61
Kurotani K, Akter S, Kashino I, Goto A, Mizoue T, Noda M, Sasazuki S, Sawada N, Tsugane S. Quality of diet and mortality among Japanese men and women: Japan Public Health Center based prospective study. BMJ 2016;352:i1209
Leenders M, Sluijs I, Ros MM, Boshuizen HC, Siersema PD, Ferrari P, Weikert C, Tjonneland A, Olsen A, Boutron-Ruault MC, et al. Fruit and vegetable consumption and mortality: European prospective investigation into cancer and nutrition. Am J Epidemiol 2013;178:590–602
Martínez-González MA, Guillén-Grima F, De Irala J, Ruíz-Canela M, Bes-Rastrollo M, Beunza JJ, López del Burgo C, Toledo E, Carlos S, Sánchez-Villegas A. The Mediterranean diet is associated with a reduction in premature mortality among middle-aged adults. J Nutr 2012;142:1672–8
Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Br J Nutr 2009;102:285–92
Nguyen B, Bauman A, Gale J, Banks E, Kritharides L, Ding D. Fruit and vegetable consumption and all-cause mortality: evidence from a large Australian cohort study. Int J Behav Nutr Phys Act 2016;13:9
Olsen A, Egeberg R, Halkjaer J, Christensen J, Overvad K, Tjonneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr 2011;141:639–44
Oyebode O, Gordon-Dseagu V, Walker A, Mindell JS. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: analysis of Health Survey for England data. J Epidemiol Community Health 2014;68:856–62
Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 1996;144:501–11
Shi Z, Zhang T, Byles J, Martin S, Avery JC, Taylor AW. Food habits, lifestyle factors and mortality among oldest old Chinese: the Chinese Longitudinal Healthy Longevity Survey (CLHLS). Nutrients 2015;7:7562–79
Stefler D, Pikhart H, Kubinova R, Pajak A, Stepaniak U, Malyutina S, Simonova G, Peasey A, Marmot MG, Bobak M. Fruit and vegetable consumption and mortality in Eastern Europe: longitudinal results from the Health, Alcohol and Psychosocial Factors in Eastern Europe study. Eur J Prev Cardiol 2016;23:493–501
Strandhagen E, Hansson PO, Bosaeus I, Isaksson B, Eriksson H. High fruit intake may reduce mortality among middle-aged and elderly men. The Study of Men Born in 1913. Eur J Clin Nutr 2000;54:337–41
Tognon G, Lissner L, Saebye D, Walker KZ, Heitmann BL. The Mediterranean diet in relation to mortality and CVD: a Danish cohort study. Br J Nutr 2014;111:151–9
Tucker KL, Hallfrisch J, Qiao N, Muller D, Andres R, Fleg JL. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: the Baltimore Longitudinal Study of Aging. J Nutr 2005;135:556–61
Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr 1999;2:477–87
Zhang X, Shu XO, Xiang YB, Yang G, Li H, Gao J, Cai H, Gao YT, Zheng W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr 2011;94:240–6
Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J Nutr 2014;144:673–80
Limongi F, Noale M, Gesmundo A, Crepaldi G, Maggi S; for the ILSA Working Group. Adherence to the Mediterranean diet and all-cause mortality risk in an elderly Italian population: data from the ILSA study. J Nutr Health Aging 2016 Sept 89 (Epub ahead of print; DOI: 10.1007/s12603-016-0808-9)
Prinelli F, Yannakoulia M, Anastasiou CA, Adorni F, Di Santo SG, Musicco M, Scarmeas N, Correa Leite ML. Mediterranean diet and other lifestyle factors in relation to 20-year all-cause mortality: a cohort study in an Italian population. Br J Nutr 2015;113:1003–11
Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ 1996;312:478–81
Hodgson JM, Prince RL, Woodman RJ, Bondonno CP, Ivey KL, Bondonno N, Rimm EB, Ward NC, Croft KD, Lewis JR. Apple intake is inversely associated with all-cause and disease-specific mortality in elderly women. Br J Nutr 2016;115:860–7
Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–11
Eslamparast T, Sharafkhah M, Poustchi H, Hashemian M, Dawsey SM, Freedman ND, Boffetta P, Abnet CC, Etemadi A, Pourshams A, et al. Nut consumption and total and cause-specific mortality: results from the Golestan Cohort Study. Int J Epidemiol 2016 Mar 5 (Epub ahead of print; DOI: 10.1093/ije/dyv365)
Gopinath B, Flood VM, Burlutksy G, Mitchell P. Consumption of nuts and risk of total and cause-specific mortality over 15 years. Nutr Metab Cardiovasc Dis 2015;25:1125–31
Luu HN, Blot WJ, Xiang YB, Cai H, Hargreaves MK, Li H, Yang G, Signorello L, Gao YT, Zheng W, et al. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern Med 2015;175:755–66
Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR Jr.. Health benefits of nuts: potential role of antioxidants. Br J Nutr 2006;96(Suppl 2):S52–60
Bonaccio M, Di Castelnuovo A, De Curtis A, Costanzo S, Bracone F, Persichillo M, Donati MB, de Gaetano G, Iacoviello L. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: prospective results from the Moli-sani study. Br J Nutr 2015;114:804–11
Fernández-Montero A, Bes-Rastrollo M, Barrio-López MT, Fuente-Arrillaga Cde L, Salas-Salvadó J, Moreno-Galarraga L, Martínez-González MA. Nut consumption and 5-y all-cause mortality in a Mediterranean cohort: the SUN project. Nutrition 2014;30:1022–7
Guasch-Ferré M, Bulló M, Martínez-González MÁ, Ros E, Corella D, Estruch R, Fitó M, Arós F, Wärnberg J, Fiol M, et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med 2013;11:164
Hshieh TT, Petrone AB, Gaziano JM, Djousse L. Nut consumption and risk of mortality in the Physicians’ Health Study. Am J Clin Nutr 2015;101:407–12
Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart 1997;78:450–5
Sluik D, Boeing H, Li K, Kaaks R, Johnsen NF, Tjonneland A, Arriola L, Barricarte A, Masala G, Grioni S, et al. Lifestyle factors and mortality risk in individuals with diabetes mellitus: are the associations different from those in individuals without diabetes? Diabetologia 2014;57:63–72
van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: a cohort study and meta-analysis. Int J Epidemiol 2015;44:1038–49
Bongard V, Arveiler D, Dallongeville J, Ruidavets JB, Wagner A, Simon C, Marecaux N, Ferrieres J. Food groups associated with a reduced risk of 15-year all-cause death. Eur J Clin Nutr 2016;70:715–22
Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol 2002;156:824–31
Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, Zheng W, Shu XO. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr 2014;100:693–700
Yamasaki K, Kayaba K, Ishikawa S. Soy and soy products intake, all-cause mortality, and cause-specific mortality in Japan: the Jichi Medical School Cohort Study. Asia Pac J Public Health 2015;27:531–41
Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009;338:b2337
Nakamura Y, Okamura T, Tamaki S, Kadowaki T, Hayakawa T, Kita Y, Okayama A, Ueshima H. Egg consumption, serum cholesterol, and cause-specific and all-cause mortality: the National Integrated Project for Prospective Observation of Non-communicable Disease and Its Trends in the Aged, 1980 (NIPPON DATA80). Am J Clin Nutr 2004;80:58–63
Qureshi AI, Suri FK, Ahmed S, Nasar A, Divani AA, Kirmani JF. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med Sci Monit 2007;13:CR1–8
Djoussé L, Gaziano JM. Egg consumption in relation to cardiovascular disease and mortality: the Physicians’ Health Study. Am J Clin Nutr 2008;87:964–9
Bonthuis M, Hughes MC, Ibiebele TI, Green AC, van der Pols JC. Dairy consumption and patterns of mortality of Australian adults. Eur J Clin Nutr 2010;64:569–77
Elwood PC, Pickering JE, Fehily AM, Hughes J, Ness AR. Milk drinking, ischaemic heart disease and ischaemic stroke I. Evidence from the Caerphilly cohort. Eur J Clin Nutr 2004;58:711–7
Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology 2000;11:440–5
Goldbohm RA, Chorus AM, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr 2011;93:615–27
Huang LY, Wahlqvist ML, Huang YC, Lee MS. Optimal dairy intake is predicated on total, cardiovascular, and stroke mortalities in a Taiwanese cohort. J Am Coll Nutr 2014;33:426–36
Michaëlsson K, Wolk A, Langenskiöld S, Basu S, Warensjö Lemming E, Melhus H, Byberg L. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ 2014;349:g6015
Ness AR, Smith GD, Hart C. Milk, coronary heart disease and mortality. J Epidemiol Community Health 2001;55:379–82
Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med 2007;44:305–10
Soedamah-Muthu SS, Masset G, Verberne L, Geleijnse JM, Brunner EJ. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br J Nutr 2013;109:718–26
van Aerde MA, Soedamah-Muthu SS, Geleijnse JM, Snijder MB, Nijpels G, Stehouwer CD, Dekker JM. Dairy intake in relation to cardiovascular disease mortality and all-cause mortality: the Hoorn Study. Eur J Nutr 2013;52:609–16
Wang C, Yatsuya H, Tamakoshi K, Iso H, Tamakoshi A. Milk drinking and mortality: findings from the Japan collaborative cohort study. J Epidemiol 2015;25:66–73
Bell GA, Kantor ED, Lampe JW, Kristal AR, Heckbert SR, White E. Intake of long-chain omega-3 fatty acids from diet and supplements in relation to mortality. Am J Epidemiol 2014;179:710–20
Bellavia A, Larsson SC, Wolk A. Fish consumption and all-cause mortality in a cohort of Swedish men and women. J Intern Med 2017;281:86–95
Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 1997;336:1046–53
Engeset D, Braaten T, Teucher B, Kühn T, Bueno-de-Mesquita HB, Leenders M, Agudo A, Bergmann MM, Valanou E, Naska A, et al. Fish consumption and mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Eur J Epidemiol 2015;30:57–70
Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol 2004;160:1005–10
Gillum RF, Mussolino M, Madans JH. The relation between fish consumption, death from all causes, and incidence of coronary heart disease. the NHANES I Epidemiologic Follow-up Study. J Clin Epidemiol 2000;53:237–44
Kappeler R, Eichholzer M, Rohrmann S. Meat consumption and diet quality and mortality in NHANES III. Eur J Clin Nutr 2013;67:598–606
Lee JE, McLerran DF, Rolland B, Chen Y, Grant EJ, Vedanthan R, Inoue M, Tsugane S, Gao YT, Tsuji I, et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr 2013;98:1032–41
Letois F, Mura T, Scali J, Gutierrez LA, Feart C, Berr C. Nutrition and mortality in the elderly over 10 years of follow-up: the Three-City study. Br J Nutr 2016;116:882–9
Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Tamaki S, Okayama A. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med 2005;118:239–45
Osler M, Andreasen AH, Hoidrup S. No inverse association between fish consumption and risk of death from all-causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol 2003;56:274–9
Owen AJ, Magliano DJ, O’Dea K, Barr EL, Shaw JE. Polyunsaturated fatty acid intake and risk of cardiovascular mortality in a low fish-consuming population: a prospective cohort analysis. Eur J Nutr 2016;55:1605–13
Stefler D, Malyutina S, Kubinova R, Pajak A, Peasey A, Pikhart H, Brunner EJ, Bobak M. Mediterranean diet score and total and cardiovascular mortality in Eastern Europe: the HAPIEE study. Eur J Nutr 2017;56:421–9
Takata Y, Zhang X, Li H, Gao YT, Yang G, Gao J, Cai H, Xiang YB, Zheng W, Shu XO. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am J Epidemiol 2013;178:46–57
Villegas R, Takata Y, Murff H, Blot WJ. Fish, omega-3 long-chain fatty acids, and all-cause mortality in a low-income US population: results from the Southern Community Cohort Study. Nutr Metab Cardiovasc Dis 2015;25:651–8
Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol 2008;52:988–96
Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. JAMA 1998;279:23–8
Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995;91:645–55
Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol 2001;154:809–16
Nilsson LM, Winkvist A, Brustad M, Jansson JH, Johansson I, Lenner P, Lindahl B, Van Guelpen B. A traditional Sami diet score as a determinant of mortality in a general northern Swedish population. Int J Circumpolar Health 2012;71:1–12
Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from two prospective cohort studies. Arch Intern Med 2012;172:555–63
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V, et al. Meat consumption and mortality–results from the European Prospective Investigation into Cancer and Nutrition. BMC Med 2013;11:63
Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med 2009;169:562–71
Bellavia A, Stilling F, Wolk A. High red meat intake and all-cause cardiovascular and cancer mortality: is the risk modified by fruit and vegetable intake? Am J Clin Nutr 2016;104:1137–43
Barrington WE, White E. Mortality outcomes associated with intake of fast-food items and sugar-sweetened drinks among older adults in the Vitamins and Lifestyle (VITAL) study. Public Health Nutr 2016;19:3319–26
Odegaard AO, Koh WP, Yuan JM, Pereira MA. Beverage habits and mortality in Chinese adults. J Nutr 2015;145:595–604
Tasevska N, Park Y, Jiao L, Hollenbeck A, Subar AF, Potischman N. Sugars and risk of mortality in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2014;99:1077–88
Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, Geleijnse JM. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr 2011;93:158–71
Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617
Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 2016;14:207
Schwingshackl L, Hoffmann G, Missbach B, Stelmach-Mardas M, Boeing H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Des 2017;23:1016–1027
Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–99
Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–33
Domingo JL. Nutrients and chemical pollutants in fish and shellfish. Balancing health benefits and risks of regular fish consumption. Crit Rev Food Sci Nutr 2016;56:979–88
Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Clin Pract 2016;70:791–805
Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010;23:65–134
Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr 2013;4:384S–92S
Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187–225
DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab 2000;24:794–800
Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014;17:2769–82 0
Drewnowski A. Diet image: a new perspective on the food-frequency questionnaire. Nutr Rev 2001;59:370–2
Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr 2008;87:1107–17

Return to NUTRITION
Since 6-05-2017


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |