

Dietary Amelioration of Locomotor,
Neurotransmitter and Mitochondrial AgingThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: Exp Biol Med (Maywood). 2010 (Jan); 235 (1): 66–76 ~ FULL TEXT
Vadim Aksenov, Jiangang Long, Sonali Lokuge, Jane A Foster, Jiankang Liu and C David Rollo
Department of Biology,
McMaster University
1280 Main St W,
Hamilton, Ontario, Canada
Aging degrades motivation, cognition, sensory modalities and physical capacities, essentially dimming zestful living. Bradykinesis (declining physical movement) is a highly reliable biomarker of aging and mortality risk. Mice fed a complex dietary supplement (DSP) designed to ameliorate five mechanisms associated with aging showed no loss of total daily locomotion compared with >50% decrement in old untreated mice. This was associated with boosted striatal neuropeptide Y, reversal of age-related declines in mitochondrial complex III activity in brain and amelioration of oxidative stress (brain protein carbonyls).
Supplemented mice expressed approximately 50% fewer mitochondrial protein carbonyls per unit of complex III activity. Reduction of free radical production by mitochondria may explain the exceptional longevity of birds and dietary restricted animals and no DSP is known to impact this mechanism. Functional benefits greatly exceeded the modest longevity increases documented for supplemented normal mice.
Regardless, for aging humans maintaining zestful health and performance into later years may provide greater social and economic benefits than simply prolonging lifespan. Although identifying the role of specific ingredients and interactions remains outstanding, results provide proof of principle that complex dietary cocktails can powerfully ameliorate biomarkers of aging and modulate mechanisms considered ultimate goals for aging interventions.
Keywords : aging, locomotion, mitochondria, protein carbonyls, neuropeptide Y, free radicals, energy regulation, growth hormone, mice, dietary supplement
From the FULL TEXT Article:
Introduction
Declining locomotor activity with age (bradykinesis) is universal from nematodes to insects to vertebrates. [1–8] In man, declining activity contributes to ‘metabolic syndrome’, and in advanced years, frailty. Metabolic syndrome (high abdominal fat, insulin resistance, hypertension, arteriosclerosis and elevated free radical processes) afflicts ~50% of North Americans >60 years of age. [9] Associated risks include type II diabetes, stroke, heart attack and cancer. [8, 10] Declining motor function also correlates with disability risk. [11] Frailty, a leading cause of mortality and institutionalization, afflicts 37% or more for those over 85 years. [12] Deteriorating neurotransmitter systems mediating arousal and activity (particularly in the nigrostriatum and cerebellum) is a primary cause. [8, 13, 14] Aging skeletal, muscular and cardiovascular systems also contribute but exercise may be offsetting. [5, 6, 15]
We developed a dietary supplement (DSP) targeting five key mechanisms of aging (oxidative stress, inflammation, mitochondrial function, insulin resistance and membrane integrity) [16] and tested it on normal mice (Nr) and transgenic growth hormone mice (Tg) that show greatly reduced motor activity. The growth hormone axis modulates aging [17–19] and Tg express elevated free radical processes in brain [17, 20] and accelerated aging. [18, 19, 21] The DSP abolished age-related declines in Tg cognition, extended longevity (Nr ~11% and Tg ~28%) and offset radiation-induced apoptosis and DNA damage in both genotypes. [16, 22–24]
Here we report impacts of the DSP on motor function and the first results addressing impacts on mitochondria. The most dramatic impacts were in Nr mice. Typical of aging, the oldest untreated Nr mice showed >50% reduction in locomotion compared with their youth (~3 h/day less movement). This was paralleled by loss of mitochondrial complex III activity and elevated protein carbonylation in brain homogenates. Mitochondria, and particularly complex III activity, are highlighted in aging and free radical generation and protein carbonyls are a recognized biomarker of free radical stress. The DSP abolished declines in daily locomotion in association with increased mitochondrial complex III activity and reduced protein carbonyls in brain homogenates. The DSP reduced the ratio of mitochondrial carbonyls to complex III activity by ~50% in both genotypes, indicating that effective ingredients crossed the blood–brain barrier and penetrated mitochondria. We further considered that alterations in motor activity would involve striatum, and that growth hormones might act on striatal somatostatin neurons known to co-express neuropeptide Y (NPY) and nitric oxide synthase (NOS). We had a sensitive probe for NPY mRNA and consequently applied it in the striatum and cortex. Significant increases in NPY mRNA were found in supplemented Nr mice, but growth hormone (GH) transgenesis had no impact.
The cause(s) and amelioration of aging remain controversial, particularly with regard to DSPs. [25–29] Our results approach dietary restriction for functional biomarkers of behavior, neurochemistry, mitochondria and oxidative stress. Functional benefits greatly exceeded the 11% lifespan extension obtained for supplemented Nr mice. [22] Prolonging youthful function in populations with expanding elderly complements, however, may be of greater value than simply extending decrepit lifespans. The DSP strongly ameliorated normal functional aging and is likely to benefit age-associated pathologies. Moreover, supplements do not require drastic dieting or induction of states mimicking diapause or dwarfism.
Materials and methods
Animals and diets

Table 1 Breeding and husbandry of random bred C57BL/6J*SJL Nr and Tg mice were previously described. [16, 30] Protocols adhered to Canada Council on Animal Care guidelines. Our DSP contained 30 ingredients available without prescription (Table 1). Dosages were derived from recommended human doses adjusted for body size and the 10-fold higher metabolic rate of mice. [16] A slurry of the DSP was soaked onto small pieces of bagel. Mice from the breeding colony were randomly assigned at weaning and for life to either the DSP treatment group (one dose/day) or remained untreated. Bagel bits were avidly eaten ensuring accurate dosing. Nr and Tg mice in appropriate age ranges were randomly selected from control and supplemented populations for various assays.
Behavioral assessment
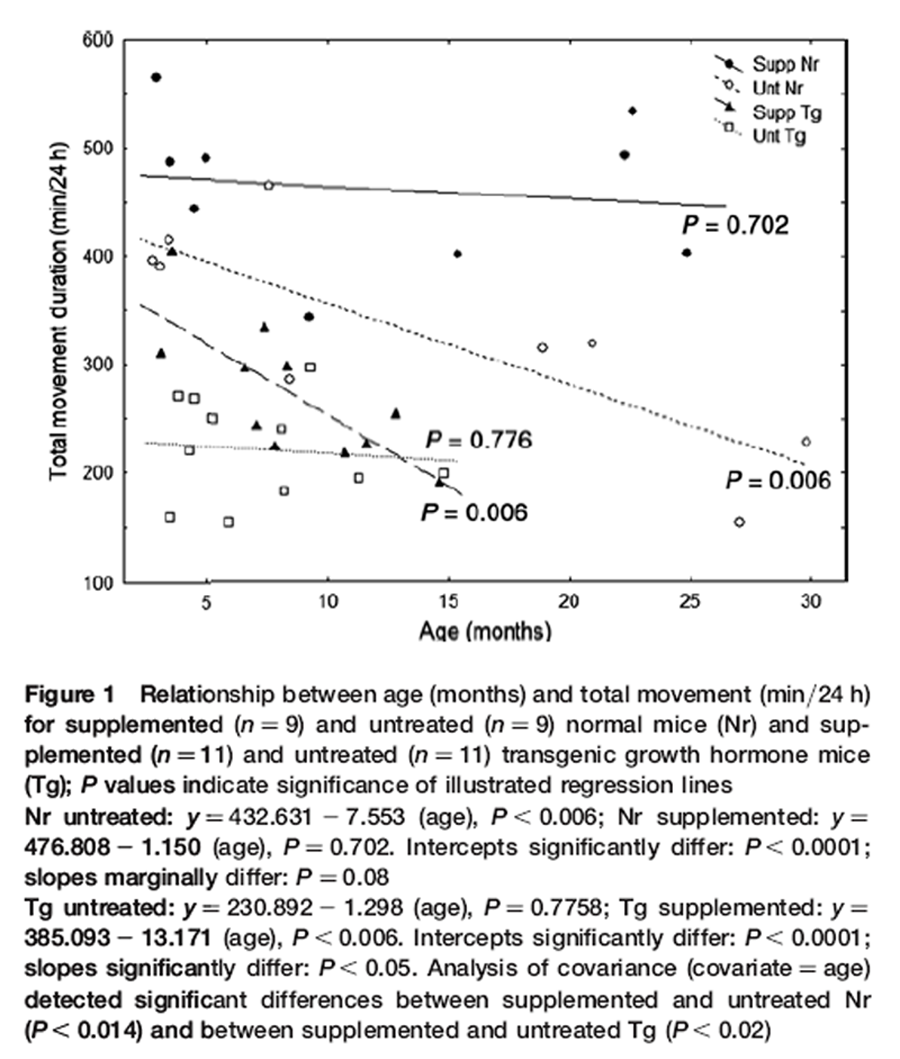
Figure 1 The duration of total daily movement was assessed in transparent acrylic arenas. Four inter-connected chambers contained a running wheel, a jar with nesting material, a food dish or water bottle. [31] Mice were acclimated for 48 h (12 h light:12 h dark photoperiod) then individually videotaped for 24 h with an infrared-sensitive camera. [31] Each mouse was used only once. Groups consisted of nine supplemented and nine untreated Nr male mice ~three to 30 months old, and 11 supplemented and 11 untreated male Tg mice ~3–15 months old. Mice were screened for health, vision and auditory responsiveness. Duration moving about the arena, wheel running and climbing on the roof were counted at one second intervals over 24 h, and then added to obtain duration of total daily movement. Each point in Figure 1 is one mouse.
Neuropeptide Y
DSP impacts on motor activity were likely to involve striatum which contains somatostatin neurons likely to be affected in Tg. We had a sensitive probe for NPY which is colocalized with somatostatin in striatum so we quantified NPY in striatum and cortex. Samples were obtained from eight supplemented and five untreated Nr female mice (5–25 months old) and six supplemented and eight untreated Tg females (5–15 months old). Mice were decapitated during the mid-photophase; brains were removed on ice and immediately placed in isopentane (–60°C) for 5 s and stored at –80°C.
Two coronal brain slices (10 µm thick and 100 µm apart) were obtained by cryostat at Bregma 0.9832 and thaw mounted onto gelatin-coated slides. NPY ribonucleotide antisense probes labeled with 35S radioisotope were applied. [33, 34] Slide-mounted sections were fixed in 4% formaldehyde in phosphate-buffered saline, acetylated with 0.25% acetic anhydride in 0.1 mol/L triethanolamine-HCl (pH 8.0), dehydrated in increasing concentrations of ethanol and delipidated with chloroform. Tissue sections were hybridized (approximately 500,000 counts per minute (CPM)/section) for 18 h at 55°C in a humidified chamber with radiolabeled riboprobe diluted in hybridization buffer (0.6 mol/L NaCl, 10 mmol/L Tris pH 8.0, 1 mmol/L ethylenediaminetetraacetic acid (EDTA) pH 8.0, 10% dextran sulfate, 0.01% sheared salmon sperm DNA, 0.5% total yeast RNA, type XI, 0.01% yeast transfer RNA and 1x Denhardt’s solution). Slides were washed in 20 µg/mL ribonuclease solution for 30 min to reduce nonspecific binding followed by 1 h each in 2x saline sodium citrate (SSC) at 50°C, 0.2x SSC at 55°C and 0.2x SSC at 60°C. Slides were dehydrated through a graded series of ethanol and air dried for auto-radiography. Hybridization of target tissues with an S35-labeled sense probe showed no signal, confirming specificity.
Slides were exposed to film in X-ray cassettes (Kodak BioMax MRw film, Eastman Kodak, Rochester, NY, USA) and developed for 18 h. All slides were processed in the same in situ hybridization experiment with the same probe and with film in adjacent cassettes at the same time. Images were digitized using a Qicam® camera. NPY mRNA density was represented as grayscale images (ImageJw software). For statistical analyses samples from whole brain, striatum and a 300 x 300 pixel sample of cortex were averaged from two consecutive slices to obtain the best estimate of NPY mRNA density.
Mitochondrial complex III activity
Different mice were used for mitochondrial and behavioral studies. Brains were removed on ice and stored at –80°C. Mitochondria were prepared by Paula’s method [35] and complex III activity was determined as described in Sun et al. [36] Numbers per group (male mice) were as follows: untreated Tg, eight; supplemented Tg, 11; untreated Nr, 10; and supplemented Nr, 11. Briefly, complex III activity was measured in a mixture containing 250 mmol/L sucrose, 1 mmol/L EDTA, 50 mmol/L KPi, 2 mmol/L KCN, 50 µmol/L cytochrome c, and 0.1% bovine serum albumin (with pH adjusted to 6.5 to reduce auto-oxidation of reduced CoQ1). The reaction was initiated with 40 µg/mL brain mitochondria and 50 µmol/L reduced CoQ1 (final concentration) and increased absorption at 550 nm was recorded for 2 min.
Slot-blot assays of carbonyls in brain homogenates and mitochondria
Protein carbonyls were measured by slot-blot [37] and relative density was obtained via optical scans. Measurements were made for both brain homogenates and the mitochondrial fraction. Carbonyl assays were from the same brains used for complex III assessment.
Statistical analyses
Analysis of covariance (ANCOVA) (age as covariate) was applied to assess impacts of genotype and diet. A multiple slopes model was employed as appropriate. ANOVA was used where age was not significant. Although genotypes were compared where appropriate, Nr and Tg mice were largely analyzed separately because different trends between genotypes otherwise reduced resolution. Post hoc comparisons employed Student–Newman–Keuls (SNK) tests as appropriate. Age-related trends were also characterized with linear and polynomial regression. Where applicable, planned comparisons were carried out with subsets of data divided into younger (<450 days) or older (>450 days) mice. A dividing point of 450 days best captured the very oldest ages of Tg and middle aged Nr. All data are described as means ± standard error. Where age was a covariate, means were calculated for a common age across groups. Analyses were performed with Statistica® software.
Results
Total daily movement
Nr mice were much more active than Tg (pooled Nr versus pooled Tg data: P < 0.001). The DSP had dissimilar agerelated effects on genotypes and reduced longevity of Tg was also apparent (Figure 1). Activity was elevated in both genotypes but especially in older Nr mice. In Tg, however, activity was higher in youth (Figure 1). Consequently, Nr and Tg were analyzed separately.
For Nr, bradykinesis progressed from the youngest ages of untreated mice resulting in >50% decline in activity by 24 months of age (Figure 1, regression: P< 0.006). The DSP elevated activity of Nr mice at all ages and virtually abolished bradykinesis (Figure 1, regression: P = 0.702). At 24 months, activity of supplemented Nr was ~3 h/day longer than untreated Nr. This reflected that untreated Nr activity was ~66% of supplemented Nr mice at 24 months (Figure 1). Regressions for Nr groups differed significantly in intercepts (P< 0.00001) and slopes differed marginally (untreated b = –7.55; supplemented b = –1.15; P = 0.08). Variance suggests that slopes would be better resolved with a larger sample size. Since slopes were only marginally resolved, we applied both multiple- and same-slope models of ANCOVA (covariate = age). Significant differences for treatment were obtained with either approach (same-slope ANCOVA: df = 15, P< 0.003; separate-slope ANCOVA: df = 14, P< 0.014). Thus, the most conservative estimate resolved a highly significant impact of the DSP on duration of daily movement in Nr mice (P< 0.014).
Untreated Tg expressed chronically low activity across all ages (comparable to 30 months old untreated Nr). At four months, supplemented Tg were ~45% more active than untreated Tg. The subsequent decline in activity for supplemented Tg (slope = –13.17, P< 0.006) was much steeper than for untreated Nr (slope = –7.553) and by 13 months (senescence for Tg) supplemented and untreated Tg were equally hypoactive (Figure 1). Regression lines for Tg groups differed significantly in intercepts (P< 0.00001) and slopes (untreated slope = 21.30; supplemented slope = –13.17; P< 0.05). Separate slopes ANCOVA resolved a significant impact of the DSP (P< 0.015).
Total locomotion encompassed all measures of movement including exercise. Exercise duration did decline with age, but remained higher in supplemented Nr mice across all ages (data not shown). Reductions in bouts of intense activity were offset by increases in moderate movement such that duration of total locomotion remained constant with age (Figure 1).
Neuropeptide Y mRNA
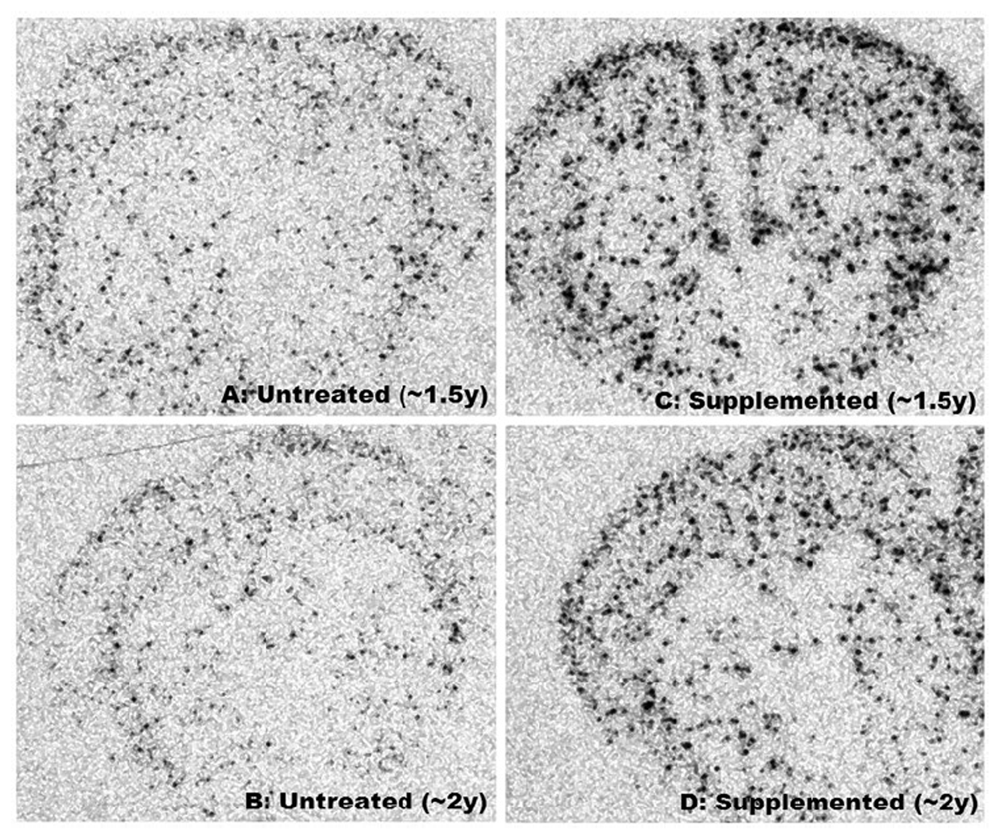
Figure 2

Figure 2 text Improved motor function of supplemented mice was associated with enhanced expression of NPY mRNA throughout striatum and cortex (Figure 2). Differences in intensity, size and number of foci between supplemented and untreated Nr were visually striking (Figure 2) and ANCOVA (separate slopes model) detected significant impacts of the DSP in whole brain (P< 0.041), striatum (P< 0.04) and cortex (P< 0.016) of Nr. In all three brain regions age-related increases in NPY mRNA were statistically resolved in supplemented Nr (linear regression: whole brain: r = 0.733, P< 0.039; striatum: r = 0.719, P< 0.044; cortex: r = 0.796, P< 0.018; n = 8) but no pattern was detected in untreated Nr (linear regression: all brain regions: P > 0.25). Slopes for supplemented Nr differed significantly from corresponding controls (whole brain: P< 0.038; striatum: P< 0.028; cortex: P< 0.009). Changes paralleled increasing mitochondrial complex III activity in aging supplemented Nr mice (see Figure 3).
Although sample sizes were small, comparison of the oldest three Nr mice in each treatment (all >450 days old) indicated that NPY was 23% higher in the cortex of supplemented Nr (supplemented: 64.8 ± 6.0 relative units; untreated: 49.9 ± 6.2 relative units, n = 6, P< 0.04). A similar trend for Tg was not statistically resolved. NPY mRNA did not differ significantly between Nr and Tg.
Brain mitochondrial complex III activity
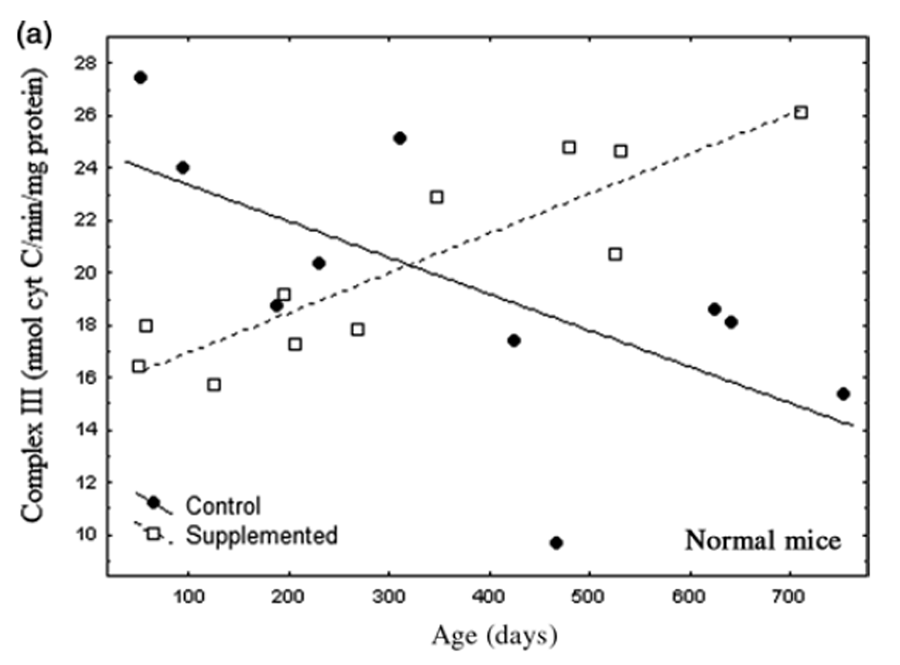
Figure 3 A
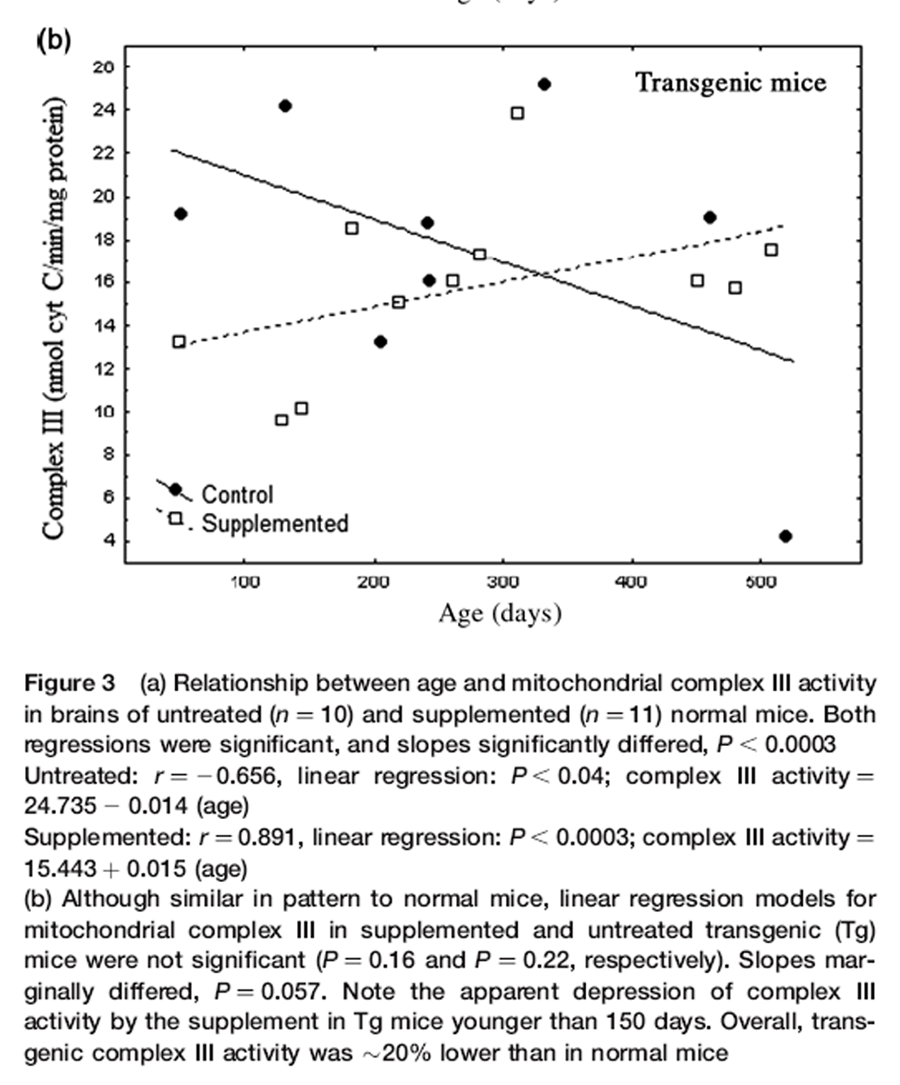
Figure 3 B text Nr and Tg showed similar changes in complex III activity with age and supplementation (Figures 3a and b). Patterns of complex III activity with age had diametrically opposite slopes between supplemented and untreated mice. A general linear model (separate slopes) detected a powerful effect of the DSP (P< 0.001) and an age x DSP x genotype interaction (P< 0.004). Reciprocal slopes for complex III activity in supplemented versus untreated Nr mice were each statistically resolved and they significantly differed (untreated: slope = –0.015; supplemented: slope = 0.015, P< 0.00034). Such complexity meant that, depending on age, the supplement had negative (youth), neutral (one-year-old) or positive (older mice) impacts on complex III activity relative to controls. Moreover, overall means did not reflect the remarkable degree of impact.
We expected that complex III would reflect ATP availability likely to contribute to levels of striatal and motor activity. Complex III activity in untreated Nr mice declined over 24 months to 46% of young mice, closely parallel to bradykinesis (compare Figures 1 and 3a). Supplemented Nr mice showed a linear 56% gain in complex III activity from 2 to 24 months of age. At ~24 months supplemented Nr mice had 85% more complex III activity than untreated Nr. Although mitochondrial function was elevated in old supplemented Nr compared with controls, complex III activity was relatively reduced in young supplemented Nr (Figure 3a). Thus, despite close association of complex III activity with locomotor declines in untreated Nr, supplemented Nr mice maintained constantly high motor activity that did not reflect rising activity of complex III (Figure 1). Thus, there was no simple relationship between complex III activity and behavior.
Trends in Tg complex III activity were very similar to Nr mice (Figure 3b). Regression slopes were only marginally resolved (untreated slope = –0.020; supplemented: slope = 0.012, P = 0.057), but suggests that impacts of the DSP were similar in both genotypes. Overall complex III activity, however, was lower than in Nr mice (reflecting an age x DSP x genotype interaction, P< 0.029). Comparison of mean complex III activity indicated that overall activity in Tg was ~20% lower than in Nr mice (Tg = 16.42 ± 0.96 versus Nr = 20.29 ± 0.90 ng cytochrome c/min/mg protein). Although low complex III activity is consistent with low Tg locomotion, elevated motor activity in young supplemented Tg (Figure 1) was associated with relatively lower complex III activity, similar to the pattern seen in supplemented Nr.
Protein carbonyls in brain homogenates

Table 2
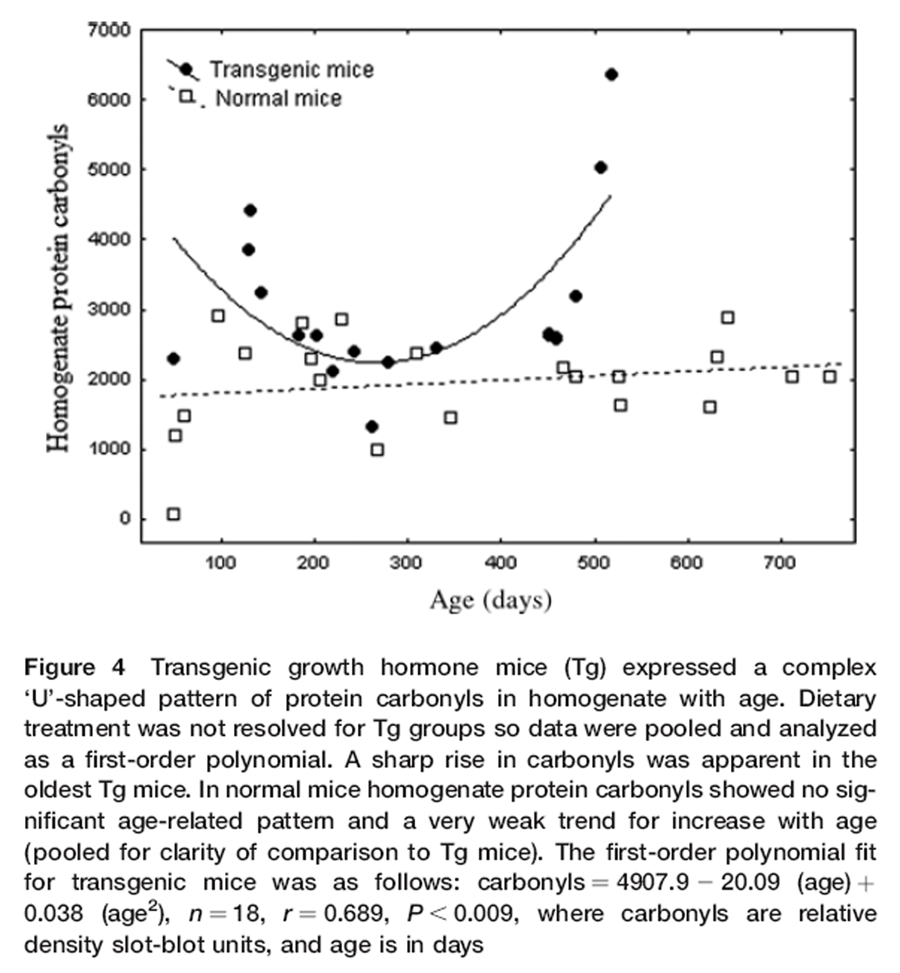
Figure 4 Supplemented Nr mice had 28% less protein carbonyls in brain homogenates than untreated Nr (P< 0.048; Table 2), but carbonyls showed no age-related trends in either Nr groups. The levels of carbonyls in untreated Nr mice were ~30% lower than those of untreated Tg, but this did not achieve significance (Table 2). Supplemented Tg also showed a non-significant trend for reduced carbonylation in brain homogenates compared with untreated Tg (~15%; Table 2).
Combining all Tg revealed a ‘U’-shaped pattern of homogenate carbonyls with elevations in youth (under 150 days old) and (especially) in mice older than 400 days (Figure 4). Tg carbonyls exponentially increased beyond an age of 400–450 days (Figure 4) corresponding to the period of rapid die-off. In light of these complex aging patterns, we selected a subset of data restricted to Nr and Tg mice older than 450 days. This statistically resolved the exceptional increases in homogenate carbonyls in senescing Tg (3947.6 ± 743.5 relative units) compared with stable carbonyl levels in aging Nr mice that were nearly 50% lower (2070.0 ± 128.5: t-test, P< 0.006).
Mitochondrial protein carbonyls
Although age-related trends were apparent in mitochondrial protein carbonyls, no groups were statistically resolved. For Nr mice, however, pooling treatments resolved a significant linear regression of mitochondrial carbonyls with age (r = 0.5595, n = 17, P< 0.02). Age-related changes in carbonyls were further confirmed by separating pooled Nr data into young (<450 days old) and older (.450 days old) categories. Young Nr had mitochondrial carbonyls (2085 ± 403) of only 34% of those observed in older Nr mice (6095 ± 1003, P< 0.0015). Increased mitochondrial carbonyls in aged Nr mice resembled the rapid late-life rise in Tg homogenate carbonyls, although not as severe. In contrast, untreated Tg expressed a trend for age-related decline in mitochondrial carbonyls. Analysis of variance (ANOVA) did not resolve an impact of genotype on mitochondrial protein carbonyls, but the DSP had a very powerful effect (P< 0.0088). This was especially apparent in Tg where supplemented mice had carbonyl levels only ~47% of those of untreated Tg (P< 0.0073) (Table 2). Nr supplemented mice had carbonyl levels only 64% of untreated Nr but this was not resolved.
Comparing protein carbonyls across the lifetime of genotypes (see Table 2) obscures the enormity of early oxidative stress in untreated Tg. Thus, analysis of lifetime data did not differentiate genotype, but when the analysis was limited to mice younger than 450 days, highly significant impacts of genotype (P< 0.0039) and a genotype x diet interaction (ANOVA, P< 0.0014) emerged. SNK resolved a 2.4-fold elevation of mitochondrial protein carbonyls in untreated Tg (6026 ± 591 relative units) compared with supplemented Tg (2501 ± 639) or either group of Nr (i.e. all three latter groups were at least 60% lower than untreated Tg).
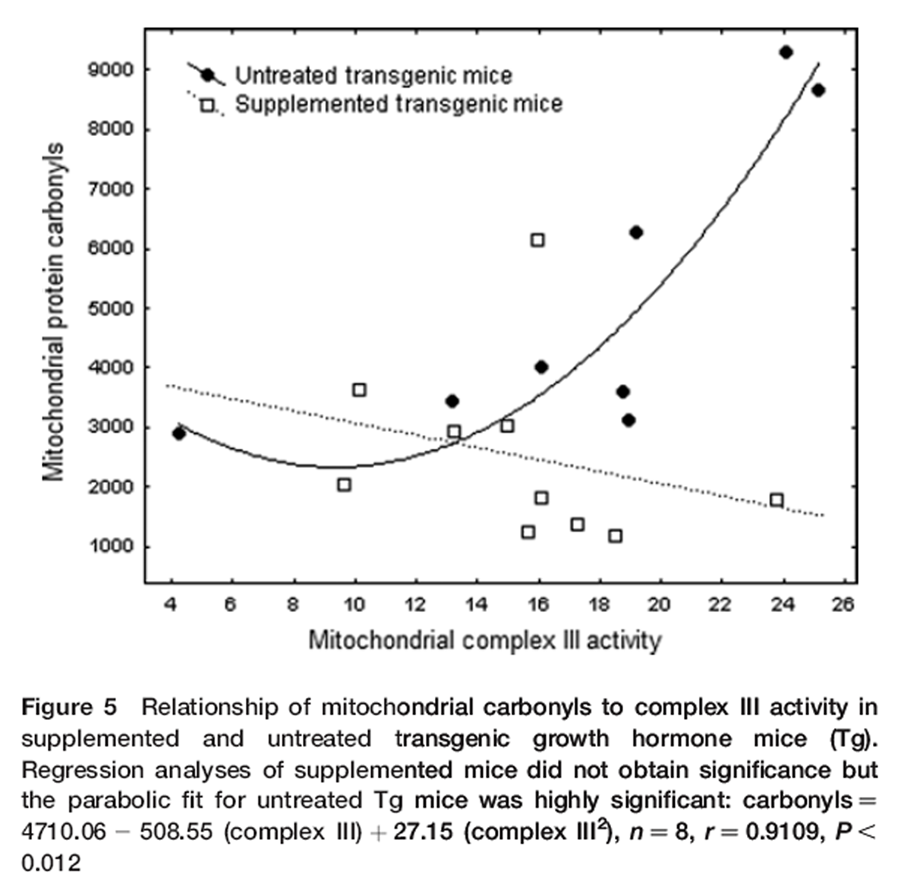
Figure 5 The relationship of mitochondrial activity to oxidative stress is critically related to aging. Consequently, we examined mitochondrial protein carbonyls in relation to complex III activity via regression analyses. Untreated Tg expressed strong increases in mitochondrial carbonyls with increasing complex III activity, whereas insignificant trends in supplemented Tg and either Nr group were neutral or declining. A first-order polynomial found that ~80% of the variance (r2 = 0.828, P< 0.012) in untreated Tg carbonyls was explained by complex III activity. [38] The strength of this result suggests that this mechanism explains accelerated aging of Tg (Figure 5).
To further explore oxidative stress relative to complex III activity, we divided mitochondrial carbonyls by complex III activity to obtain a composite variable. An Arcsine(Sqrt) transformation corrected for ratio-scale data. [38] ANOVA did not resolve genotype but diet proved significant (P< 0.028). The relative proportion of carbonyls associated with mitochondrial complex III activity was reduced by 52% and 54% in supplemented Nr and Tg mice, respectively – virtually identical. For untreated Tg, reduction of carbonyls per unit of mitochondrial activity by the DSP was greater at higher levels of complex III activity. At highest complex III activity carbonyls in supplemented Tg were only 20% of untreated Tg (Figure 5).
Discussion
Total locomotion
Bradykinesis is evident in 1-year-old mice and 20-year-old humans, [39, 40] but supplemented Nr mice showed no decline even at 24 months (Figure 1). Declines in untreated Nr began in youth and progressed to >50% loss of activity by 24 months (Figure 1, regression: P< 0.006). We know of no other treatment that ameliorates bradykinesis to this degree. Improved motor function in aging may be obtained by dietary restriction [2, 7, 41] or exercise (especially if coupled with N-acetyl cysteine and creatine). [15, 42] Flavinoids, antioxidants, [14, 43–45] L-deprenyl (monoamine oxidase inhibitor) [46, 47] and environmental enrichment [26] are also beneficial. L-DOPA increases locomotion in conditions of depleted dopamine (DA). [8]
Rats supplemented with α-lipoic acid and acetyl-L-carnitine showed 30% declines in activity in aged rats compared with 70% loss in controls. [1, 48, 49] Locomotion of young rats was increased by ~32%. [50] Our DSP abolished age-related declines and boosted activity of young mice (Figure 1). The DSP ameliorated but did not prevent declines in intense exercise implicating cardio-skeletal-muscular competence. Thus, further benefit might be obtained by exercise. [8, 42]
Despite amelioration of bradykinesis, mitochondrial function, oxidative damage and neurotransmitter declines, the DSP only extended Nr longevity by a modest 11%. [22] Female mice selected for high activity expressed deferred senescence but accelerated late mortality. [4] Alternatively, long-lived Drosophila ‘Methuselah’ mutants express oxidative stress resistance but no improvement in bradykinesis. [51] This resembles Tg where the supplement increased activity only in youth (Figure 1) despite amelioration of cognitive aging and a 28% increase in longevity. [16, 22] Dietary restriction benefits Drosophila in early life but negatively impact late-life stress resistance. [52] Similarly, acetyl-Lcarnitine improved cognition and survivorship but not age-related sensory–motor deficits in rats. [53] Increased longevity of dwarf rats via GH manipulation was also accompanied by functional impairments. [54] Thus, aging functions are somewhat dissociable from one another and longevity. [7, 51, 52, 55, 56]
Neuropeptide Y mRNA
NPY is co-localized in striatal interneurons with somatostatin and neuronal NOS. [57–59] We applied NPY mRNA as a biomarker for these neurons since NPY regulates foraging [60] and is altered in Parkinson’s disease. [61] The DSP strongly altered NPY mRNA (Figure 2). Similar increases occur in number, size and intensity of foci of somatostatin stimulated by GH excess in the hypothalamus [62] but NPY showed no alterations in Tg here. Bradykinesis is associated with declines in DA [8] and parallel loss of striatal NPY. [63] DA neurons are susceptible to oxidative stress which the DSP reduces. [8, 64] Striatal DA of Tg is ~40% that of Nr, which undoubtedly contributes to hypoactivity. [30] Striatal DA and NPY neurons are closely associated and NPY regulates DA synthesis and release. [65, 66] The broad distribution of NPY/NOS foci (Figure 2) could facilitate nitric oxide (NO) release mediating general arousal and waking. [57, 58] NO inhibits DA reuptake, elevating extracellular DA. [59, 67] NO promotes motor activity and can contribute to hyperactivity. [68, 69] Elevation of blood flow and metabolism by NO could also be important in aging. [70]
Mitochondrial complex III activity
Declining mitochondrial function is a biomarker of aging implicated in free radical generation. [29, 41, 48, 71, 72] Complex III activity in brains of old mice falls to ~60% of youthful levels [73] and was 46% of youth in untreated Nr at 24 months. In contrast, supplemented Nr showed remarkable 56% gains in complex III activity from 2 to 24 months of age. Tg had ~20% lower complex III activity than Nr (overall), and although complex III activity rose in supplemented Tg, levels did not exceed those of old untreated Nr (compare Figures 3a and b).
The DSP increased mitochondrial activity but reduced free radical processes relative to complex III activity (see below). Increased ATP would support functionality of aging mice. Oddly, the DSP lowered complex III activity in young mice (Figure 3), suggesting greater benefits at older ages. Mitochondrial status in youth can influence later-life functions however, [74] so benefits of youthful supplementation to older ages requires assessment.
Homogenate protein carbonyls
Protein carbonyls reflect oxidative damage associated with enzyme dysfunction, protein accumulation, cellular inclusions, extracellular deposits, neurodegeneration and aging. [37] Oxidative stress generally increases with age although most studies do not separate cytosolic and mitochondrial compartments. The DSP reduced homogenate carbonyls in Nr but age-related trends were absent (Figure 4). We documented increasing superoxide radical and lipid peroxidation with age in Tg and Nr mice, [20] suggesting that constant carbonyls in Nr homogenate likely reflects sustained proteosome function.
Although GH can ameliorate some symptoms of aging, considerable evidence suggests GH accelerates aging. [17–19, 21, 75, 76] Insulin-like growth factor 1 (IGF-1) and insulin signaling via the PI3K pathway modulate aging and many models of life extension (e.g. dwarf mice) express reduced PI3K signaling. Growth factors signal via free radicals and chronic low radiation stimulates growth. [76] GH, insulin and IGF-1 are elevated in Tg and IGF-1 regulates other growth factors. [17, 76, 77] Oxidative stress and accelerated aging of Tg20 are consistent with the free radical theory.
Protein carbonyls in Tg homogenates expressed a ‘U’ shaped pattern with the highest levels in youth and old age. Beyond ~400 days carbonyls rose precipitously (Figure 4), even though some mitochondrial measures were lowest at these ages. Free radicals in Tg homogenate may involve extramitochondrial sources like NAD(P)H oxidase or low ATP may compromise the proteosome. Accumulation of oxidized/ inactivated proteins is a reliable biomarker (and possible mechanism) of aging. Carbonyls negatively correlate with cognitive and motor functions in aging rodents and protein accumulation in aged gerbil brain were cleared by an antioxidant that restored youthful functions. [43, 78] Aging is linked to capacities for repair and turnover such as autophagy, lysosomal function and proteosome activity. [79] Aging mice exhibit increases in oxidized cysteine and ubiquinated proteins whereas long-lived mole rats maintain proteosome function and forestall bradykinesis for >20 years. [80] Reversibly oxidized cysteine residues and ATP regulate enzymes and signaling cascades, so ATP shortfalls and oxidative stress in old Tg (Figure 4) would synergistically disrupt cell functions and energy balance. [8, 81, 82]
Tg show constitutively elevated DNA damage as indicated by γH2AX foci and 8-oxodG levels as well as hypersensitivity to radiation-induced DNA damage and apoptosis. [23, 24] Knockdown of MTH1, the main sanitizing substrate for 8-oxodG (and oxidized ATP) induces replicative senescence and DNA damage even if free radicals, antioxidants and mitochondrial activity are unaltered. Thus, the nucleotide pool is also a major target of oxidation. [83]
Mitochondrial protein carbonyls
Reduction of mitochondrial carbonyls by the DSP indicates penetration of the blood–brain barrier and mitochondria (Table 2, P< 0.009), key goals of aging interventions. [84–86] Catalase expressed in mitochondria extended longevity of mice by ~17%.87 The DSP reduced carbonyls by ~36% and ~53% in Nr and Tg, respectively. This was statistically resolved for Tg (P< 0.008) but not for Nr mice (Table 2). Rising carbonyls and declining complex III activity in old untreated Nr (Figure 3a) suggest reduced ATP production potentially impacting numerous functions. In supplemented Nr, increased metabolic rate as reflected in higher physical (Figure 1) and mitochondrial activity (Figure 3) likely offset the degree of free radical reductions and limited gains in lifespan to 11%.
Differences in mitochondrial carbonyls between untreated Tg and Nr groups were not resolved across lifetimes partly because increases in carbonyls (Table 2) occurred at different ages. When analysis was limited to mice <450 days old strong genotype (P< 0.004), and genotype x diet interactions (ANOVA, P< 0.0015) emerged. SNK found untreated Tg (6026 ± 591 relative units) differed from other groups that all had at least 60% fewer carbonyls (2501 ± 639 relative units or less).
Age-related trends in mitochondrial carbonyls were not significant in either Nr group, but regression analysis of pooled data did detect increases with age (n = 17, r = 0.559, P< 0.02). Dividing mice into those younger or older than 450 days showed that carbonyls in youth were only 34% of older mice (P< 0.0017). Untreated Tg expressed high levels of mitochondrial carbonyls (Table 2), but these occurred in youth (unlike Nr). Lowest levels occurred in old Tg with low complex III activity. Early elevation of GH, anabolism and oxidative stress may reduce subsequent ATP availability and accelerate aging of Tg. [17, 18, 75, 76]
Youthful elevation of carbonyls in untreated Tg was associated with highest complex III activity (Figure 3). Overall, however, Tg complex III activity was ~20% lower than age-matched Nr. Even the highest Tg complex III activity fell in the Nr range despite being associated with high mitochondrial carbonyls. Further analyses revealed that untreated Tg mitochondria are associated with exceptional levels of protein carbonyls that dramatically rise with increasing complex III activity (Figure 5, r = 0.91). Extra-mitochondrial sources of free radicals88 or diversion of energy away from longevity assurance mechanisms could also contribute. [75]
In some circumstances high mitochondrial activity can generate low levels of free radicals, thereby increasing ATP production without accelerating aging. [15, 72, 89–91] Reduced oxidative stress relative to mitochondrial activity contributes to exceptional lifespans of birds or dietary restricted rodents. [17, 76] ANOVA of the ratio of mitochondrial carbonyls to complex III activity showed that the DSP reduced the ratio from 311.3 ± 45.9 relative units in untreated to 166.2 ± 40.8 for supplemented mice (i.e. supplemented Nr and Tg generated ~50% fewer carbonyls per unit of complex III activity; P, 0.021). For the highest complex III activity in Tg, the DSP reduced carbonyls by a remarkable 80% (Figure 5), increased longevity by 28% [22] and elevated youthful locomotion (Figure 1). Supplemented Tg still died earlier than Nr, however, and their bradykinesis progressed rapidly (Figure 1). Alternatively, increased complex III activity and reduced carbonylation per unit of complex III activity in supplemented Nr likely explains how both functional gains and modestly increased longevity were obtained.
Divergence of homogenate versus mitochondrial carbonyls
If mitochondria are the main source of free radicals then cytosolic and mitochondrial oxidative stress should be correlated. [92–94] Instead, age-related patterns of carbonyls in homogenate and mitochondria strongly differed (Table 2, Figures 4 and 5). Homogenates included mitochondria so estimates of divergence are conservative. Homogenate carbonyls were ~63% of those in mitochondria overall (P< 0.012, t-test), but there was no correlation between homogenate and mitochondrial levels (r = 0.06, P > 0.05). Even reduction of mitochondrial carbonyls in supplemented Tg by ~50% was not reflected in homogenate (Figure 4, Table 2). Caenorhabditis elegans also shows increased carbonyls with age in the mitochondria, but not in the cytoplasm, [28] whereas long-lived MCKL1 mice express elevated oxidative stress in the mitochondria but reductions in the cytosol. [95]
Cytosolic sources of free radicals may also be important. Thus, angiotensin II (a model for growth factor signaling) generates waves of free radicals derived from NAD(P)H oxidase and NOS. Resulting oxidative stress can derive mitochondrial DNA damage, mitochondrial free radical generation and ultimately, apoptosis. Thus, growth factor signaling can damage mitochondria and compromise energy production. [88]
Bradykinesis is an excellent aging biomarker since it is linked to metabolic rate, feeding, fat storage, brain neurotransmitters, cardiovascular and skeletal–muscular systems and mitochondria. A linkage to brain PI3K signaling is also suggested by knockout of insulin receptor substrate-2 (a PI3K element modulated by the GH axis). This altered energy balance and extended mouse longevity by 18%. Obesity and hyperinsulinemia were offset by increased activity, amelioration of bradykinesis at 22 months, elevated glucose utilization and reduced free radical stress. [96]
Accelerated aging of Tg may involve diversion of resources away from longevity assurance systems to growth. [75, 97, 98] This predicted that Tg would express elevated free radicals that was strongly confirmed. [20] Complex III activity was reduced in untreated Tg indicative of energy shortfalls. Energy limitation was also suggested by dietary preferences for carbohydrate99 and a carbohydrate-biased metabolism. [21] The levels of ATP in Tg skeletal muscle were 51% that of Nr. [20] Our results suggest that accelerated aging of Tg is strongly linked to free radical generation at complex III (Figure 5). A universal correlate of growth factor signaling via PI3K is mammalian target of rapamycin (mTOR). mTOR modulates growth, mitochondrial activity and ATP production and is associated with reduced longevity. [92] GH/IGF-1 likely regulate mitochondrial coupling and associated free radical generation via this pathway [100] Remarkably, the DSP elevated mitochondrial activity (energy) and reduced free radical processes, thus ameliorating two key mechanisms linked to aging and its dysregulation in Tg.
Author contributions:
All participated in design, interpretation and review of the manuscript. VA and J Long equally contributed to behavioral and mitochondrial work. SL and JF conducted the neurotransmitter work. CDR, VA and J Liu wrote the paper and analysed the data. J Liu contributed to mitochondrial aspects and CDR contributed to theory, development of the supplement and behavioral studies.
ACKNOWLEDGEMENTS
We thank an anonymous reviewer and Dr M Friedlander for guidance that greatly improved the paper. This study was supported by a grant to CDR (Natural Sciences and Engineering Research Council of Canada).
REFERENCES
Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci 1998;95:9562–6
Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc 2000;32:1623–9
Ridgel AL, Ritzmann RE. Insights into age-related locomotor declines from studies of insects. Ageing Res Rev 2005;4:23–39
Bronikowski AM, Morgan TJ, Garland T Jr, Carter PA. The evolution of aging and age-related physical decline in mice selectively bred for high voluntary exercise. Evolution 2006;60:1494–508
Martin I, Grotewiel MS. Oxidative damage and age-related functional declines. Mech Ageing Dev 2006;127:411–23
Wolkow CA. Identifying factors that promote functional aging in Caenorhabditis elegans. Exp Gerontol 2006;41:1001–6
Altun M, Bergman E, Edstrom E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol Behav 2007;92:911–23
Rollo CD. Dopamine and aging: intersecting facets. Neurochem Res 2009;34:601–29
Ford ES. Prevalence of the metabolic syndrome defined by the international diabetes federation among adults in the US. Diabetes Care 2005;28:2745–9
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–42
Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol 2005;60(A): 1437–46
Rockwood K, Howlett SE, MacKnight C, Beattie B, Bergman H, Hebert R, Hogan DB, Wolfson C, McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: Report from the Canadian study of health and aging. J Gerontol Ser A Biol Sci Med Sci 2004;59:1310–7
Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriat Psychiat 2002;17:359–70
Joseph JA, Shukitt-Hale B, Casadesus G, Fisher D. Oxidative stress and inflammation in brain aging: nutritional considerations. Neurochem Res 2005;30:927–35
Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol 2005;40:173–80
Lemon JA, Boreham DR, Rollo CD. A dietary supplement abolishes age-related cognitive decline in transgenic mice expressing elevated free radical processes. Exp Biol Med 2003;228:800–10
Rollo CD. Overview of research on giant transgenic mice with emphasis on the brain and aging. In: Samaras T, ed. Human Body Size and the Laws of Scaling. New York: Nova Biomedical Publishers, 2007:235–60
Bartke A. Growth hormone and aging: A challenging controversy. Clin Interv Aging 2008;3:659–65
Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol 2009;299:64–71
Rollo CD, Carlson J, Sawada M. Accelerated aging of giant transgenic mice is associated with elevated free radical processes. Can J Zool 1996;74:606–20
Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol Ser A Biol Sci Med Sci 2009;64:443–51
Lemon JA, Boreham DR, Rollo CD. A complex dietary supplement extends longevity of mice. J Gerontol 2005;60A:275–9
Lemon JA, Rollo CD, McFarlane NM, Boreham DR. Radiation-induced apoptosis in mouse lymphocytes is modified by a complex dietary supplement: the effect of genotype and gender. Mutagenesis 2008;23:465–72
Lemon JA, Rollo CD, Boreham DR.
Elevated DNA Damage in a Mouse Model of Oxidative Stress: Impacts of Ionizing Radiation and a Protective Dietary Supplement
Mutagenesis. 2008 (Nov); 23 (6): 473–482Roth GS, Lane MA, Ingram DK. Caloric restriction mimetics: the next phase. Ann NY Acad Sci 2006;1057:365–71
Head E. Combining an antioxidant-fortified diet with behavioral enrichment leads to cognitive improvement and reduced brain pathology in aging canines. Ann NY Acad Sci 2007;1114:398–406
Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, Matscheski A, Vanfleteren JR, Gems D. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 2008;22:3236–324
Gems D, Doonan R. Oxidative stress and aging in the nematode Caenorhabditis elegans. In: Miwa S, Beckman KB, Muller FL, eds. Oxidative Stress in Aging: From Model Systems to Human Diseases. Totowa, NJ: Humana Press, 2008:81–110
Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature 2008;454:1065–71
Chaudhry AM, Marsh-Rollo SE, Aksenov V, Rollo CD, Szechtman H. Modifier selection by transgenes: the case of growth hormone transgenesis and hyperactive circling mice. Evol Biol 2008;35:267–86
Lachmansingh E, Rollo CD. Evidence for a trade-off between growth and behavioural activity in giant ‘Supermice’ genetically engineered with extra growth hormone genes. Can J Zool 1994;72:2158–68
Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd edn. Oxford, UK: Elsevier, 2001
Foster JA, Herkenham M. Induced neuronal expression of class I major histocompatibility complex (MHC) mRNA in acute and chronic inflammation models. J Neuroimmunol 2002;131:83–91
Bercik P, Verdu EF, Foster JA, Lu J, Scharringa A, Kean I, Wang L, Blennerhassett P, Collins SM. Persistent dyspeptic feeding behavior in mice following eradication of Helicobacter pylori infection: role of gut–brain axis. Am J Physiol Int Comp Physiol 2009;296:R587–R594
Keeney PM, Xie J, Capaldi RA, Bennett JP Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 2006;26:5256–64
Sun L, Luo C, Long J, Wei D, Liu J. Acrolein is a mitochondrial toxin: effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion 2006;6:136–42
Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: Relevance to Alzheimer’s disease. Neurobiol Aging 2008;29:51–70
Zar JH. Biostatisitical Analysis. Engelwood Cliffs, NJ: Prentice Hall, 1974
Elias PK, Elias MF, Eleftheriou BE. Emotionality, exploratory behavior, and locomotion in aging inbred strains of mice. Gerontologia 1975;21:46–55
Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, Gash D. Motor slowing and Parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol Ser A Biol Sci Med Sci 2000;55:B473–80
Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science 2005;310:1641
Droge W. Oxidative aging and insulin receptor signaling. J Gerontol Biol Sci Med Sci 2005;60(A):1378–85
Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-a-phenylnitrone. Proc Natl Acad Sci 1991;88:3633–6
Navarro A, Gomez C, Sanchez-Pino MJ, Gonzalez H, Bandez MJ, Boveris AD, Boveris A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol 2005;289:R1392–9
Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of agerelated markers in a short-lived vertebrate. Curr Biol 2006;16:296–300
Ebadi M, Sharma S, Shavali S, El Refaey H. Neuroprotective actions of selegiline. J Neurosci Res 2002;67:285–9
Khaldy H, Escames G, Leon J, Bikjdaouene L, Acuna-Castroviejo D. Synergistic effects of melatonin and deprenyl against MPTP-induced mitochondrial damage and DA depletion. Neurobiol Aging 2003;24:491–500
Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann NY Acad Sci 2002;959:133–66
Liu J, Head E, Kuratsune H, Cotman CW, Ames BN. Comparison of the effects of L-carnitine and acetyl- L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann NY Acad Sci 2004;1033:117–31
Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN. Feeding acetyl- L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci 2002;99:1870–5
Cook-Weins E, Grotewiel M. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp Gerontol 2002;37:1347–57
Burger JMS, Hwangbo DS, Corby-Harris V, Promislow DEL. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell 2007;6:63–71
Markowska AL, Ingram DK, Barnes CA, Spangler EL, Lemken VJ, Kametani H, Yee W, Olton DS. Acetyl- L-carnitine 1: Effects on mortality, pathology and sensory–motor performance in aging rats. Neurobiol Aging 1990;11:491–8
Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology 2005;146:2920–32
Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 2004;431:1095–9
Simon AF, Liang DT, Krantz DE. Differential decline in behavioral performance of Drosophila melanogaster with age. Mech Ageing Dev 2006;127:647–51
Figueredo-Cardenas G, Morello M, Sancesario G, Bernardi G, Reiner A. Colocalization of somatostatin, neuropeptide Y, neuronal nitric oxide synthase and NADPH-diaphorase in striatal interneurons in rats. Brain Res 1996;735:317–24
Marino J, Cudeiro J. Nitric oxide-mediated cortical activation: a diffuse wake-up system. J Neurosci 2003;23:4299–307
Kiss JP, Zsilla G, Vizi ES. Inhibitory effect of nitric oxide on dopamine transporters: interneuronal communication without receptors. Neurochem Int 2004;45:485–9
Colton CA, Vitek MP. NPY and Chronic Neurodegenerative Disease NPY Family of Peptides in Neurobiology, Cardiovascular and Metabolic Disorders: From Genes to Therapeutics. Switzerland: Birkhauser Verlag, 2006
Cannizzaro C, Tel BC, Rose S, Zeng BY, Jenner P. Increased neuropeptide Y mRNA expression in striatum in Parkinson’s disease. Mol Brain Res 2003;110:169–76
Hurley DL, Bartke A, Wagner TE, Wee BEF, Phelps CJ. Increased hypothalamic somatostatin expression in mice transgenic for bovine or human GH. J Neuroendocrinol 1994;6:539–48
Cha JI, Hong JJ, Lee YI, Lee BR, Jo SS, Baek SH. Immunohistochemical study on the changes of neuropeptide Y immunoreactive neurons in the corpus striatum and motor system of aged rat. Kor J Anat 1997;30:215–24
Michel PP, Ruberg M, Hirsch E. Dopaminergic neurons reduced to silence by oxidative stress: an early step in the death cascade in Parkinson’s disease? Science STKE 2006(332):pe19
Adewale AS, Macarthur H, Westfall TC. Neuropeptide Y induced modulation of dopamine synthesis in the striatum. Regul Peptides 2005;129:73–8
Adewale AS, MacArthur H, Westfall TC. Neuropeptide Y-induced enhancement of the evoked release of newly synthesized dopamine in rat striatum: Mediation by Y2 receptors. Neuropharmacology 2007;52:1396–402
Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci 2001;24:211–5
Grammatikopoulos G, Pignatelli M, D’Amico F, Fiorillo C, Fresiello A, Sadie AG. Selective inhibition of neuronal nitric oxide synthesis reduces hyperactivity and increases non-selective attention in the Naples high-excitability rat. Behav Brain Res 2002;130:127–32
Szentirmai E, Krueger JM. Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Regul Integr Comp Physiol 2006;291:R473–80
Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev 2005;4:195–212
Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol 2004;287:R1244–9
Navarro A, Boveris A. Brain mitochondrial dysfunction in aging: conditions that improve survival, neurological performance and mitochondrial function. Front Biosci 2007;12:1154–63
Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys 2000;373:16–22
Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science 2002;298:2398–401
Rollo CD. Phenotypes: Their Epigenetics, Ecology and Evolution. London: Chapman & Hall, 1994
Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev 2002;4:55–61
Rovira II, Finkel T. Reactive oxygen species as signaling molecules. In: Miwa S, Beckman KB, Muller FL, eds. Oxidative Stress in Aging: From Model Systems to Human Diseases. Totowa, NJ: Humana Press, 2008:293–307
Forester MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci 1996;93:4765–9
Buffenstein R, Edrey YH, Yang T, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. AGE 2008;30:99–109
Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, Ward W, Richardson A, Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci 2009;106:3059–64
Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 2007;6:361–70
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 2004;287:C817–33
Rai P, Onder TT, Young JJ, McFaline JL, Pang B, Dedon PC, Weinberg RA. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc Natl Acad Sci 2009;106:169–74
Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem 2004;279:37575–87
Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta Mol Basis Dis 2006;1762:256–65
Murphy MP, Smith RAJ. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Ann Rev Pharmacol Toxicol 2007;47:629–56
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005;308:1909–11
Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol 2008;294:C413–22
Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med 1998;25:740–7
Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 2004;3:87–95
Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 2007;292:C670–86
Schieke SM, Phillips D, McCoy JP Jr, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem 2006;281:27643–52
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005;120:483–95
Shadel GS. Expression and maintenance of mitochondrial DNA: new insights into human disease pathology. Am J Pathol 2008;172:1445–56
Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1þ/2 mice. J Biol Chem 2008;283:26217–27
Taguchi A,Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 2007;317:369–72
Kajiura LJ, Rollo CD. A mass budget for transgenic ‘Supermice’ engineered with extra rat growth hormone genes: evidence for energetic limitation. Can J Zool 1994;72:1010–7
Kajiura LJ, Rollo CD. The ontogeny of resource allocation in giant transgenic rat growth hormone mice. Can J Zool 1996;74:492–507
Rollo CD, Kajiura LJ, Wylie B, D’Souza S. The growth hormone axis, feeding, and central allocative regulation: lessons from giant transgenic growth hormone mice. Can J Zool 1999;77:1861–73
Rollo CD, Lai M, Whitehead K, Perreault ML, Lemon J, Chaudhry AM. Thermoregulation of transgenic growth hormone mice. Can J Zool 2004;82:934–49

Return to NUTRITION
Since 8–03-2018


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |