FROM:
Genes Nutr 2007 (Dec); 2 (3): 295-305 ~ FULL TEXT
G. Filomeni, I. Graziani, G. Rotilio, and M. R. Ciriolo
Department of Biology,
University of Rome "Tor Vergata",
via della Ricerca Scientifica, 00133,
Rome, Italy
Polyphenols represent a large class of plant-derived molecules with a general chemical structure that act as potent free radical scavengers. They have long been recognized to possess several therapeutic activities ranging from anti-thrombotic to antioxidant. Moreover, the capability of polyphenols to act as reducing or oxidizing molecules depends on the presence of environmental metals and on the concentrations used. In this work we demonstrated that the stilbene trans-resveratrol was able to commit human breast cancer MCF-7 cells to apoptosis. Mainly, we evidenced a pivotal role of the mitochondria in this phenomenon as cytochrome c release into the cytosol was found after the treatment. We further showed that trans-resveratrol was able to affect cellular redox state. In particular, it induced an early production of ROS and lipid oxidation, and only later compromised the GSH/GSSG ratio. This mode of action was mirrored by a temporally different activation of JNK and p38(MAPK), with the former rapidly induced and the latter weakly activated at long intervals. The results obtained demonstrate a pro-apoptotic activity for trans-resveratrol, and suggest a preferential activation of different classes of MAP kinases in response to different oxidative stimuli (ROS versus GSH/GSSG alteration).
From the FULL TEXT Article:
Introduction
Apoptosis is a programmed mode of cell death induced physiologically either during organ development, or for the maintenance of cellular homeostasis by means of the elimination of damaged or unnecessary cells [54, 61]. Recently, it has been demonstrated that phosphorylation/de-phosphorylation state of some regulatory proteins are crucial events along the pathways controlling cell growth and apoptosis. A well established apoptotic signaling cascade is that regulated by mitogen activated protein (MAP) kinases, such as the p42/44 extracellular signal-related kinases (ERK1/2), c-Jun N-terminal protein kinase (JNK) and p38MAPK, which directly modulate the phospho-active levels of pro-apoptotic factors [38]. MAP kinases are serine/threonine kinases that are regulated by a variety of extracellular stimuli including growth factors, mitogens, cytokines and environmental stresses [39]. Oxidative stress-mediated apoptosis is induced by phosphorylative cascades; indeed, in the last few years a hypothesis has emerged that reactive oxygen species (ROS) are not only the downstream damaging species produced by radical chain reactions, but also the second messengers of signaling networks [15].
Considerable scientific interest in the anticancer therapy has been focused, for long time, on the identification of compounds able to efficiently commit cells to apoptosis; in this context several chemotherapeutic agents can generate ROS at high extent, by catalyzing one-electron redox cycles with oxygen [55]. The specificity of action of these compounds towards tumor histotypes is supposed to be due to: (i) low concentration of antioxidant enzymes in transformed cells; (ii) high rate of proliferation with respect to differentiated cells, a feature that does not allow tumor cells repairing oxidative injuries, especially those involving DNA [23, 32, 49]. More recently, there has been a focus of attention on natural anti-oxidant molecules that, besides their role in scavenging-free adicals, could function as pro-oxidants, when highly concentrated or in the presence of transition metals [51]. Previous studies have indicated that some nutraceuticals exhibit potent anti-tumor properties and can modulate apoptosis, differentiation and cell cycle, probably by virtue of their anti-oxidant functions. However, in vitro experiments reported that most of them behave as potent pro-oxidants molecules [6, 9, 40]. For example, organosulfur molecules from garlic, such as the oil-soluble diallyl disulfide [12, 13], diallyl trisulfide [22], or the water-soluble S-allylmercaptocysteine [63] have been demonstrated to induce growth arrest and apoptosis dose-dependently by increasing ROS production.
Among nutraceuticals, polyphenols represent the most intriguing and studied class of compounds that can be therapeutics for a large spectrum of the most common diseases including cancer [20]. Nevertheless, despite several studies regarding their function, the anti-oxidant/pro-oxidant properties of these compounds remain somewhat debatable and the detailed molecular mechanisms of their effects continue to be largely unknown. A pro-apoptotic function has been often proposed for many polyphenols [21, 35, 42]; however, although a huge amount of data support these assumptions, the molecular mechanism(s) by which apoptosis is triggered are long to be elucidated.
One of the well-characterized polyphenols is trans-resveratrol (3,5,4'-trihydroxy-trans-stilbene), a phytoalexin found in edible material, such as grape skin, peanuts and red wine. Jang and coworkers demonstrated that trans-resveratrol is an anticancer compound able to inhibit each phase of cell transformation both in in vitro and in in vivo models. Particularly, by acting as antioxidant, anti-mutagen as well as by inducing phase II drug-metabolizing enzymes, trans-resveratrol inhibits the initiation phase of tumorigenesis; concomitantly, it mediates the anti-inflammatory response and induces cell differentiation, thereby inhibiting tumor promotion and progression, respectively [28]. Subsequently, other groups reported the anti-proliferative and pro-apoptotic activity of trans-resveratrol in several tumor cell lines [2, 27, 41, 56], suggesting that it can function by modulating and interacting with a broad range of cellular targets, ranging from cell surface receptors [5, 7] to caspases [43]; from mitochondria [8] and mitochondria-associated proteins [43, 50, 60], to intracellular protein kinases, such as protein kinase B (PKB)/Akt, PKC, MAP kinases [34, 57, 58, 62, 65] and transcription factors (e.g. p53, pRb, c-Jun and NF-κB) [30, 37, 45, 59].
Although resveratrol, and polyphenols in general have been reported to be antioxidants, the idea that they can function as pro-oxidant compounds is emerging. Galati and colleagues demonstrated that several flavonoids, including trans-resveratrol, show anti-cancer properties when present at high concentration, by virtue of their capability to react with cellular peroxidases or with cellular thiols, thereby transforming into highly reactive phenoxyl radicals [17, 18]. The capability of these phenoxyl radicals to selectively deplete the cells of glutathione (GSH), to form GSH-conjugates or to oxidize GSH into the disulfide form of the tripeptide (GSSG) mainly depends on their chemical structure, in particular it seems to correlate with the number and the position of hydroxyl groups [19].
Besides this hypothesis of a direct production of oxyradicals (especially superoxide, O2-), trans-resveratrol and other polyphenols could induce the production of ROS by means of several indirect pathways, such as those downstream of surface receptors, which have been often suggested to be associated with an NAD(P)H-dependent ROS production. It has been also demonstrated that, under the phenoxyl radical form, polyphenols can cause mitochondrial toxicity by collapsing the mitochondrial transmembrane potential, thereby allowing ROS being generated at high extent [19]. Moreover it has been reported that several natural-occurring flavonoids, and trans-resveratrol itself, are able to affect mitochondrial respiration [24] by inhibiting NADH oxidase, succinoxidase and ATP synthesis [3], thus inducing the generation of partially reduced oxygen species [26, 48]. Particularly, a direct relationship between flavonoids redox potential and the above mentioned mitochondrial phenomena, has been proposed [25].
An intimate relationship between ROS and activation of phosphorylative cascades in the apoptotic response downstream polyphenols treatment has been argued [52] and, especially for what trans-resveratrol concerns, several pathways involving MAP kinases have been proposed to be activated upon pharmaceutical (micro-molar) doses, as modulators of apoptosis [24]. Therefore, in this study, we have focused on the role of oxidative stress and oxidative stress-induced MAPK as downstream effectors of high (pharmaceutical) doses of trans-resveratrol. In particular, we demonstrate that both JNK and p38MAPK were involved in the cytotoxic effects of trans-resveratrol, although with a temporally different activation, and sensitivity to ROS production or GSH/GSSG alteration.
Materials and methods
Cell culture
Human breast cancer cells MCF-7 were purchased from the American Type Culture Collection and grown in minimum essential medium (SIGMA, St.Louis, MO, USA) supplemented with 1% sodium pyruvate, 1% non-essential amino acids, 1 mg/l bovine insulin 10% fetal calf serum, at 37°C in an atmosphere of 7.5% CO2 in air. Cells were routinely trypsinized, plated at 4 × 104/ cm2 flasks. Cell viability was assessed by Trypan blue exclusion.
Treatments
About 5 mg/ml solution of trans-resveratrol (SIGMA) was prepared just before the experiments by dissolving the powder in dimethyl sulfoxide. Treatments were performed with different amounts of trans-resveratrol ranging from 6.25 to 50 µg/ml at 37°C in medium supplemented with serum. Unless specified, the concentration of trans-resveratrol selected for all the experiments was 50 µg/ml since it gives a valuable degree of apoptosis on MCF-7. As control, equal amount of dimethyl sulfoxide (0.1%) was added to untreated cells.
Treatment with the cell permeable JNK and p38MAPK inhibitors, SP600125 and SB203580 (Calbiochem-Novabiochem, La Jolla, CA, USA), respectively, were performed at concentration of 10 µM because lower concentrations did not show significant inhibition of MAP kinases phosphorylation and higher concentrations were toxic. SP600125 and SB203580 were added 1 h before the addition of trans-resveratrol and maintained throughout the experiment.
Analysis of cell viability and apoptosis
Adherent (after trypsinization) and detached cells were combined, washed in PBS and counted after Trypan blue staining by optic microscope on hemocytometer. Nuclei were detected by fluorescent microscopy using vital staining assay with DNA-specific cell-permeable dye Hoechst 33342. Images of cells were rapidly digitized with a Cool Snap video camera connected to Nikon Eclipse TE200 epifluorescence microscopy. All images were captured under constant exposure time, gain, and offset. Cells were also stained with 50 µg/ml propidium iodide (dissolved in 0.1% Triton X-100) prior to analysis by a FACScalibur instrument (Becton Dickinson, San Josè, CA, USA). The percentages of cells in each phase of cell cycle were evaluated according to Nicoletti et al. [47] by calculating peak areas of nuclei with different amounts of DNA, Alternatively, cells were washed with PBS, stained with an annexin V-FITC/propidium iodide kit (Bender MedSystem, Vienna, Austria) and analyzed by FACScalibur instrument.
Measurements of ROS levels and oxidative damages
Detection of intracellular ROS was performed as previously described [9]. Briefly, cells were incubated with 50 µM 2’,7’-dichlorodihydrofluorescein diacetate (DHCF-DA) (Molecular Probes, Eugene, OR, USA) (dissolved in dimethyl sulfoxide) for 30 min at 37°C followed by treatment with trans-resveratrol. Treatment with 100 µM tert-butyl hydroperoxide was used as a positive control.
Carbonylated proteins were detected using the Oxyblot Kit (Intergen, Purchase, NY, USA) after reaction with 2,4-dinitrophenylhydrazine (DNP) for 15 min at 25°C. Samples were resolved on 12% SDS-polyacrylamide gels and DNP-derivatized proteins were identified by immunoblot using an anti-DNP antibody.
Levels of malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) were measured by a colorimetric method using the Lipid Peroxidation Assay Kit (Calbiochem-Novabiochem) according to manufacturer instructions. Lipid peroxidation was evaluated with reference to standard curves obtained with known amounts of MDA and 4-HNE and expressed as µmol MDA+4-HNE/mg protein.
Glutathione determination
Intracellular glutathione was assayed upon formation of S-carboxymethyl derivatives of free thiols with iodoacetic acid, followed by the conversion of free amino groups to 2,4-dinitrophenyl derivatives by the reaction with 1-fluoro-2,4-dinitrobenzene. Cells were lysed by repeated cycles of freezing and thawing under liquid nitrogen. Lysates were then utilized for GSH and GSSG assay as previously described [16]. Data are expressed as nmoles of GSH or GSSG/mg protein.
Preparation of cell lysates and Western blot analyses
Cytosolic caspase-9, cytochrome c and β-actin determination
Cells were washed with PBS and collected by centrifugation at 700×g for 7 min at 4°C. The cell pellet was resuspended in extraction buffer containing 220 mM mannitol, 68 mM sucrose, 50 mM PIPES-KOH, pH 7.4, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol (DTT), and protease inhibitors [11]. After 30 min incubation on ice, cells were homogenized with a glass Dounce. Cell homogenates were spun at 14,000×g for 15 min at 4°C; supernatants and pellets were collected and stored at –80°C until analysis by gel electrophoresis; 20 µg of cytosolic protein or 10 µg of pellets extracts were loaded onto each lane of a 12% SDS-polyacrylamide gel, separated, and then blotted to nitrocellulose membrane (Bio-Rad). Purified mouse anti-cytochrome c (PharMingen, San Diego, CA, USA), anti-caspase-9 (Upstate Biotechnology, Lake Placid, NY, USA) and anti-β-actin (SIGMA) monoclonal antibodies were used as primary antibodies (1:3,000).
Phospho-JNK, phospho-p38MAPK, phospho-c-Jun
Cell pellet was resuspended in lysis buffer containing 62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 50 mM DTT, 0.01% bromophenol blue, sonicated for 15 s to shear DNA and reduce sample viscosity. About 20 µl of sample was loaded on 12% polyacrylamide gel and transferred onto a nitrocellulose membrane (BioRad). Polyclonal anti-phospho-p38MAPK (Cell Signaling, Beverly, MA, USA), and monoclonal anti-phospho-JNK and c-Jun (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used as primary antibodies (1:1,000). The protein complex formed upon specific secondary antibody treatments (1:5,000), was identified using a Fluorchem Imaging system (Alpha Innotech–Analitica De Mori, Italy) after incubation with ChemiGlow chemiluminescence substrate (Alpha Innotech). Densitometric analyses were calculated using Quantity One Software (Bio-Rad) and data were normalized with respect to the actin band. Data are reported as arbitrary units.
Proteins were determined by the method of Lowry et al. [36].
Data presentation
All experiments were done at least 5 different times (n = 5) unless otherwise indicated. The results are presented as means ± SD. Statistical evaluation was carried out by ANOVA, followed by correction with Bonferroni’s. Comparisons were considered to be significant at P < 0.05.
GSH/GSSG alteration.
Results
trans-Resveratrol induces apoptosis in MCF-7 cells via the mitochondrial pathway
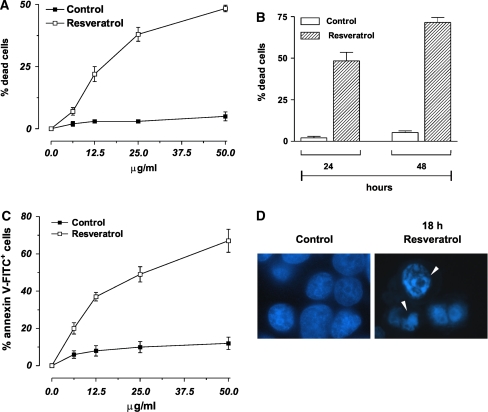 Figure 1
Figure 1
|
We studied the effects of trans-resveratrol treatment on cell viability of human breast cancer cells MCF-7. Figure 1a shows the percentages of Trypan blue-positive cells after 24 h of treatment with different concentrations of trans-resveratrol. The data reported evidenced that trans-resveratrol decreased cell viability in a dose- and time-dependent manner (Fig. 1a, b). In particular, at concentration of 50 µg/ml, the percentage of dead cells at 24 h was about 48%, phenomenon that became more evident after 48 h of treatment, reaching values of about 72% (Fig. 1b).
In order to distinguish which kind of cell death was induced under our experimental conditions (apoptosis versus necrosis), MCF-7 cells were treated for 24 h with the different concentrations of trans-resveratrol and then utilized for cytofluorimetric analyses upon staining with annexin V-FITC. Figure 1c shows the percentages of annexin V-FITC positive cells the amount of which paralleled the data previously obtained by direct counts upon Trypan blue exclusion (Fig. 1a). Moreover, fluorescence microscope analysis of nuclei upon staining with DNA-specific dye Hoechst 33342, showed that MCF-7 cells, treated for 18 h with 50 µg/ml trans-resveratrol, exhibited areas of condensed chromatin confirming that this compound is able to commit MCF-7 cell to death with the features of apoptosis (Fig. 1d).
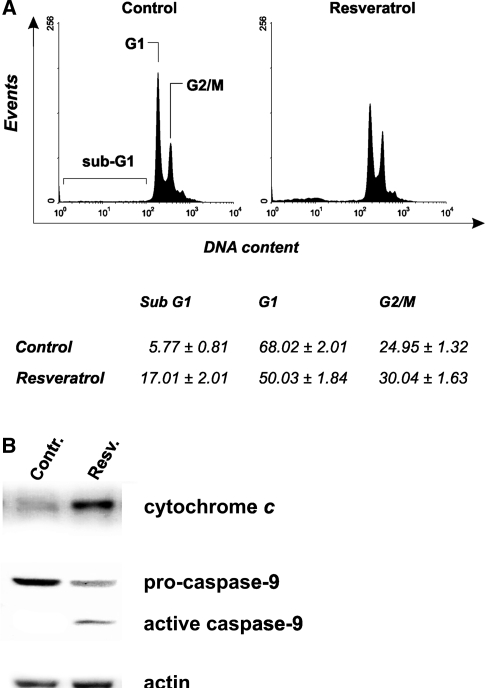 Figure 2
Figure 2
|
MCF-7 cells represent a well-established hystotype lacking of caspase-3, for this reason, they undergo apoptosis without showing inter-nucleosomal cleavages [29]. This was confirmed by cytofluorimetric analyses of cell cycle carried out upon staining with propidium iodide. As shown in Fig. 2a the representative histograms of MCF-7, cells treated for 24 h with 50 µg/ml trans-resveratrol, evidenced a low but significant arrest of the cell cycle in G2/M phase (see table below). As a consequence of the deficiency of caspase-3 activity, the rate of sub-G1 (apoptotic) cells was lower with respect to the data obtained by the previous analyses (see Figs. 1, 2).
To characterize the mechanism through which apoptosis occurs, MCF-7 cells were treated with 50 µg/ml trans-resveratrol and used for sub-cellular fractionation in order to isolate mitochondria and the cytosolic fractions. Figure 2b shows the Western blot analyses of cytosolic cytochrome c and caspase-9 after 18 h of treatment; in particular, cytochrome c was efficiently released from mitochondria, and caspase-9 activated, indicating an efficient induction of the intrinsic mitochondrial pathway. These results suggest that, also in the absence of an active caspase-3, the execution of the apoptotic program could occur as already demonstrated [29].
Trans-Resveratrol induce alteration of intracellular redox state
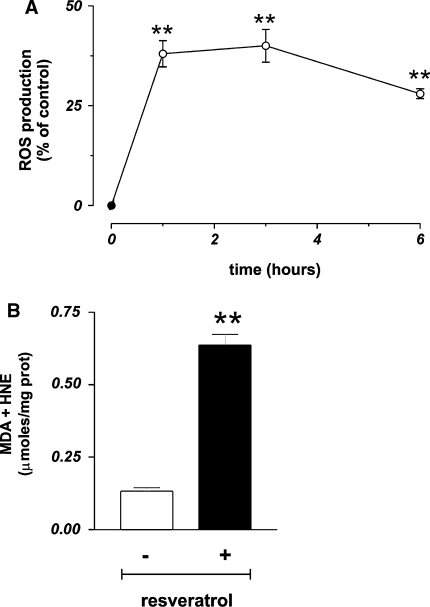 Figure 3
Figure 3
|
As previously reported, trans-resveratrol can produce ROS, directly by reacting as phenoxyl radical with O2 [17, 18], or indirectly by impairing mitochondrial integrity [19, 26, 48]. In order to investigate whether ROS could be produced under our experimental conditions, we performed cytofluorimetric analyses of MCF-7 cells pre-loaded with the ROS-sensitive fluorophore DHCF-DA. Figure 3a shows that trans-resveratrol was able to induce a high production of ROS (+37.2%) that was maintained up to 3 h with a slow decrease only after 6 h of treatment.
This result prompted us to investigate on the molecular targets of ROS by determining the content of protein oxidation and the extent of lipid peroxidation. No increase in carbonyl content was evidenced by Western blot analyses (data not shown); conversely, the levels of lipid peroxides significantly changed. Figure 3b shows that 24 h of treatment with 50 µg/ml trans-resveratrol induced a fivefold increase of lipid peroxides, indicating that lipids were one of the main targets of this compound under our experimental conditions.
 Figure 4
Figure 4
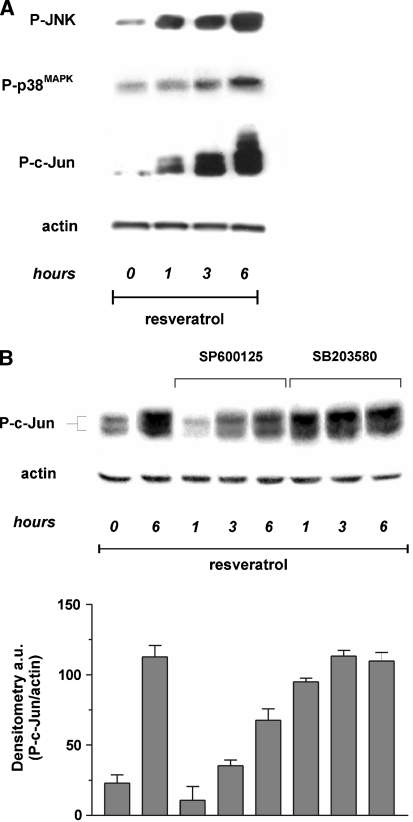 Figure 5
Figure 5
|
A sustained flux of ROS usually results in an imbalance of the intracellular redox state, which is promptly buffered by glutathione, the most abundant low molecular weight thiol inside the cell [14]; therefore we analysed the concentration of both GSH and GSSG. MCF-7 cells were treated with 50 µg/ml trans-resveratrol and used for the determination of the tripeptide by HPLC technique. Figure 4a shows that trans-resveratrol was able to profoundly influence the ratio [GSSG]/[2GSH], since GSH levels decreased, while GSSG concentration increased in a time-dependent manner (Fig. 4b). Such a phenomenon was particularly significant starting from 3-6 h of treatment, suggesting a sequence of oxidative events in which the upstream production of ROS represents the primary inducer, and changes in GSH redox state the downstream outcome.
Trans-Resveratrol induce a selective activation of JNK and p38MAPK
We have previously demonstrated that different oxidizing agents can trigger apoptosis by inducing JNK or p38MAPK-governed phosphorylative cascades. In order to investigate whether apoptosis was related to the activation of the MAP kinases pathway, we treated MCF-7 cells with 50 µg/ml trans-resveratrol for 6 h and then performed Western blot analyses of phospho-activated isoforms of the pro-apoptotic members of the MAP kinases family, JNK and p38MAPK. Figure 5a shows that trans-resveratrol was able to rapidly activate JNK and p38MAPK, although with different kinetics and to different extent. Particularly, the phosphorylation of JNK was time-dependent and started to be operative after 1 h of treatment, conversely the lower induction of p38MAPK could be observed only at 3-6 h of treatment. The results obtained allow to speculate that, while the activation of JNK is very rapid and associated with the increase of ROS concentration, the activation of p38MAPK could reasonably represent an event principally related to late alterations of the intracellular redox state, such as those in [GSSG]/[2GSH] ratio, thus suggesting a specific response to different types of oxidative stress, i.e. production of ROS versus alteration of [GSSG]/[2GSH] ratio.
To investigate the specific contribution of JNK and p38MAPK on c-Jun phosphorylation, we incubated MCF-7 cells with the specific inhibitors of JNK and p38MAPK, SP600125 and SB203580, respectively, and further analysed the phospho active levels of c-Jun. Western blot reported in Fig. 5b shows that, the incubation with SP600125 determined a time-shifted phosphorylation of c-Jun, suggesting that JNK was the earlier, but not the sole mediator of trans-resveratrol-induced apotosis. Conversely, the inhibition of p38MAPK did not prevent the phosphorylation of c-Jun (Fig. 5b), suggesting that, under our experimental conditions, p38MAPK had an additive, rather than a principal role in phosphorylating c-Jun.
The inhibition of JNK and p38MAPK inhibits apoptosis induced by trans-resveratrol
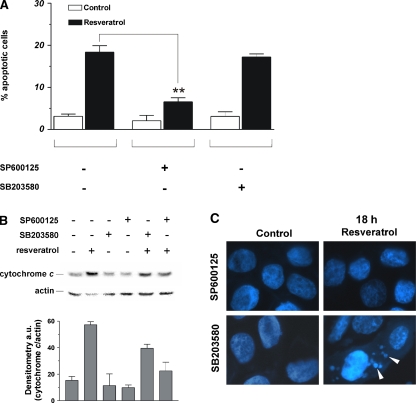 Figure 6
Figure 6
|
The involvement of JNK and p38MAPK in the induction of apoptosis upon treatment with trans-resveratrol was investigated by incubating MCF-7 cells with specific inhibitors of JNK and p38MAPK. Figure 6a shows the cytofluorimetric analyses upon propidium iodide staining; in particular, the inhibition of JNK induced a significant decrease of apoptotic cells, indicating that JNK-dependent phosphorylative cascade plays a pivotal role in the cell death induced by trans-resveratrol. On the other hand, the inhibition of p38MAPK did not affect the content of apoptotic cells after treatment with trans-resveratrol, indicating that p38MAPK pathway was not effective in the induction of cell death. Moreover, Western blot analyses showed that the inhibition of p38MAPK only partially decreased the level of cytosolic cytochrome c (–30%), whereas the inhibition of JNK induced a significant decrease of the protein in the cytosol (–65%), confirming the pivotal role played by the latter kinase in the apoptotic process. This trend was confirmed by fluorescence microscopy analyses of MCF-7 nuclei stained with Hoechst 33342. Figure 6c shows that, after incubation with SP600125, chromatin distribution within the nuclei was not affected by trans-resveratrol, whereas the inhibition of p38MAPK-mediated pathway was not able to efficiently prevent the canonical apoptotic changes of chromatin. In particular, apoptotic bodies were still visible after 18 h of treatment (white arrows).
Discussion:
trans-Resveratrol has been recently reported to possess several beneficial effects for human health. Besides its antioxidant activity, it has been suggested that trans-resveratrol inhibits each phase of tumor growth, by interacting with several cellular targets, thereby activating cell cycle arrest and death. At molecular level, it has been suggested that high concentration of polyphenols can induce the production of ROS, rather than reinforcing the antioxidant defense, and it has been hypothesized that this pro-oxidant function can play a pivotal role in resveratrol-induced apoptosis in tumor cells [19]. In fact, the generation of phenoxyl radicals of polyphenols by the peroxidase–H2O2 system, which co-oxidizes cellular glutathione or NADH, accompanied by O2 uptake to form ROS has been demonstrated [17, 18]. In addition, polyphenols have been proposed to induce oxidative stress indirectly by targeting mitochondrial electron transport chain, thereby generating a downstream flux of ROS [26, 48]. In agreement with previous works, which identified a role for oxyradicals in cytotoxicity of polyphenols [6, 46, 19], we observed an early increase of ROS levels after treatment with high doses of trans-resveratrol in human breast cancer cells MCF-7. The involvement of ROS has been proposed as upstream event occurring during treatment with high (micro-molar) concentrations of trans-resveratrol [1, 21], as well as with other polyphenols, including epigallocatechin-3-gallate [44, 53], woodfordin I [35] and quercetin [33, 64]. In our experimental system ROS burst occurs precociously (within the first hour) and the detrimental effects produced were confirmed by the increase in lipid peroxidation by-products, MDA and 4-HNE. On the other hand, no damage on protein in terms of carbonyls content was observed, implying that the hydrophobicity of trans-resveratrol renders the lipids the preferential targets of its pro-oxidant action. It is worth to notice that, even though the pro-oxidant effects observed in this study were referred to the highest dose of trans-resveratrol (50 µg/ml), we suggest that apoptosis observed at the lowest dose (6.25 µg/ml) is still mediated by ROS production and oxidative insults. However, since differentiated cells are equipped with high levels of both enzymatic and non-enzymatic antioxidants, we can presume that, as previously demonstrated with other diet-derived pro-oxidant molecules [12], trans-resveratrol could be non toxic towards normal tissues.
We also found that trans-resveratrol induced a significant alteration of GSH/GSSG ratio in agreement with the knowledge that the phenoxyl radical form of polyphenols can produce ROS in association with a selective depletion of intracellular GSH, to form GSH-conjugates or GSSG. However, under our experimental conditions, GSH depletion was a late event, which allows speculating that it represents just a epiphenomenon rather than the pivotal redox change governing resveratrol-induced apoptosis.
 Figure 7
Figure 7
|
The direct relationship between alteration of intracellular redox state and activation of MAP kinases-mediated phosphorylative pathways has been exhaustively demonstrated [15]. Moreover, a role for polyphenols in the activation of different MAP kinases members has been suggested as necessary event for downstream induction of apoptosis in cancer cells [31]. Here we reported that the oxidative stress deriving from treatments with trans-resveratrol is differently associated with MAP kinases activation. In particular, trans-resveratrol seems to preferentially induce the JNK/c-Jun-mediated phosphorylative cascade as the principal mediator of apoptosis. Moreover, if we consider the relationship with the oxidative alterations observed (ROS or GSH-dependent) we could hypothesize a selective activation of different MAP kinases, with JNK more responsive to the increase of ROS concentration and p38MAPK preferentially responsive to the alteration of GSH redox state. This different sensitivity of MAP kinases to ROS- or GSH-dependent intracellular redox changes is in line with our recent results suggesting that different types of oxidative stress could preferentially drive different phosphorylative pathways [10, 12, 13]. On this basis, we can outline the results obtained as shown in Fig. 7. In particular trans-resveratrol activates JNK and p38MAPK, but its different capability to alter intracellular redox environment concur to make JNK activation the principal response, and p38MAPK a redundant kinase for the induction of cell death. Finally, further works should be done in animal models in order to determine the concentrations that allow trans-resveratrol to behave as chemotherapeutic other than antioxidant.
Acknowledgments
This work was partially supported by grants from FIRB, MIUR, and Ministero della Sanità.
Footnotes
G. Filomeni and I. Graziani are recipients of fellowships from the Italian Association for Cancer Research (AIRC-FIRC).
References:











