Nonpharmacological Treatment of Army Service Members with
Chronic Pain Is Associated with Fewer Adverse Outcomes
After Transition to the Veterans Health AdministrationThis section is compiled by Frank M. Painter, D.C.
Send all comments or additions to: Frankp@chiro.org




FROM: J General Internal Medicine 2020 (Mar); 35 (3): 775–783 ~ FULL TEXT
OPEN ACCESS Esther L. Meerwijk, PhD, MSN , Mary Jo Larson, PhD, MPA, Eric M. Schmidt, PhD, Rachel Sayko Adams, PhD, MPH, Mark R. Bauer, MD, Grant A. Ritter, PhD, Chester Buckenmaier III, MD, and Alex H. S. Harris, PhD, MS
VA Health Services Research & Development,
Center for Innovation to Implementation (Ci2i),
VA Palo Alto Health Care System,
Menlo Park, CA, USA.
esther.meerwijk@va.gov
FROM: Nahin ~ Pain 2017BACKGROUND: Potential protective effects of nonpharmacological treatments (NPT) against long-term pain-related adverse outcomes have not been examined.
OBJECTIVE: To compare active duty U.S. Army service members with chronic pain who did/did not receive NPT in the Military Health System (MHS) and describe the association between receiving NPT and adverse outcomes after transitioning to the Veterans Health Administration (VHA).
DESIGN AND PARTICIPANTS: A longitudinal cohort study of active duty Army service members whose MHS healthcare records indicated presence of chronic pain after an index deployment to Iraq or Afghanistan in the years 2008-2014 (N = 142,539). Propensity score-weighted multivariable Cox proportional hazard models tested for differences in adverse outcomes between the NPT group and No-NPT group.
EXPOSURES: NPT received in the MHS included acupuncture/dry needling, biofeedback, chiropractic care, massage, exercise therapy, cold laser therapy, osteopathic spinal manipulation, transcutaneous electrical nerve stimulation and other electrical manipulation, ultrasonography, superficial heat treatment, traction, and lumbar supports.
MAIN MEASURES: Primary outcomes were propensity score-weighted proportional hazards for the following adverse outcomes: (a) diagnoses of alcohol and/or drug disorders; (b) poisoning with opioids, related narcotics, barbiturates, or sedatives; (c) suicide ideation; and (d) self-inflicted injuries including suicide attempts. Outcomes were determined based on ICD-9 and ICD-10 diagnoses recorded in VHA healthcare records from the start of utilization until fiscal year 2018.
KEY RESULTS: The propensity score-weighted proportional hazards for the NPT group compared to the No-NPT group were 0.92 (95% CI 0.90-0.94, P < 0.001) for alcohol and/or drug use disorders; 0.65 (95% CI 0.51-0.83, P < 0.001) for accidental poisoning with opioids, related narcotics, barbiturates, or sedatives; 0.88 (95% CI 0.84-0.91, P < 0.001) for suicide ideation; and 0.83 (95% CI 0.77-0.90, P < 0.001) for self-inflicted injuries including suicide attempts.
CONCLUSIONS: Nonpharmacological treatments (NPT) provided in the Military Health System (MHS) to service members with chronic pain may reduce risk of long-term adverse outcomes.
KEYWORDS: adverse outcomes; chronic pain; nonpharmacological treatment; opioids; veterans
From the FULL TEXT Article:
INTRODUCTION
Chronic pain is a costly public health issue that is associated with many adverse outcomes including chronic opioid use and suicide. [1] Deployment to conflict zones places military service members at risk for chronic pain, which often persists after they leave military service and transition their healthcare from the Military Health System (MHS) to the Veterans Health Administration (VHA). [2, 3] Twenty-nine percent to 44% of active duty service members reported chronic pain after deployment to conflict zones in Iraq or Afghanistan, and 48 to 60% of VHA primary care patients reported chronic pain. [4–7] Chronic pain is a well-established risk factor for suicide ideation and suicide attempts, as well as for opioid use disorder and opioid-related overdose, especially in the presence of already existing substance use disorder. [8, 9]
Chronic pain is often managed with prescription opioids which, especially at higher doses and/or longer duration of use, have been associated with increased risk for substance use disorders, opioid-related overdose, self-inflicted injuries, and suicide attempts. [10–16] In addition to opioids, chronic pain can be managed with nonpharmacological treatments (NPT). [17–19] These include treatments like exercise therapy and chiropractic manipulation, as well as less common treatments, like yoga, massage, and acupuncture. [20] Compelling evidence for a moderate effect on clinical outcomes was found for exercise and spinal manipulation in nonmilitary samples with chronic low back pain, although the effect on pain intensity was small to moderate and mostly short-term. [21, 22] Recent research in active duty service members showed that early NPT was associated with a lower risk of military duty limitations, and facilities where NPT was more common were less likely to initiate long-term opioid treatment for their patients. [23, 34]
If NPT is used to manage chronic pain, in addition to or instead of opioids, thismay not only have an effect on pain and functional status, but also on adverse outcomes that are associated with chronic pain and opioid use, such as substance use disorders, drug overdose, and self-inflicted injuries. The potential long-term protective effect of NPT against adverse outcomes has not been examined. The purpose of the current analyses was to compare active duty U.S. Army service members with chronic pain who did and did not receive NPT in the MHS and describe the association between receiving NPT in theMHS and adverse outcomes observed after transition to the VHA, specifically alcohol and drug abuse or dependence, accidental or intentional drug poisoning, suicide ideation, and self-inflicted injuries.
Studying these outcomes broadens our knowledge of the potential impacts of NPT beyond their effect on pain and provides valuable information to support clinical decision-making regarding chronic pain management. Studying outcomes after transition to VHA allows for longterm observation and highlights the potential cross-system impacts of NPT. We hypothesized that the use of NPT in the MHS would be associated with a lower likelihood of adverse outcomes in the VHA. These analyses are part of the Substance Use and Psychological Injury Combat Study (SUPIC), the largest longitudinal, observational study to date of pain management and behavioral health conditions using MHS data from U.S. Army service members returning from deployments in support of Operations Enduring Freedom (OEF), Iraqi Freedom (OIF), and New Dawn (OND). [25]
METHODS
The current study used a longitudinal cohort design where active duty service members with chronic pain were identified through their healthcare records in the military data repository and other DoD sources compiled by SUPIC. [25] MHS data up to the end of 2015 were included. Quasi-experimental methods were used to determine outcomes for identified service members who enrolled in VHA and based on clinical encounters registered in the VHA corporate data warehouse. Healthcare records related to outpatient visits and inpatient stays were used.
Cohort Description

Figure 1 Active duty Army service members with chronic pain after an index OEF/OIF/OND deployment ending between October 1, 2007, and September 30, 2014, were included (N = 286,885). The relative timing of deployment end date, chronic pain diagnosis, NPT treatment, and VHA outcome measurement is shown in Figure 1. Chronic pain was operationalized as a recurrence at least 90 days apart within any of ten clusters of International Classification of Diseases (ICD-9) diagnoses known to be associated with pain (e.g., nontraumatic joint disorders, musculoskeletal disorders). Similar diagnosis clusters have been previously used to identify chronic pain in health record data. [26, 27] The specific codes used for this study are described in detail elsewhere. [26] In addition to diagnoses in a service member’s health records, any 60-days supply of opioids prescribed in a 3-month period or a 90-days supply in 12 months was taken as an indication of chronic pain. [28] We determined days supply across non-injectable opioids prescribed in the MHS including codeine, dihydrocodeine, fentanyl, hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, oxymorphone, and tapentadol.
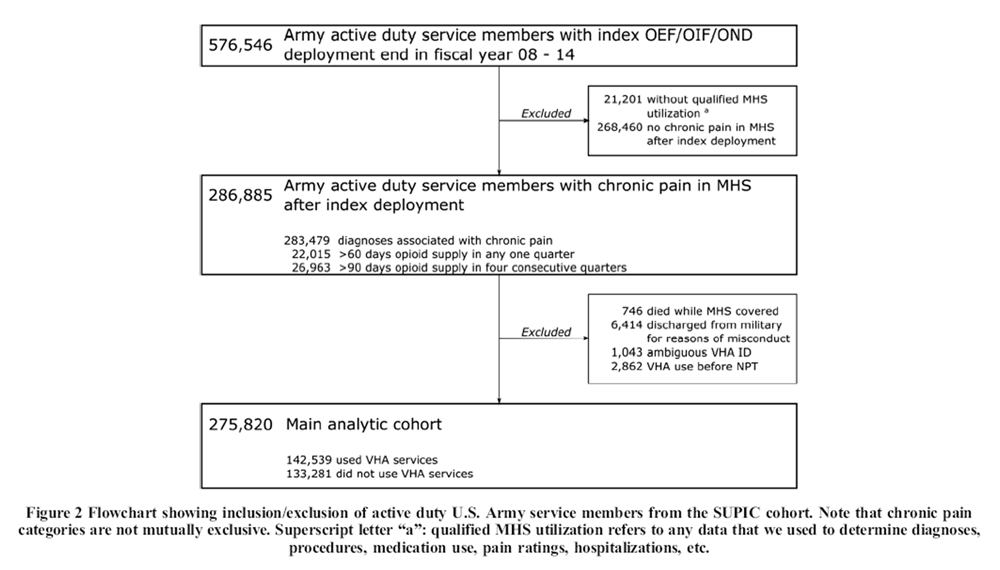
Figure 2 We excluded servicemembers who were discharged from the military for reasons we classified as misconduct and service members who died while receiving care in the MHS, as they were unlikely to have substantial VHA records. To avoid cases where events occurred in reverse order, we also excluded service members who received VHA care before receiving NPT in the military. After applying exclusion criteria, 275,820 Army service members with chronic pain remained: 142,539 who received care in VHA and 133,281 who did not (see Fig. 2).
Independent Variable: Receipt of NPT in the MHS
For each servicemember, we determined if they received any NPT (yes/no) in the MHS after their index deployment. NPT were identified in the MHS data repository using ICD-9 diagnosis codes, Current Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS) codes. NPT were defined as acupuncture/dry needling, biofeedback, chiropractic care, massage, exercise therapy, cold laser therapy, osteopathic spinal manipulation, transcutaneous electrical nerve stimulation (TENS) and other electrical manipulation, ultrasonography, superficial heat treatment, traction, other physical therapy, and lumbar supports. The specific codes used are described in detail elsewhere. [29]
Dependent Variables: Adverse Outcomes in the VHA
Main outcomes after service members enrolled in VHA were determined based on ICD-9 and ICD-10 diagnosis codes recorded in VHA healthcare records until fiscal year 2018 (see eTable 1 in the Supplement). Outcomes included diagnoses of alcohol and/or drug disorders (yes/no), poisoning with opioids, related narcotics, barbiturates, or sedatives (yes/no, separately for accidental and intentional poisoning), suicide ideation (yes/ no), and self-inflicted injuries including suicide attempts (yes/ no). The outcome for alcohol and/or drug disorders combined abuse and dependence and excluded tobacco use disorder.
Propensity Score Weighting
To account for differences between service members with chronic pain who received NPT in the MHS and those who did not, we used propensity score-weighted analyses. Propensity scores represent the probability of group membership, in our studymembership of the group who receivedNPT, and were estimated using the following demographic, clinical, and military service characteristics recorded between the end of the index deployment and the last quarter of MHS utilization or the end of 2015, whichever came first: age, gender, race, marital status, rank/pay grade, fiscal year of index deployment, total days being deployed, days of deployment as of the index deployment, length of observation in the MHS, presence of any diagnoses for the following disorders: adjustment, depression, anxiety, or posttraumatic stress (PTSD), traumatic brain injury (TBI), alcohol use disorder (AUD), or substance use disorder (SUD), whether specialty services were received for mental health or substance use, use of prescription opioids (average dailymorphine equivalents and days supply), sum of appointment days with low pain, sum of appointment days with moderate pain, sum of appointment days with severe pain, and number of inpatient days and hospital discharges. Characteristics that increase with increasing MHS observation time (e.g., days supply opioids, number of inpatient days) were normalized by the length of a service member’s observation in the MHS. Propensity scores were then used to determine inverse probability of treatment weights (IPTW), which were used in our final analyses to balance group differences. [30] To avoid undue influence from extreme weights, we truncated the weights to 10, as 90% of our cohort had a weight less than 10. [31] To account for potential differences between soldiers who do and do not enroll in VHA, we used a multinomial propensity score model to determine IPTW for four groups: (1) NPT and enrolled in VHA; (2) No-NPT and enrolled in VHA; (3) NPT and not enrolled in VHA; and (4) No-NPT and not enrolled in VHA.
Analysis

Table 1 Primary statistical analysis involved time-to-event analysis comparing the two groups that enrolled in VHA (NPT vs. No-NPT). Data were right censored. For each of the outcomes, we report a propensity score-weighted log-rank test for differences in the Kaplan-Meier survival curves and propensity score-weighted multivariable Cox proportional hazard models. We assessed both weighted and unweighted Kaplan- Meier survival curves (see Supplement) and found the weighted curves to support the proportional hazards assumption. The log-rank test combines results of χ2 tests of the probability of an event of interest between two groups across time. [32] Cox proportional hazard models estimate the relative difference (NPT vs. No-NPT) in rates at which events occur across time, while accounting for covariates. Because the No-NPT group is weighted to balance the NPT group, the estimated model coefficient for the group variable represents the average adjusted difference among those exposed to NPT. As the NPT group and the No-NPT group were still significantly different after applying IPTW in age, the length of observation in the MHS, presence of TBI, and the number of inpatient days and the sums of appointment days with low, moderate, and severe pain (see Table 1), we included those variables as covariates in our final analyses, following Ridgeway et al. [33] As MHS alcohol and drug use diagnoses were available, we limited analyses for that particular outcome to only those service members who had not been diagnosed with alcohol and/or drug abuse or dependence while in the MHS, essentially focusing on new-onset alcohol and drug use disorders in VHA (n = 86,773 with NPT, n = 18,789 No-NPT).
Descriptive statistics showed that 26,103 (9.5%) active duty service members with chronic pain received NPT before they were diagnosed with chronic pain. Running our primary analyses without these service members did not substantially change our results (data not shown), and these service members were retained in our analytic cohort.
As a secondary analysis and to address potential alternative interpretations of our results, we added additional covariates from each service member’s VHA healthcare records to the Cox proportional hazard models. Specifically, we added length of observation in the VHA, exposure to NPT in the VHA, and days supply of opioids in the VHA, based on the same specifications that were used to determine these variables in the MHS.
All analyses were done in R version 3.5.3 and IPTW were determined with the function ‘mnps’ from the R package ‘twang’. [34] All propensity score-weighted analyses were done with functions from the ‘Survey’ package and P < 0.05 was considered statistically significant. Approval for this study was granted by the Brandeis University Committee for Protection of Human Subjects, the Stanford University and VA Palo Alto Health Care System Institutional Review Boards, and the Human Research Protection Program at the Office of the Assistant Secretary of Defense for Health Affairs/ Defense Health Agency (OASD/DHA). The DHA Privacy and Civil Liberties Office executed an annual Data Sharing Agreement.
RESULTS

Table 2

Table 3

Table 4 Table 1 shows the demographic and military variables as well as the clinical history based on MHS records of service members in our cohort. The table also shows that propensity score weighting was successful in balancing the NPT and the No- NPT groups on most variables. The median age in the cohort was 26 (range 18–67), and most service members were married (63.2%) and ranked as enlisted (91.7%). The median total duration of deployments was 446 days (range 30–3856) and the median duration of observation in theMHS after the index deployment was 1274 days (range 0–2912). Table 2 shows the distribution of diagnoses associated with pain and exposure to NPT for each cluster of diagnoses, and Table 3 shows the rate of each NPT modality.
Alcohol and/or drug use disorders were the most frequent adverse outcome (n = 28,614; 20.1%; median time to event 264 days), followed by suicide ideation (n = 7648; 5.4%; median time to event 581 days) and self-inflicted injuries including suicide attempts (n = 1621; 1.1%; median time to event 621 days). Poisoning with opioids, related narcotics, barbiturates, or sedatives was least frequent (intentional poisoning: n = 270, 0.2%, median time to event 786 days; accidental poisoning: n = 147, 0.1%, median time to event 1045 days). Table 4 shows the results of the time-to-event analyses for the models with and without additional VHA covariates. The propensity score-weighted log-rank test showed significant differences in the Kaplan-Meier survival curves between the NPTand No-NPT groups for all outcomes, except for intentional poisoning with opioids, related narcotics, barbiturates, or sedatives. The Cox proportional hazard models showed that, while accounting for covariates, the relative rate at which events occurred (NPT vs. No-NPT) was significantly less than 1.0. The proportional hazard for the NPT group was 0.92 for alcohol and/or drug use disorders, 0.65 and 0.82 for accidental and intentional poisoning with opioids, related narcotics, barbiturates, or sedatives, 0.88 for suicide ideation, and 0.83 for self-inflicted injuries including suicide attempts, compared to the No-NPT group. The Cox proportional hazard models additionally adjusted for VHA covariates showed only marginally lower proportions, compared to the models without VHA covariates. We also did propensity score-weighted logistic regressions for these adverse outcomes and found essentially the same results (see eTable 2 in the Supplement) .
We ran a chi-square analysis on the proportion of service members who died after transition to the VHA before any of our outcomes of interest occurred and found that slightly more service members died in the No-NPT group compared to the NPT group (0.5% vs. 0.4%, χ2 = 11.16, P < 0.001).
DISCUSSION
The purpose of this study was to compare active duty U.S. Army service members with chronic pain who did and did not receive NPT in theMHS and describe the association between receiving NPT in the MHS and adverse outcomes observed in the VHA. The results corroborated our hypothesis that use of NPT in the MHS would be inversely associated with adverse outcomes in the VHA. Active duty service members with chronic pain who received NPT in the MHS were at significantly lower risk in the VHA for new-onset alcohol and/or drug use disorder, poisoning with opioids, related narcotics, barbiturates, or sedatives, suicide ideation, and self-inflicted injuries including suicide attempts. These results were observed when we used propensity score-weighted Cox proportional hazardmodels and were further supported by propensity score-weighted logistic regression.
Note that we did not study individual NPT modalities. If some modalities did not protect against adverse outcomes, our resultsmay understate the effect for NPT modalities that did protect against adverse outcomes. It is well-known that ICD codes for suicide ideation, suicide attempts, and other self-inflicted injuries are underreported in electronic medical records. [35–37] As such, the rates of these adverse outcomes based on our data may be lower than the actual rates. However, we have no reason to believe that the amount of underreporting differed between those who did and did not receive NPT. Consequently, we think it unlikely that relying on ICD codes has affected our findings for suicide ideation and self-inflicted injuries.
From other research into NPT, it might be inferred that service members who use NPT may be healthier than those who do not use NPT and as such might be expected to be at lower risk for adverse outcomes. [38] However, we did not observe this pattern in our data. Service members who received NPT in the military more often reported their highest pain level as low, moderate, or severe, were more often hospitalized and had longer inpatient stays, and were more likely to be diagnosed with mental disorders (except alcohol use disorder) than service members who had not received NPT. A study by Han et al. also found higher pain intensity and a higher likelihood of mental disorders in veterans who received NPT in the VHA, compared to those who did not receive NPT. [17] The proportion of service members who received NPT and who died before an adverse event occurred was slightly lower than for those who had not received NPT. This reinforces our confidence in the favorable effect of NPT, as proportionally more who received NPT remained at risk.
We are not aware of other studies of chronic pain and NPT that examined the transition from the MHS to the VHA. By combining data from two healthcare systems, we could study long-term outcomes and examine the cross-system impacts of NPT. The use of propensity score weights and additional VHA covariates in the analyses accounted for group differences and increased our confidence that the observed protective associations with adverse outcomes in the VHA are indeed due to NPT received in the MHS. While our analysis methods were rigorous, there are several unobserved factors that we could not control for and should be considered limitations of the study. The vastmajority of NPTwas administered after service members were diagnosed with chronic pain; however, we do not know whether NPT was specifically provided to treat chronic pain.
It could also be that some service members in our cohort used other pain treatments of a psychobehavioral nature (e.g., cognitive behavioral therapy for chronic pain) or of a pharmacological nature (e.g., NSAIDs, acetaminophen, gabapentinoids, serotonin-norepinephrine reuptake inhibitors), or any self-administered treatment not captured in the medical record. If these other pain treatments decreased pain levels or the number of days with pain, it would be reasonable to conclude that at least some of the observed effects were caused by these other pain treatments. Yet, the NPT group reported more days with pain, regardless of pain level, weakening the argument that other pain treatments may have been responsible for the observed effects. Also, service members with more combat exposure during their deployments are at higher risk for suicidal behavior. [39, 40]
While our analyses accounted for covariates of combat exposure (e.g., TBI and PTSD), we did not control for combat exposure itself. It is possible that combat exposure was not balanced between the NPTand No-NPT group, thus potentially affecting the observed associations between NPT and suicide ideation, and self-inflicted injuries in our study. As the prevalence of individual NPTmodalities in the MHS varied widely, it is possible that our results were driven by some NPT modalities more than others, or the dose in which they were received, and that the protective association of NPT may be more apparent for some chronic pain conditions than for others. We were unable to control for the severity of any of the mental health conditions that we included as covariates, nor did we control for physical health conditions associated with adverse outcomes (see, for example, Ahmedani et al.), [41] although these aspects may have been reflected in the number of hospitalizations and number of inpatient days. Overall, we do not expect that an imbalance of physical health conditions is likely to have affected our results, as our cohort was relatively young (median age 26) and healthy (they had been deployed to Iraq or Afghanistan).
Our results suggest that nonpharmacological treatments (NPT) provided to active duty service members with chronic pain may reduce their odds of longterm adverse outcomes. Given known associations of these adverse outcomes with morbidity and mortality, providing NPT to service members with chronic pain could potentially save lives. Our results provide further support for the role of NPT as a risk mitigation strategy when long-term opioid therapy is initiated, which is only briefly mentioned in the VA/DoD Clinical Practice Guideline for Opioid Therapy for Chronic Pain. [16] Given that our findings may have been driven by some NPT modalities more than others, the dose in which these modalities were received, or unmeasured confounding, more research is needed to clarify these effects. As confounders may change during NPT (e.g., daily dose of opioids), it may be important to include time-varying covariates in follow-up research.
Supplementary material
11606_2019_5450_MOESM1_ESM.docx
eFigure 1. Trends in Self-Reported Prediabetes by Age (Panel A),
Body Mass Index Category (Panel B), and
Race/Ethnicity Group (Panel C)
eTable 1. Demographic and Clinical Characteristics of U.S. Adults with
Self-Reported Prediabetes by Race/Ethnicity,
NHANES 2005-2014Acknowledgments:
We thank Richard Gromadzki and Andrea Linton with AXIOM Resource Management, Inc., who compiled the DoD data used in these analyses, and the Defense Health Agency’s Privacy and Civil Liberties Office, which provided access to the DoD data.
Funding Information
This research was supported by grants NIDA R01DA030150 and NCCIH R01AT008404 from the National Institutes of Health (M.J. Larson) and a Research Career Scientist Award RCS-14-232 from the Veterans Health Administration Health Services Research and Development Service (A.H. Harris).
Compliance with Ethical Standards:
Approval for this study was granted by the Brandeis University Committee for Protection of Human Subjects, the Stanford University and VA Palo Alto Health Care System Institutional Review Boards, and the Human Research Protection Program at the Office of the Assistant Secretary of Defense for Health Affairs/ Defense Health Agency (OASD/DHA).
Conflict of Interest:
The authors declare that they do not have a conflict of interest.
References:
Institute of Medicine.
Relieving Pain in America: A Blueprint for Transforming Prevention,
Care, Education, and Research
Washington, DC: The National Academies Press, 2011.Spelman JF, Hunt SC, Seal KH, Burgo-Black AL.
Post deployment care for returning combat veterans.
J Gen Intern Med 2012;27(9):1200-9. https://doi.org/10.1007/s11606-012-2061-1.Seal KH, Bertenthal D, Barnes DE, Byers AL, Strigo I, Yaffe K, et al.
Association of Traumatic Brain Injury With Chronic Pain in Iraq and Afghanistan Veterans: Effect of Comorbid Mental Health Conditions.
Arch Phys Med Rehabil. 2017;98(8):1636-45. https://doi.org/10.1016/j.apmr.2017.03.026.Helmer DA, Chandler HK, Quigley KS, Blatt M, Teichman R, Lange G.
Chronic widespread pain, mental health, and physical role function in OEF/OIF veterans.
Pain Med. 2009;10(7):1174-82. https://doi.org/10.1111/j.1526-4637.2009.00723.x.Toblin RL, Quartana PJ, Riviere LA, Walper KC, Hoge CW.
Chronic pain and opioid use in US soldiers after combat deployment.
JAMA. 2014;174(8):1400-1. https://doi.org/10.1001/jamainternmed.2014.2726.Crosby FE, Colestro J, Ventura MR, Graham K.
Survey of pain among veterans in Western New York.
Pain Manag Nurs. 2006;7(1):12-22. https://doi.org/10.1016/j.pmn.2005.12.001.Kerns RD, Otis J, Rosenberg R, Reid MC.
Veterans’ reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system.
J Rehabil Res Dev. 2003;40(5):371-9.Racine M.
Chronic pain and suicide risk: A comprehensive review.
Prog. Neuropsychopharmacol. Biol Psychiatry. 2018;87(Pt B):269–4LL
https://doi.org/10.1016/j.pnpbp.2017.08.020.Kaye AD, Jones MR, Kaye AM, Ripoll JG, Galan V, Beakley BD, et al.
Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: Part 1.
Pain Physician. 2017;20(2S):S93-S109.Paulozzi LJ, Zhang K, Jones CM, Mack KA.
Risk of adverse health outcomes with increasing duration and regularity of opioid therapy.
J Am Board Fam Med. 2014;27(3):329-38. https://doi.org/10.3122/jabfm.2014.03.130290.Morasco BJ, O'Hearn D, Turk DC, Dobscha SK.
Associations between prescription opioid use and sleep impairment among veterans with chronic pain.
Pain Med. 2014;15(11):1902-10. https://doi.org/10.1111/pme.12472.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al.
Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan.
JAMA. 2012;307(9):940-7. https://doi.org/10.1001/jama.2012.234.Barth KS, Guille C, McCauley J, Brady KT.
Targeting practitioners: A review of guidelines, training, and policy in pain management.
Drug Alcohol Depend. 2017;173 Suppl 1:S22-s30
https://doi.org/10.1016/j.drugalcdep.2016.08.641.Macey TA, Morasco BJ, Duckart JP, Dobscha SK.
Patterns and correlates of prescription opioid use in OEF/OIF veterans with chronic noncancer pain.
Pain Med. 2011;12(10):1502-9
https://doi.org/10.1111/j.1526-4637.2011.01226.x.The Opioid Therapy for Chronic Pain Work Group
Healthcare Inspection - VA Patterns of Dispensing Take-Home Opioids and Monitoring Patients on Opioid Therapy:
Department of Veterans Affairs - Office of Inspector General,, I
nspections OoH;2014 May 14, 2014.
Report No.: Report No. 14-00895-163.U.S. Department of Veterans Affairs - Office of Inspector General
VA/DoD Clinical Practice Guideline for Opioid Therapy for Chronic Pain
U.S. Department of Veterans Affairs - Department of Defense.
Version 3.0 – 2017Han L, Goulet JL, Skanderson M, Bathulapalli H, Luther SL, Kerns RD, et al.
Evaluation of Complementary and Integrative Health Approaches Among US Veterans
with Musculoskeletal Pain Using Propensity Score Methods.
Pain Med. 2018. https://doi.org/10. 1093/pm/pny027.The Diagnosis and Treatment of Low Back Pain Work Group.
VA/DoD Clinical Practice Guideline for Diagnosis and Treatment of Low Back Pain
Washington, DC: The Office of Quality, Safety and Value, VA, &
Office of Evidence Based Practice, U.S. Army MedicalCommand, 2017, Version 2.0.Buckenmaier CC, 3rd, Galloway KT, Polomano RC, Deuster PA.
Pain as a Barrier to Human Performance: A Focus on Function for Self-Reporting Pain With the Defense Veterans Pain Rating Scale
J Spec Oper Med. 2016;16(2):82-7.National Center for Complementary and Integrative Health.
Complementary, Alternative, or Integrative Health: What’s In a Name?
Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al.
Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2017 (Apr 4); 166 (7): 493–505Chou R, Huffman LH; American Pain Society.
Nonpharmacologic Therapies for Acute and Chronic Low Back Pain:
A Review of the Evidence for an American Pain Society/
American College of Physicians Clinical Practice Guideline
Annals of Internal Medicine 2007 (Oct 2); 147 (7): 492–504Larson MJ, Adams RS, Ritter GA, Linton A, Williams TV, Saadoun M, et al.
Associations of Early Treatments for Low-Back Pain with Military Readiness Outcomes.
J Altern Complement Med. 2018;24(7):666-76
https://doi.org/10.1089/acm.2017.0290.Carey EP, Nolan C, Kerns RD, Ho PM, Frank JW.
Association Between Facility-Level Utilization of Non-pharmacologic Chronic Pain Treatment and Subsequent Initiation of Long-Term Opioid Therapy.
J Gen Intern Med. 2018;33(Suppl 1):38-45
https://doi.org/10.1007/s11606-018-4324-y.Larson MJ, Adams RS, Mohr BA, Harris AH, Merrick EL, Funk W, et al.
Rationale and methods of the Substance Use and Psychological Injury Combat Study (SUPIC): a longitudinal study of Army service members returning from deployment in FY2008-2011.
Subst Use Misuse. 2013;48(10):863-79
https://doi.org/10.3109/10826084.2013.794840.Reif S, Adams RS, Ritter GA, Williams TV, Larson MJ.
Prevalence of Pain Diagnoses and Burden of Pain
Among Active Duty Soldiers, FY2012
Military Medicine 2018 (Sep 1); 183 (9-10): e330–e337Dobscha SK, Morasco BJ, Kovas AE, Peters DM, Hart K, McFarland BH.
Short-term variability in outpatient pain intensity scores in a national sample of older veterans with chronic pain.
Pain Med 2015;16(5):855-65.
https://doi.org/10.1111/pme.12643.Tian TY, Zlateva I, Anderson DR.
Using electronic health records data to identify patients with chronic pain in a primary care setting.
J Am Med Inform Assoc 2013;20(e2):e275-80.
https://doi.org/10.1136/amiajnl-2013-001856.VannemanME, LarsonMJ, Chen C, Adams RS,Williams TV, Meerwijk E, et al.
Treatment of Low Back Pain With Opioids and Nonpharmacologic Treatment Modalities for Army Veterans.
Med Care. 2018.
https://doi.org/10.1097/MLR.0000000000000977.Linden A, Adams JL.
Using propensity score-based weighting in the evaluation of health management programme effectiveness.
J Eval Clin Pract. 2010;16(1):175-9.
https://doi.org/10.1111/j.1365-2753.2009.01219.x.Austin PC, Stuart EA.
Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies.
Stat Med. 2015;34(28):3661-79.
https://doi.org/10.1002/sim.6607.Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, Wang EW.
A practical guide to understanding Kaplan-Meier curves.
Otolaryngol Head Neck Surg. 2010;143(3):331-6.
https://doi.org/10.1016/j.otohns.2010.05.007.Ridgeway G, McCaffrey D, Morral A, Burgette L, Griffin BA.
Toolkit for Weighting and Analysis of Nonequivalent Groups: A tutorial for the twang package. 2017.R Core Team. R:
A language and environment for statistical computing.
Vienna: R Foundation for Statistical Computing: 2018.
https://www.Rproject.org.Anderson HD, Pace WD, Brandt E, Nielsen RD, Allen RR, Libby AM, et al.
Monitoring suicidal patients in primary care using electronic health records.
J Am Board Fam Med. 2015;28(1):65-71.
https://doi.org/10.3122/jabfm.2015.01.140181.Hedegaard H, Schoenbaum M, Claassen C, Crosby A, Holland K, Proescholdbell S.
Issues in Developing a Surveillance Case Definition for Nonfatal Suicide Attempt and Intentional Self-harm Using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Coded Data.
Natl Health Stat Rep. 2018(108):1-19.Hoffmire C, Stephens B, Morley S, Thompson C, Kemp J, Bossarte RM. VA
Suicide Prevention Applications Network: A National Health Care System-Based Suicide Event Tracking System.
Public Health Rep. 2016;131(6):816-21.
https://doi.org/10.1177/0033354916670133.Micek MA, Bradley KA, Braddock CH, 3rd, Maynard C, McDonell M, Fihn SD.
Complementary and alternative medicine use among Veterans Affairs outpatients.
J Altern Complement Med. 2007;13(2):190-3.
https://doi.org/10.1089/acm.2006.6147.Dillon KH, CunninghamKC, Neal JM, Wilson SM, Dedert EA, Elbogen EB, et al.
Examination of the indirect effects of combat exposure on suicidal behavior in veterans.
J Affect Disord. 2018;235:407-13.
https://doi.org/10.1016/j.jad.2018.04.031.Cesur R, Chesney A, Sabia JJ.
Combat Exposure, Cigarette Consumption, and Substance Use.
Econ Inq. 2016;54(3):1705-26.
https://doi.org/10.1111/ecin.12312.Ahmedani BK, Peterson EL, Hu Y, Rossom RC, Lynch F, Lu CY, et al.
Major Physical Health Conditions and Risk of Suicide.
Am J Prev Med. 2017;53(3):308-15.
https://doi.org/10.1016/j.amepre.2017.04.001.

Return to LOW BACK PAIN
Return NON-PHARMACOLOGIC THERAPY
Return to CHIROPRACTIC CARE FOR VETERANS
Since 11-12-2019


| Home Page | Visit Our Sponsors | Become a Sponsor |
Please read our DISCLAIMER |